The role of water in the formation of crystal structures: a case study of valnemulin hydrochloride†
Shuyu
Li
a,
Ting
Wang
*ab,
Xin
Huang
ab,
Lina
Zhou
ab,
Mingyu
Chen
a,
Wanying
Liu
a,
Xiunan
Zhang
a,
Yuyuan
Dong
a and
Hongxun
Hao
 *abc
*abc
aNational Engineering Research Center of Industrial Crystallization Technology, School of Chemical Engineering and Technology, Tianjin University, Tianjin, 300072, China. E-mail: hongxunhao@tju.edu.cn
bState Key Laboratory of Chemical Engineering, Tianjin University, Tianjin 30072, China
cSchool of Chemical Engineering and Technology, Hainan University, Haikou 570208, China
First published on 16th November 2020
Abstract
In this work, a crystalline dihydrate form of valnemulin hydrochloride (VHW) was discovered and characterized. The single crystal data of VHW were determined and analyzed. Compared with the two valnemulin hydrochloride solvates reported previously, VHW exhibits better stability. From the crystal structure analysis of the three crystal forms and molecular conformation analysis, it was found that water molecules play a key role in the formation of crystalline state valnemulin hydrochloride. At the same time, it was found that except for packing, conformational similarity also has a certain effect on the polymorphic transformation behaviors of different forms of valnemulin hydrochloride.
Introduction
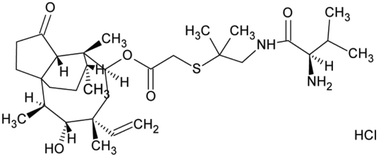
Polymorphism refers to a crystal system in which a substance can exist in a structure characterized by different crystal cells, but each form having exactly the same element composition.1 For solvates, the elemental composition of their cells also consists of one or more solvent molecules.2 When the solvent molecule in the crystal structure is water, it is named as hydrate.3–7 As a ubiquitous substance in nature, water is small in size and strong in hydrogen bonding ability, which makes it an ideal medium to connect drug molecules to form a stable crystal structure.8–10 As a result, hydrates are the most common type of solvated organic crystals.1 Solvates or hydrates have many applications in practice.11,12 Hence, it is important to determine the relative stability of solvate/hydrate for practical purposes. For the study of the relative stability of solvate/hydrate, the role of solvent/water must be considered.13In this work, to better understand the role of solvents during the formation of solvate/hydrate and the effect of the existence of solvent molecules on the properties of the host molecule, valnemulin hydrochloride (VH) was used as a model compound to investigate the effect of water on the formation of crystal structures. Valnemulin hydrochloride is a new semisynthetic pleuromutilin derivative, whose molecular structural formula is shown in Fig. 1. It is mainly used to prevent and cure mycoplasma disease and Gram-positive bacterial infection in pigs, cattle, sheep and poultry.14–17 At present, amorphous valnemulin hydrochloride is mainly used as a commercial material. This amorphous form has many shortcomings, such as low stability, strong hygroscopicity, low bulk density and heavy irritating taste.18 Many efforts have been made to prepare crystalline state product of valnemulin hydrochloride. However, only two kinds of crystalline solvates, methanol–water solvate (VHWMS) and ethanol–water solvate (VHWES), have been successfully developed and characterized.19 Although the hygroscopicity of these two solvates are better than that of amorphous valnemulin hydrochloride, their thermal stabilities are still not ideal and they will lose some associated solvents continuously at around 40 °C, which limits their applications.
In this study, one new form of valnemulin hydrochloride, valnemulin hydrochloride dihydrate (VHW), was developed for the first time and was characterized. Since there are water molecules in all crystal forms of valnemulin hydrochloride at present, it is speculated that water plays a key role in the crystal formation of valnemulin hydrochloride. Therefore, the single crystal structures of different crystals are determined and analyzed to explore the role of water in the stabilization of these structures. At the same time, the change of molecular conformation in different forms was also analyzed. The transformation process of VHW to VHWMS and VHWES was investigated by infrared and Raman spectroscopy and discussed in detail. The results show that, in addition to stacking similarity, the conformation may also have significant impact on the crystallization kinetics.
Experimental
Materials
VH with a purity of 99.0% (mass fraction) was supplied by Hubei Longxiang Pharmaceutical Co. Ltd. of China. The solvents, including ethanol and methanol, are analytical grade reagents and were obtained from Kemiou Chemical Reagent Co. Ltd. and were used without any further purification. Deionized water was prepared using an ultrapure water system (YPYD Co., China) in the laboratory and used throughout.Preparation of different crystal forms
The polymorph screening trials9 includes evaporation, cooling crystallization, slurry crystallization, antisolvent precipitation, liquid-assisted grinding experiments and crystallization with additives (detailed procedures in the ESI†). Finally, three crystal forms were obtained, including VHW, VHWES and VHWMS.Characterization methods
Powder X-ray diffraction (PXRD) was used to identify the samples on Rigaku D/max-2500 (Rigaku) using Cu Kα radiation (0.15405 nm) in the 2θ range of 2–40° and a scanning speed rate of 8° min−1.FTIR spectroscopy (Bruker, Germany) with a resolution of 4 cm−1 and 24 scans per spectrum ranging from 4000 cm−1 to 360 cm−1 was used to identify the different crystal forms.
Thermal analysis
Differential scanning calorimetry (DSC) measurements were conducted on DSC 1/500 (Mettler Toledo, Switzerland) with a dry nitrogen purge flow of 100 mL min−1. About 5–10 mg of the powder samples were placed into aluminum pans and analyzed within the temperature range from 303 to 423 K at a heating rate of 5 K min−1. TGA was performed on TGA/DSC STARev System (Mettler Toledo, Switzerland) with a nitrogen purge flow of 20 mL min−1, and the temperature range was from 303 to 573 K at a heating rate of 10 K min−1.The solid-state dehydration behaviors of VHWES and VHW were observed under a hot-stage microscope (HSM, Olympus UMAD3). The sample was heated from 303.15 to 473.15 K with a heating rate of 5 K min−1. Photos were recorded using a polarized light microscope.
Dynamic vapor sorption (DVS)
The water sorption/desorption experiments of VH were performed on a dynamic vapor sorption analyzer (VTI-SA, VTI Corporation, United States). The experiments were conducted over a relative humidity (RH) range of 5 to 95% by a step of 5%. RH was maintained until the weight change is less than 0.01 wt% per minute or the measurement last maximum step length (60 min). The temperature was set at 298.15 K, and solid forms were analyzed by PXRD after experiments.Single crystal X-ray diffraction (SCXRD)
The single crystals of VHW were obtained by using a solvent evaporation method (detailed procedures in the ESI†). Single crystal X-ray diffraction (SCXRD) measurements were conducted on a Rigaku Saturn 70 CCD diffractometer using Mo Kα radiation (λ = 1.54184 Å) with a graphite monochromator. Integration and scaling of the intensity data were accomplished using the SAINT program. The structure was solved using the SHELXS-2014 suite of programs, and refinement was conducted using SHELXL-2018. Anisotropic displacement parameters were refined for all nonhydrogen atoms. All hydrogen atoms were located by Fourier-difference synthesis and fixed geometrically according to the environment with predefined isotropic thermal factors. The data were corrected considering the effects of absorption using SADABS.Study on the process of solvent-mediated polymorphic transformation
The process of solvent-mediated polymorphic transformation was in situ monitored by attenuated total reflectance Fourier transform infrared (ATR-FTIR) and Raman spectroscopy.20–22Raman spectra were collected with a Kaiser Raman RXN2 system (Kaiser Optical System, Inc., Ann Arbor, MI, USA). The solid powders were analyzed with a PhAT probe which was perpendicularly fixed above the solid powders at 2–3 mm. In the transformation experiments, the immersion MR probe was inserted into the crystallizer and immersed into the suspension. Tin foil was used for shading during the whole experiment process. The Raman spectra were collected in the range of 100 cm−1 to 3200 cm−1 with a resolution of 0.5 cm.
FTIR spectra were recorded on an ATR-FTIR spectrometer (ReactIRTM45, Mettler-Toledo) equipped with a Duradisc Dicomp probe for the solution samples. For each sample, 32 scans were collected over a spectral range from 800 to 4000 cm−1 at a resolution of 4 cm−1 to study the change of solution concentration in the process of crystal transformation.
Results and discussion
Identification and characterization of the different crystal forms
Fig. S1a† shows the XRD spectra of the three crystal forms. It was found that the peak positions of VHWES and VHWMS are basically the same, which are the same as those reported in previous literature.19 Compared with VHWES and VHWMS, VHW is apparently different, indicating that this hydrate is a new crystal form. Fig. S1b† shows the comparison between the XRD data of experimental sample and the simulated XRD data from the corresponding single crystal data, verifying the reliability of the XRD results.Fig. S2† shows the infrared spectra of the three crystal forms and amorphous form. VHWES and VHWMS have a small peak at 3476 cm−1, corresponding to the water molecule –OH stretching vibration of the two solvates. However, this peak does not appear in amorphous form. The related –OH vibration of the water molecule in VHW has a red shift compared with the two solvates, and a wide peak appears at 3422 cm−1, indicating that there is a stronger hydrogen bond interaction between the water molecules and API molecules.23
Thermal stability analysis
Fig. S3† shows the thermal analysis results of the three crystal forms. TG results suggested that all of the three crystal forms lost weight, indicating the loss of solvent during heating (Fig. S3b†). The melting peaks of the three crystal forms overlap with the desolvation peaks, and the peak of VHW appears in a higher temperature range (Fig. S3a†). Combined with the infrared data (Fig. S2†), it can be speculated that this is due to the stronger hydrogen bonding between the water and API molecules in VHW, which makes the crystal structure more stable.In order to verify that the desolvation process and melting occurred at the same time, hot-stage microscopy was used to monitor the melting process. Fig. 2 and 3 are the hot-stage microscopy images describing the melting process of VHW and VHWES, respectively. For VHW, the color of the crystal began to darken at 323 K, indicating the occurrence of desolvation. When the temperature reached 379 K, the color of the crystal further darkened, during which the small crystal at the edge of the crystal begins to melt. As the temperature rises, the solvent removal and melting processes further proceed and at 433 K the crystal completely melts and the solvent is removed completely. The melting process of VHWES is similar to that of VHW, in which the desolvation is accompanied by melting. The only difference is that both the desolvation temperature and melting temperature of VHWES are lower than those of VHW. Cracks on the crystal surface can be observed at 307 K. As the temperature rises, a slight melting phenomenon was observed at 353 K, and the crystal almost has no color at 379 K. At 393 K, the melting and desolvation processes are almost done. The thermal analysis data and hot-stage microscopy data confirm that the stability of VHW is better than that of VHWES.
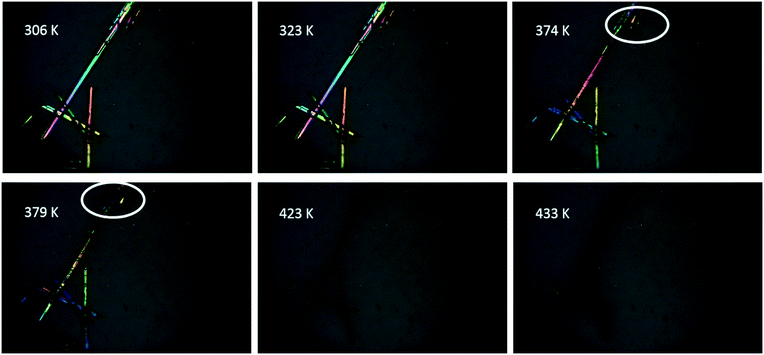 | ||
| Fig. 2 Hot-stage microscopy images of the solid-state dehydration of VHW during the heating process. The two white circles suggest the melting of the small crystal. | ||
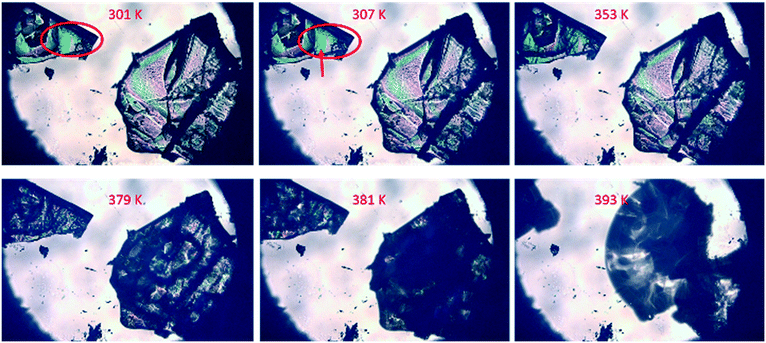 | ||
| Fig. 3 Hot-stage microscopy images of the solid-state desolvation of VHWES during the heating process. In the red circle, cracks on the crystal surface can be observed at 307 K. | ||
The water vapor adsorption and desorption experiments were also carried out for the three crystal forms and the DVS diagrams are illustrated in Fig. S4.† It can be found that the DVS curves of VHWES and VHWMS are relatively close. For the adsorption process, both VHWES and VHWMS exhibit relatively obvious moisture absorption when the relative humidity is 5%, after which it continues to rise slowly from 5% to 80%. When the humidity is greater than 80%, the moisture absorption increases sharply, reaching 6.5% and 8.4%, respectively. For the desorption process, both VHWES and VHWMS cannot lose water totally, even when the humidity is reduced to 5%. 3.3% and 5.9% of water are still maintained for VHWES and VHWMS, respectively. In addition, the PXRD results showed that the products after the adsorption and desorption experiments of VHWES and VHWMS were amorphous (Fig. S5†). Compared with VHWES and VHWMS, VHW maintained relatively stable properties throughout the process. Even at 95% relative humidity, the moisture absorption capacity was only 1.9%, and only 0.6% of water was kept after the desorption process. Furthermore, the solid appearance did not change after the end of the experiment. The PXRD results show that the crystal form is still VHW (Fig. S5†), indicating its better stability. From the thermal stability and hygroscopicity analysis, it can be concluded that VHW is more stable.
Crystal structure analysis
Single crystal data of VHWES and VHWMS, together with the newly obtained single crystal data of VHW, are presented in Table 1. It can be found that VHWES and VHWMS belong to the monoclinic system and P21 space group, which are consistent with the data reported previously.19 There are both water molecules and solvent molecules inside the crystal lattice of VHWES and VHWMS, and the ratio of VH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent is 1
solvent is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Different from the above two solvates, the hydrate VHW belongs to the triclinic system, P1 space group, and the ratio of water molecules to VH molecules is 2
1. Different from the above two solvates, the hydrate VHW belongs to the triclinic system, P1 space group, and the ratio of water molecules to VH molecules is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
| Name | VHWES | VHWMS | VHW |
|---|---|---|---|
| Empirical formula | C33H61ClN2O7S | C32H59ClN2O7S | C31H57ClN2O7S |
| Formula weight | 665.3 | 651.3 | 637.3 |
| Temperature (K) | 113(2) | 113(2) | 160(10) |
| Wavelength (Å) | 0.71073 | 0.71073 | 1.54184 |
| Crystal system, space group | Monoclinic, P2(1) | Monoclinic, P2(1) | Triclinic, P1 |
| Unit cell dimensions (Å) | a = 8.4013(17) | a = 8.3555(17) | a = 9.60590(10) |
| b = 16.424(3) | b = 16.471(3) | b = 15.35080(10) | |
| c = 13.536(3) | c = 13.319(3) | c = 19.7270(2) | |
| Unit cell dimensions (deg) | α = 90 | α = 90 | α = 105.9470(10) |
| β = 104.34(3) | β = 105.13(3) | β = 93.0280(10) | |
| γ = 90 | γ = 90 | γ = 108.1380(10) | |
| Z | 2 | 2 | 3 |
| Z′ | 1 | 1 | 3 |
| Crystal size | 0.20 × 0.18 × 0.12 mm | 0.20 × 0.18 × 0.12 mm | 0.10 × 0.05 × 0.05 mm |
| Theta range for data collection (deg) | 1.99 to 27.97 | 1.99 to 27.97 | 2.357 to 77.546 |
| R int (%) | 6.47 | 5.96 | 7.45 |
| Flack parameter | −0.03(3) | 0.00(5) | 0.020(19) |
| Goodness-of-fit on F2 | 1.018 | 0.966 | 1.039 |
Study on the role of water in crystal structures
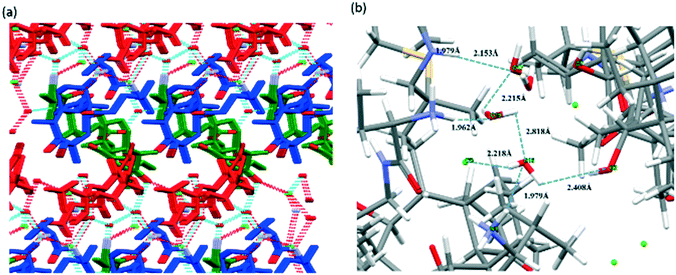
Due to the fact that water molecules exist in all of the three crystal forms of valnemulin hydrochloride, it can be inferred that water plays a very important role in the formation of crystal structures of valnemulin hydrochloride. In order to understand the effect of water on the crystal structures and the reason why VHW is more stable, the crystal structures of the three crystal forms are analyzed in detail.With the help of crystal explorer, 2D fingerprint plots of the crystals which quantitatively summarize the properties and types of intermolecular interactions and present them in a convenient graphic form can be obtained.24–26Fig. 4 illustrates the cell diagram and 2D fingerprint plot of VHWES and VHWMS. It can be seen that there are two asymmetric units in the crystal cells of the two solvates, each containing an API molecule, a water molecule and an ethanol/methanol molecule, and the 2D fingerprints plots of the two crystal forms' API molecules are almost the same. What is more, there is a similar mode of intermolecular interaction in the two crystal forms, both of which contain H⋯Cl, H⋯O, H⋯S, and H⋯H interactions. Fig. 5 suggests that the hydrogen bonds are formed between the water molecule and the protonated amino group of the API molecule, the carbonyl group on the ring and the chloride ion. These hydrogen bonds form hydrogen-bonded chains along the b-axis.
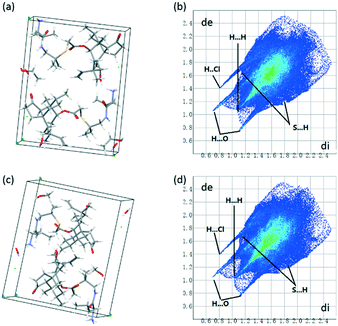 | ||
| Fig. 4 (a) Cell diagram of VHWES; (b) 2D fingerprint plot of VHWES; (c) cell diagram of VHWMS; (d) 2D fingerprint plot of VHWES. | ||
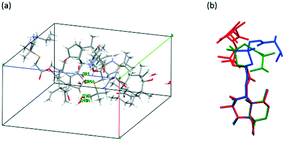 | ||
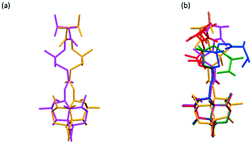
| Fig. 5 (a) Cell diagram of VHW. (b) The comparison of the molecular conformation of VHW (green, red and blue represent conformations I, II and III of VHW, respectively). | ||
And the ethanol or methanol molecules connect the adjacent hydrogen-bonded chains to form a hydrogen-bonded plane perpendicular to the c axis.
For the VHW crystal structure, there is one asymmetric unit in each cell. Three VH molecules with different conformations and six water molecules exist in one asymmetric unit, and water 16 and water 21 are disordered (Fig. 6a). Fig. 6b shows the conformational overlay diagram of the three molecule conformations, I, II and III, in the asymmetric unit (green, red and blue represent conformations I, II and III, respectively), in which conformation II has a certain degree of disorder. Through this picture, it can be found that there are three different conformations of the API molecule in VHW, which indicates that its crystal structure is more complicated.
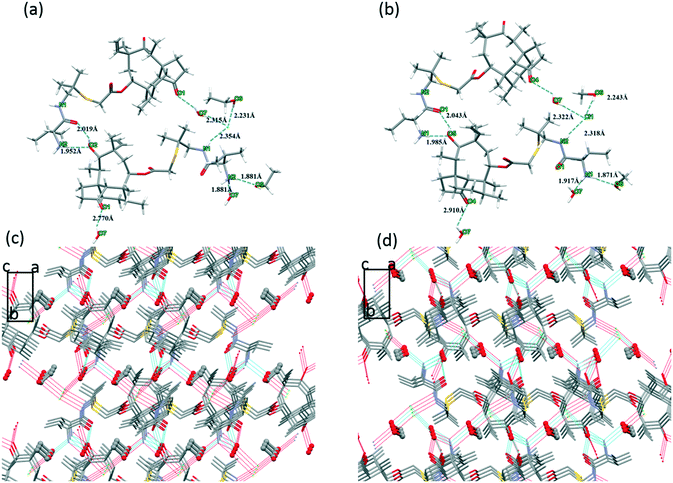 | ||
| Fig. 6 (a) Hydrogen bond interaction of VHWES. (b) Hydrogen bond interaction of VHWMS. (c) Crystal packing diagram of VHWES. (d) Crystal packing diagram of VHWMS. | ||
The interactions in VHW are very different from those of VHWMS and VHWMES. First of all, for the VH molecules, its three conformations adopt different hydrogen bonding interactions.
Fig. 7(a–c) shows the 2D fingerprint plots of the three conformations of the VH molecules in VHW. It can be found that the 2D fingerprints plots of the three conformations are quite different, indicating that there are different modes of intermolecular interaction. Fig. 7(d–f) shows the hydrogen bonding interactions of the three conformations. For conformation I (Fig. 7d), the chloride ion interacts with the protonated amino group to form N6–H⋯Cl3 hydrogen bond. N6–H⋯O19 is formed between water 19 and protonated amino groups. Carbonyl interacts with the protonated amino groups of conformation III to form N1–H⋯O5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bond. Secondary amine and water 20 form N4–H⋯O20 hydrogen bond. The carbonyl group near the ring reacts with water 20 to form O20–H⋯O4
C hydrogen bond. Secondary amine and water 20 form N4–H⋯O20 hydrogen bond. The carbonyl group near the ring reacts with water 20 to form O20–H⋯O4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bond, and the hydroxyl group on the ring interacts with water 16 to form O2–H⋯O16 hydrogen bond. The number of hydrogen bonds formed by conformation II (Fig. 7e) is less than that of conformation I. For conformation II, only the protonated amino group and carbonyl group on the ring participate in the formation of hydrogen bonds. Cl1 and Cl2 interacts with protonated amino groups to form N4–H⋯Cl1 and N4–H⋯Cl2 via hydrogen bonds. The hydroxyl group on the ring interacts with water 17 and water 18 to form O17–H⋯O7 and O7–H⋯O18. The hydrogen bond of conformation III (Fig. 7f) is different from that of the former two conformations. The protonated amino group interacts with Cl1, water 16 and the carbonyl group of conformation I to form N1–H⋯Cl1, N1–H⋯O16 and N1–H⋯O5
C hydrogen bond, and the hydroxyl group on the ring interacts with water 16 to form O2–H⋯O16 hydrogen bond. The number of hydrogen bonds formed by conformation II (Fig. 7e) is less than that of conformation I. For conformation II, only the protonated amino group and carbonyl group on the ring participate in the formation of hydrogen bonds. Cl1 and Cl2 interacts with protonated amino groups to form N4–H⋯Cl1 and N4–H⋯Cl2 via hydrogen bonds. The hydroxyl group on the ring interacts with water 17 and water 18 to form O17–H⋯O7 and O7–H⋯O18. The hydrogen bond of conformation III (Fig. 7f) is different from that of the former two conformations. The protonated amino group interacts with Cl1, water 16 and the carbonyl group of conformation I to form N1–H⋯Cl1, N1–H⋯O16 and N1–H⋯O5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonds, respectively. Secondary amine interacts with water 17 to form the N2–H⋯O17 hydrogen bond.
C hydrogen bonds, respectively. Secondary amine interacts with water 17 to form the N2–H⋯O17 hydrogen bond.
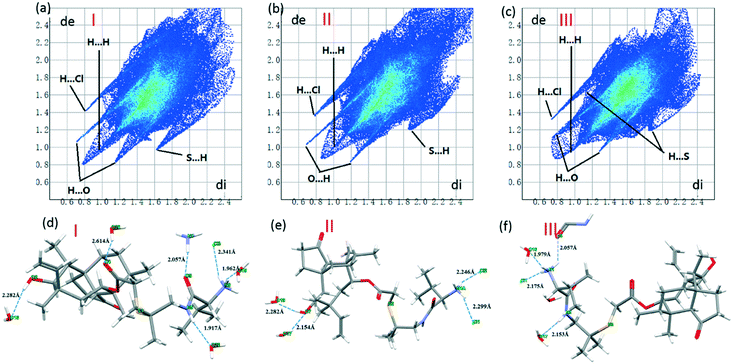 | ||
| Fig. 7 (a–c) 2D fingerprint plots of the three conformations. (d–f) The hydrogen bonding interactions of the three conformations in VHW. | ||
Fig. 8a describes the crystal structure packing diagram of VHW. Water molecules are arranged along the b-axis.
Meanwhile, the protonated amino groups, water 16, water 19, water 17, and the eight ring hydroxyl groups interact with each other to form a hydrogen bond chain (Fig. 8b).
The molecular conformations of VHWES, VHWMS and VHW are compared. Fig. 9a describes the comparison of the molecular conformation of VHWES and VHWMS. It can be seen that the molecular conformations in these two crystals are very similar and show mirror symmetry. Comparing the three conformations of VHW with the molecular conformations in VHWES and VHWMS (Fig. 9b), it can be found that VHWES is closer to the conformation of VHW than that of VHWMS. Therefore, it can be speculated that VHW may be more likely to transform into VHWES, which will be verified in the polymorphic transformation experiments.
Fig. S6† shows the relative contribution of the different interactions to the Hirshfeld surface. It can be seen that among the three crystal forms, the proportion of H⋯H interaction is relatively high, indicating that the intermolecular interaction is dominated by weaker van der Waals forces. On the other hand, there are many hydrogen bond donor–acceptor binding sites in the VH molecules, indicating that they easily form strong hydrogen bonds with strong polar solvents. Therefore, when they are dissolved in polar solvents, this hydrogen bond interaction will compete with the original van der Waals interaction. To a certain extent, this explains the phenomenon that these crystal forms have higher solubility in polar solvents.
Based on the above analysis, it can be summarized that, for VHWMS and VHWES, the carbonyl groups on the ring, Cl–, the hydroxyl groups on the ring, the protonated amino groups and imine groups are involved in the formation of hydrogen bonds with the VH molecules, and there is no interaction between the solvent molecules. For VHW, more functional groups get involved in the formation of hydrogen bonds, including Cl–, the carbonyl groups on the chain, the ester groups, the protonated amino groups, imine groups and hydroxyl groups. In addition, water molecules also form hydrogen bond interactions between themselves. Therefore, more interactions and a denser hydrogen bond network may be one of the reasons for the better stability of VHW.
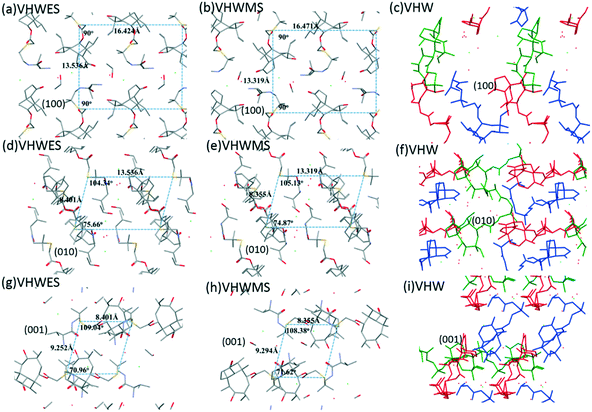
Fig. 10 shows the crystal structure stacking of the different crystal forms in the three directions. It was illustrated in the figure that the molecular arrangement directions of VHWES and VHWMS on the three crystal planes are very close, and the distance between the adjacent molecules is almost equal, indicating that the two crystal forms of VHWES and VHWMS are highly similar in crystal structure stacking. Therefore, valnemulin hydrochloride provides an interesting system to study the effect of without the influence of stacking.
At the same time, the influence of the experimental conditions on the crystal formation of VH was studied. It was found that the crystalline VH could not be obtained from ethanol or methanol by changing the experimental conditions if water was not introduced (Table S2†). Based on the analysis of the single crystal data of the three crystal forms, it could be speculated that water plays an important role in the formation of crystal structures.
To sum up, for the three crystal forms of VH, hydrogen bonding formation depends on solvent molecules, especially water molecules. When the water molecules further replace the solvent molecules, more functional groups in the VH molecules will participate in the formation of hydrogen bonds, making the crystal structure more stable. In addition to providing hydrogen bond formation sites, water molecules also help to fill the intervals in the crystal. In addition, Raj Kumar et al.27,28 have studied the effect of solvent on a crystal plane. Other studies also proved that solvent has an effect on crystal formation.29–31 Therefore, it is reasonable to speculate that crystal formation could also be affected by interactions between water and crystal faces. All these results confirm that water molecules play a key role in the formation of VH crystals.
Solution-mediated polymorphic transformations
According to previous studies, the conformation of VHWES is closer to that of VHW. In this part, the influence of the molecular conformational similarity on the polymorphic transformation process was further studied.In the solution-mediated polymorphic transformation experiment, different experimental conditions were investigated, such as temperature, supersaturation, and stirring rate (Table S3†). It was found that the transformation of VHW was not affected in the transformation process to the two kinds of solvates. Therefore, one group of experimental conditions (experiments 1 and 7) was selected to explain the effect of conformational similarity on crystallization kinetics.
Fig. S7† illustrates the solvent-mediated transformation process of VHW to VHWES in an ethanol + water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solution and to VHWMS in a methanol + water (1
1) mixed solution and to VHWMS in a methanol + water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solution, respectively. Marcus A. O'Mahony et al. reported how to use determination steps to identify SMPT by recording solution and solid phase data.32,33 It can be found that there is a long induction time before the stable crystal form appears in these two processes, and the solution concentration remains in the platform region of the metastable crystal form after the stable crystal form nucleation, indicating that these two processes are controlled by nucleation–growth. It can also be found that, under the same conditions (temperature, stirring rate, and supersaturation), transformation from VHW to VHWES is faster than that from VHW to VHWMS, suggesting that a similar conformation is conducive to the dynamics of crystal transformation.
1) mixed solution, respectively. Marcus A. O'Mahony et al. reported how to use determination steps to identify SMPT by recording solution and solid phase data.32,33 It can be found that there is a long induction time before the stable crystal form appears in these two processes, and the solution concentration remains in the platform region of the metastable crystal form after the stable crystal form nucleation, indicating that these two processes are controlled by nucleation–growth. It can also be found that, under the same conditions (temperature, stirring rate, and supersaturation), transformation from VHW to VHWES is faster than that from VHW to VHWMS, suggesting that a similar conformation is conducive to the dynamics of crystal transformation.
At the same time, although the thermal stability of VHWES and VHWMS is not as good as that of VHW, they can be obtained by sonication or by suspending VHW in mixed solvents. Previously reported studies have shown that sonication can promote the formation of metastable polymorphs since sonication could provide extra energy.34,35
Conclusions
One new dihydrate form (VHW) of valnemulin hydrochloride was discovered. The experimental results showed that the stability of VHW is better than that of the two solvates (VHWMS and VHWES) reported previously. The effect of water molecules in the formation of valnemulin hydrochloride crystals was analyzed. It was found that water molecules play a key role in stabilizing crystal structures. At the same time, it is found that in the case of almost the same packing, the conformational similarity can promote the solvent-mediated crystallization kinetics of valnemulin dihydrate. VHW will transform more quickly into VHWES which is closer to its molecular conformation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (grants 21978201 and 21908159).Notes and references
-
H. G. Brittain, Polymorphism in Pharmaceutical Solids, Informa Healthcare, New York, Londo, 2009 Search PubMed
.
- J. K. Haleblian, J. Pharm. Sci., 1975, 64, 1270–1288 Search PubMed
.
- D. Giron, C. Goldbronn, M. Mutz, S. Pfeffer, P. Piechon and P. Schwab, J. Therm. Anal. Calorim., 2002, 68, 453–465 CrossRef CAS
.
- F. Tian and J. Rantanen, Food Biophys., 2011, 6, 250–258 CrossRef
.
- A. Y. Lee, D. Erdemir and A. S. Myerson, Annu. Rev. Chem. Biomol. Eng., 2011, 2, 259–280 CrossRef CAS PubMed
.
- B. Fang, B. Hou, X. Huang, J. Wang, Y. Bao and H. Hao, Huagong Xuebao, 2019, 36, 1–8 Search PubMed
.
- X. Huang, Y. Chen, N. Su, H. Lu, J. Li, J. Li and H. Hao, Huagong Xuebao, 2019, 36, 10–23 Search PubMed
.
- A. Gillon, N. Feeder, R. Davey and R. Storey, Cryst. Growth Des., 2003, 3, 663–673 CrossRef CAS
.
- J. Aaltonen, M. Alleso, S. Mirza, V. Koradia, K. C. Gordon and J. Rantanen, Eur. J. Pharm. Biopharm., 2009, 71, 23–37 CrossRef CAS PubMed
.
- A. M. Healy, Z. A. Worku, D. Kumar and A. M. Madi, Adv. Drug Delivery Rev., 2017, 117, 25–46 CrossRef CAS PubMed
.
- S. Vippagunta, H. Brittain and D. Grant, Adv. Drug Delivery Rev., 2001, 48, 3–26 CrossRef CAS PubMed
.
- B. Zhu, J.-R. Wang, G. Ren and X. Mei, Cryst. Growth Des., 2016, 16, 6537–6546 CrossRef CAS
.
- H. Veith, C. Luebbert and G. Sadowski, Cryst. Growth Des., 2020, 20, 723–735 CrossRef CAS
.
- F. T. W. Jordan, C. A. Forrester, P. H. Ripley and D. G. S. Burch, Avian Dis., 1998, 42, 738–745 CrossRef CAS
.
- L. Chen, D. Yang, Z. Pan, L. Lai, J. Liu, B. Fang and S. Shi, Chem. Biol. Drug Des., 2015, 86, 239–245 CrossRef CAS PubMed
.
- R. Dip, Z. Nemet, B. Schiessl, U. Klein and G. Strehlau, Vet. J., 2015, 204, 309–314 CrossRef CAS PubMed
.
- J. Ouyang, B. Na, Z. Liu, L. Zhou and H. Hao, J. Solution Chem., 2019, 48, 413–426 CrossRef CAS
.
- X. Zhu, S. Xu and Q. Xu, Pharm. Dev. Technol., 2016, 21, 338–345 CrossRef CAS PubMed
.
- J. Ouyang, B. Na, L. Zhou, S. Xiao, G. Xiong and T. Jin, CrystEngComm, 2018, 20, 563–569 RSC
.
- C. Jeroen, L. Christian and M. Marco, Ind. Eng. Chem. Res., 2008, 47, 4870–4882 CrossRef
.
- A. Paudel, D. Raijada and J. Rantanen, Adv. Drug Delivery Rev., 2015, 89, 3–20 CrossRef CAS PubMed
.
- A. C. Jorgensen, C. J. Strachan, K. H. Pollanen, V. Koradia, F. Tian and J. Rantanen, J. Pharm. Sci., 2009, 98, 3903–3932 CrossRef PubMed
.
- M. Rozenberg, A. Loewenschuss and Y. Marcus, Phys. Chem. Chem. Phys., 2000, 2, 2699–2702 RSC
.
- M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378–392 RSC
.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 RSC
.
- Z. Ding, W. Su, X. Huang, B. Tian, X. Cheng, Y. Mao, G. Li, H. Liu and H. Hao, Cryst. Growth Des., 2020, 20, 1150–1161 CrossRef CAS
.
- R. Kumar and P. F. Siril, J. Drug Delivery Sci. Technol., 2020, 55, 101359 CrossRef CAS
.
- R. Kumar, P. Soni and P. F. Siril, ACS Omega, 2019, 4, 5424–5433 CrossRef CAS PubMed
.
- Z. Z. Liang, J. F. Chen, Y. Ma, W. Wang, X. L. Han, C. Y. Xue and H. Zhao, CrystEngComm, 2014, 16, 5997–6002 RSC
.
- F. Chen, T. Zhou and M. F. Wang, J. Phys. Chem. Solids, 2020, 136, 8 Search PubMed
.
- I. Hod, Y. Mastai and D. D. Medina, CrystEngComm, 2011, 13, 502–509 RSC
.
- M. A. O'Mahony, A. Maher, D. M. Croker, Å. C. Rasmuson and B. K. Hodnett, Cryst. Growth Des., 2012, 12, 1925–1932 CrossRef
.
- Z. Zheng, B. Hou, X. Cheng, W. Liu, X. Huang, Y. Bao, T. Wang, Z. Wang and H. Hao, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2020, 76, 343–352 CrossRef CAS PubMed
.
- R. Prasad, K. Panwar, J. Katla and S. V. Dalvi, Cryst. Growth Des., 2020, 20, 5169–5183 CrossRef CAS
.
- K. K. Zhang, M. C. L. Yeung, S. Y. L. Leung and V. W. W. Yam, J. Am. Chem. Soc., 2018, 140, 9594–9605 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation of crystals, Fig. S1–S7 and Tables S1–S3. CCDC 2033085. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ce01459a |
| This journal is © The Royal Society of Chemistry 2021 |