Open Access Article
Open Access ArticlePhoto-crosslinking polymers by dynamic covalent disulfide bonds†
Bianka
Sieredzinska
a,
Qi
Zhang
 a,
Keimpe J. van den
Berg
b,
Jitte
Flapper
c and
Ben L.
Feringa
a,
Keimpe J. van den
Berg
b,
Jitte
Flapper
c and
Ben L.
Feringa
 *a
*a
aStratingh Institute for Chemistry and Zernike Institute for Advanced Materials, Faculty of Science and Engineering, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands. E-mail: b.l.feringa@rug.nl
bAkzo Nobel Car Refinishes B.V., Rijksstraatweg 31, 2171 AJ Sassenheim, The Netherlands
cAkzo Nobel Decorative Coatings B.V., Rijksstraatweg 31, 2171 AJ Sassenheim, The Netherlands
First published on 3rd September 2021
Abstract
A simple and general strategy to construct photo-crosslinkable polymers by introducing sidechain 1,2-dithiolanes based on natural thioctic acid is presented. The disulfide five-membered rings act both as light-absorbing and dynamic covalent crosslinking units, enabling efficient photo-crosslinking and reversible chemical decrosslinking of polydimethylsiloxane polymers.
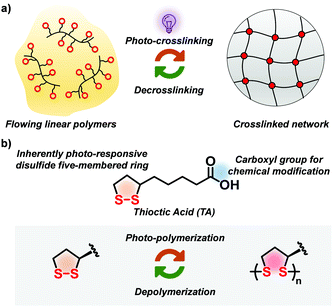
Crosslinking polymers by light provides many opportunities in order to develop smart manufacturing technologies for polymeric materials such as 3D laser printing, photo-patterning, microfabrication and coatings.1 Current major strategies include the introduction of photo-initiators which can form radicals to trigger the polymerizations and as a consequence, cure the linear polymers or resin by light irradiation.2 Although this common photo-crosslinking strategy is efficient and well established, the inevitable residues of these photo-initiators usually accelerate the undesired aging process of the materials.3 The quest for the circularity and recycling of future materials demands reversibility of cross-linking (Fig. 1a),4 while material properties should not be compromised. Meanwhile, strategies like introducing photo-switchable molecules or structural units controlling supramolecular interactions have been developed to enable the light-controlled crosslinking/decrosslinking process of polymers.5 However, such photo-crosslinking strategies require chromophores that often need tedious synthesis while many of them are not robust because of the inherently weak nature of supramolecular interactions.6 Therefore, structurally simple units with inherent photo-crosslinking capability and featuring dynamic covalent bonds that support a robust crosslinked network as well as intrinsic reversibility is a fundamental challenge for chemists.
In order to develop a robust photo-crosslinking and de-crosslinking strategy we focussed on 1,2-dithiolanes, a family of disulfide compounds with five-membered rings.7 Thioctic acid (TA) (Fig. 1b), a biologically occurring small-molecule 1,2-dithiolane, is particularly attractive8 featuring an intrinsic crosslinking and photoactive unit. In previous studies, our group has demonstrated the heat-induced ring-opening polymerization (ROP) of TA in a solvent-free state to produce poly(TA) materials that can stretch, self-heal, stick to surfaces, and even be chemically recyclable in a closed-loop manner.9 Some early-stage studies explored the photochemistry of TA,7a,10 concluding that the strained disulfide bonds can be scissored into diradicals by long-wavelength UV light, allowing to trigger the radical-initiated polymerization of TA.9,11 In other words, this photo-polymerization ability of TA is an inherent property, avoiding the need of introducing external photo-initiators or light-absorbers. To this end, we present here the use of this natural 1,2-dithiolane-based structure as a versatile dynamic covalent crosslinker for traditional polymers, to enable an inherently photo-crosslinkable, reversible and robust polymer network (Fig. 1).
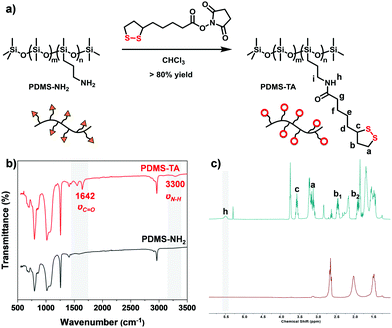
Polydimethylsiloxane (PDMS) polymers were selected due to their optical transparency and these materials sustain a broad range of practical applications including coatings, elastomers and for photo-patterning manufacturing.12 To introduce TA as the side chain of PDMS, amine-modified PDMS (PDMS-NH2) (with 6–7% molar ratio of amine units) polymers were used to react with an NHS-activated TA ester (TA-NHS) (Fig. 2a). This simple post-modification reaction afforded over 80% yield, resulting in yellow and transparent polymer liquid PDMS-TA (For details on synthesis and characterization, see ESI†). The resulting PDMS-TA polymer is highly soluble in many organic solvents and behaves like a viscous flowing liquid, indicating the uncrosslinked nature of linear polymers.
 | ||
| Fig. 2 (a) Post-modification preparation of PDMS-TA polymers; (b) IR spectra of the PDMS-NH2 and PDMS-TA polymers; (c) 1H NMR spectra of the PDMS-NH2 and PDMS-TA polymers (CDCl3, 400 MHz, 298 K). | ||
To characterize the polymer structure of the resulting PDMS-TA polymers, infrared (IR) spectroscopy was used (Fig. 2b). Compared with the initial PDMS-NH2 polymers, the functionalized PDMS-TA polymers showed distinctive vibration bands of νN–H at 3300 cm−1 and νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1650 cm−1, indicating the presence of amide bonds. The 1H NMR spectra of PDMS-TA further confirmed the distinctive proton signals of 1,2-dithiolanes in the polymers and the successful formation of amide bonds (Fig. 2c). Furthermore, considering the possibility that the amidation reactions might not proceed quantitatively, a typical amine probe molecule, 1-fluoro-2,4-dinitrobenzene (DNFB), was used to further quantify the functionalization ratio of PDMS-TA (Fig. S1, ESI†). This analysis showed that the PDMS-TA polymers had no detectable free amine groups, indicating the complete post-modification reaction.
O at 1650 cm−1, indicating the presence of amide bonds. The 1H NMR spectra of PDMS-TA further confirmed the distinctive proton signals of 1,2-dithiolanes in the polymers and the successful formation of amide bonds (Fig. 2c). Furthermore, considering the possibility that the amidation reactions might not proceed quantitatively, a typical amine probe molecule, 1-fluoro-2,4-dinitrobenzene (DNFB), was used to further quantify the functionalization ratio of PDMS-TA (Fig. S1, ESI†). This analysis showed that the PDMS-TA polymers had no detectable free amine groups, indicating the complete post-modification reaction.
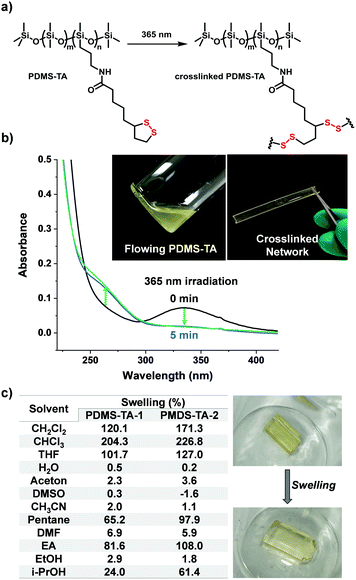
Next the photocrosslinking properties of the resulting PDMS-TA polymers were investigated (Fig. 3a). Using a UV lamp with a wavelength of 365 nm, the free flowing PDMS-TA liquid polymers were irradiated in a Teflon mould. After 1 h irradiation, transparent light yellow crosslinked materials were formed, which can be easily taken from the mould as free-standing and flexible solid samples (Fig. 3b). A similar method was also used to produce coatings on surfaces, based on this crosslinked PDMS network, with a typical contact angle of 105.8 degree (Fig. S2, ESI†).
UV-Vis absorption spectra offered evidence of the successful ring-opening crosslinking driven by light irradiation: the distinctive absorption band of 1,2-dithiolanes at 330 nm was rapidly photobleached upon 20 min UV irradiation in the solvent-free state, and the simultaneously increased absorption of ring-opened species at 250–300 nm indicated the formation of polymers or oligomers in the network (Fig. 3b). Furthermore, upon irradiation in diluted solutions the presence of sulfur radicals was observed, based on their distinctive absorption band at 500 nm (Fig. S3, ESI†),13 suggesting a photo-induced radical crosslinking mechanism.
To establish the material properties of the crosslinked system, the swelling behaviour was investigated (Fig. 3c). The resulting PDMS-TA material exhibited typical swelling properties of a PDMS network with the high swelling ability in apolar solvents (CHCl3, CH2Cl2, tetrahydrofuran, etc.) and a low swelling ratio in polar solvents (H2O, dimethyl formamide, dimethylsulfoxide, etc.). The typical swelling behaviour and robust nature (no dissolution) indicated the successful covalent crosslinking of the material. Meanwhile, the materials also exhibited a thermal decomposition temperature above 200 °C, indicating the robustness of the covalently crosslinked network (Fig. S4, ESI†). The glass transition temperature of the materials is below −20 °C according to differential scanning caborimetry (DSC) curves (Fig. S5, ESI†), which is typical for PDMS polymers.
In order to compare the effect of polymer chain length, we synthesized two types of PDMS-TA polymers with different molecular weight, distinguished by the viscosity of PDMS-NH2 precursors i.e.PDMS-TA-1 and PDMS-TA-2 (additional details see ESI†). The mechanical properties of the crosslinked PDMS-TA network were evaluated by tensile experiments (Fig. S6, ESI†). As a result, both materials exhibited typical tensile curves of a highly crosslinked polymeric network with notable Young's modulus, indicating the robust covalent crosslinking by the disulfide bonds. Due to the longer polymer chain, PDMS-TA-1 shows a higher Young's modulus (9.79 ± 0.46 MPa) than PDMS-TA-2 (5.61 ± 0.78 MPa), while the breaking elongation ratio of PDMS-TA-1 was slightly lower than that of PDMS-TA-2. The maximum strength of the two materials were similar due to the consistent crosslinking manner. Stress relaxation experiments showed steady storage modulus after relaxation (Fig. S7, ESI†), indicating the robust and steady crosslinked network due to the covalent nature of disulfide bonds.
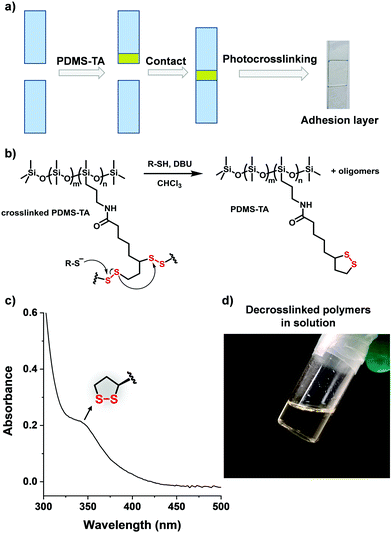
The photo-crosslinking ability of the PDMS-TA polymers also offered opportunities of designing photo-crosslinking adhesives (Fig. 4a).14 The uncrosslinked PDMS-TA polymers exhibited no adhesive ability for any surfaces because of the linear polymer chains showing free flowing behaviour. Irradiating the PDMS-TA polymer layer between two glass slides can cure the liquid layer in 1 h, resulting in robust adhering two glass surfaces mediated by a thin and transparent crosslinked network (Fig. 4a and Fig. S8, ESI†). Quantitative measurement gave the shear strength values of the crosslinked network being 0.20 ± 0.09 MPa for PDMS-TA-1, and 0.52 ± 0.06 MPa for PDMS-TA-2. The high shear strength suggest the excellent robustness of the crosslinked network especially considering the lack of (commonly required) polar groups such adhesive materials. Notably, the adhesive strength of PDMS-TA-2 was higher than that of PDMS-TA-1, which should be attributed to the higher penetration ability of shorter polymer chains that enables high contact area with the surface.
Finally we demonstrate that, due to the dynamic covalent nature of the disulfide bonds,15 the resulting crosslinked network of PDMS-TA polymers can also be decrosslinked into linear polymers in the presence of chemical initiators, e.g. thiolate. In a typical decrosslinking experiment, 1% molar ratio of benzyl mercaptane and base 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) were dissolved in 10 mL CHCl3 solution to produce thiolate (Fig. 4b). Then a piece of PDMS-TA crosslinked network was immersed into the solution, and the mixture was stirred at room temperature. In sharp contrast with the insolubility of the crosslinked network in pure CHCl3, the solid polymer sample visibly dissolved in the presence of thiolate in 48 h, forming a yellow transparent solution (Fig. 4c and d), which exhibited the distinctive absorption of the 1,2-dithiolanes monomer unit at 330 nm, indicating the successful recovery of disulfide five-membered rings from the thiolate-initiated depolymerization of poly(disulfides). The 1H NMR spectrum further supports the recovered distinctive absorptions of the five-membered rings (Ha, Hb, and Hc) (Fig. S9, ESI†). This preliminary experiment, showing mild catalytic depolymerisation, demonstrated the advantages of the dynamic covalent network based on TA towards chemically recyclable crosslinked polymers.
In conclusion, we demonstrated the successful photo-crosslinking to form a polydimethylsiloxane network enabled by introducing 1,2-dithiolane units. The inherent photo-responsive ability of 1,2-dithiolanes enables a straightforward photo-crosslinking process in solvent-free state with the distinctive advantages that the external addition of photo-initiators or light-absorbers is not required. The resulting covalent crosslinked network can be also decrosslinked under ambient conditions in the presence of thiolate initiators due to the dynamic covalent nature of disulfide bonds. We expect that this proof-of-concept study based on biobased thioctic acid can provide a general and simple strategy for the design of reversible photo-crosslinking coatings and materials.
This work is part of the Advanced Research Center for Chemical Building Blocks, ARC CBBC, which is co-founded and co-financed by the Netherlands Organization for Scientific Research (NWO, contract 736.000.000) and the Netherlands Ministry of Economic Affairs and Climate. Q. Z. acknowledges the European Union's Horizon 2020 research and innovation programme under the Marie-Skłodowska-Curie grant agreement (101025041). The authors thank Prof. K. Loos and Ing. J. van Dijken at University of Groningen for the assistance with material characterization.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) H. Xie, K. K. Yang and Y. Z. Wang, Prog. Polym. Sci., 2019, 95, 32–64 CrossRef CAS; (b) K. S. Lim, J. H. Galarraga, X. Cui, G. C. Lindberg, J. A. Burdick and T. B. Woodfield, Chem. Rev., 2020, 120, 10662–10694 CrossRef CAS PubMed; (c) H. Ge, W. Wu, Z. Li, G. Y. Jung, D. Olynick, Y. Chen, J. A. Liddle, S. Wang and R. S. Williams, Nano Lett., 2005, 5, 179–182 CrossRef CAS PubMed; (d) A. Bagheri and J. Jin, ACS Appl. Polym. Mater., 2019, 1, 593–611 CrossRef CAS; (e) N. Corrigan, J. Yeow, P. Judzewitsch, J. Xu and C. Boyer, Angew. Chem., Int. Ed., 2019, 58, 5170–5189 CrossRef CAS PubMed; (f) M. Layani, X. Wang and S. Magdassi, Adv. Mater., 2018, 30, 1706344 CrossRef PubMed; (g) M. Regehly, Y. Garmshausen, M. Reuter, N. F. König, E. Israel, D. P. Kelly, C. Chou, K. Koch, B. Asfari and S. Hecht, Nature, 2020, 588, 620–624 CrossRef CAS PubMed.
- (a) C. R. Morgan, F. Magnotta and A. D. Ketley, J. Polym. Sci., Polym. Chem. Ed., 1977, 15, 627–645 CrossRef CAS; (b) H. F. Gruber, Prog. Polym. Sci., 1992, 17, 953–1044 CrossRef CAS; (c) K. T. Nguyen and J. L. West, Biomaterials, 2002, 23, 4307–4314 CrossRef CAS PubMed; (d) J. Zhang and X. Pu, Polym. Chem., 2018, 9, 1530–1540 RSC; (e) G. Oster and N. L. Yang, Chem. Rev., 1968, 68, 125–151 CrossRef CAS.
- M. Panagiotopoulou, S. Beyazit, S. Nestora, K. Haupt and B. T. S. Bui, Polymer, 2015, 66, 43–51 CrossRef CAS.
- (a) J. B. Zimmerman, P. T. Anastas, H. C. Erythropel and W. Leitner, Science, 2020, 367, 397–400 CrossRef CAS PubMed; (b) J. C. Worch and A. P. Dove, ACS Macro Lett., 2020, 9, 1494–1506 CrossRef CAS; (c) M. Häußler, M. Eck, D. Rothauer and S. Mecking, Nature, 2021, 590, 423–427 CrossRef PubMed; (d) L. T. Korley, T. H. Epps, B. A. Helms and A. J. Ryan, Science, 2021, 373, 66–69 CrossRef CAS PubMed.
- (a) D. H. Qu, Q. C. Wang, Q. W. Zhang, X. Ma and H. Tian, Chem. Rev., 2015, 115, 7543–7588 CrossRef CAS PubMed; (b) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042–6065 RSC; (c) F. Xu, L. Pfeifer, S. Crespi, F. K. C. Leung, M. C. Stuart, S. J. Wezenberg and B. L. Feringa, J. Am. Chem. Soc., 2021, 143, 5990–5997 CrossRef CAS PubMed; (d) E. Weyandt, G. M. Ter Huurne, G. Vantomme, A. J. Markvoort, A. R. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2020, 142, 6295–6303 CrossRef CAS PubMed; (e) Y. L. Zhao and J. F. Stoddart, Langmuir, 2009, 25, 8442–8446 CrossRef CAS PubMed; (f) S. Matsumoto, S. Yamaguchi, S. Ueno, H. Komatsu, M. Ikeda, K. Ishizuka, Y. Iko, K. V. Tabata, H. Aoki, S. Ito, H. Noji and I. Hamachi, Chem. – Eur. J., 2008, 14, 3977–3986 CrossRef CAS PubMed.
- (a) D. B. Amabilino, D. K. Smith and J. W. Steed, Chem. Soc. Rev., 2017, 46, 2404–2420 RSC; (b) A. J. Savyasachi, O. Kotova, S. Shanmugaraju, S. J. Bradberry, G. M. Ó’Máille and T. Gunnlaugsson, Chemistry, 2017, 3, 764–811 CrossRef CAS.
- (a) J. A. Barltrop, P. M. Hayes and M. Calvin, J. Am. Chem. Soc., 1954, 76, 4348–4367 CrossRef CAS; (b) L. J. Reed, B. G. DeBusk, I. C. Gunsalus and G. H. F. Schnakenberg, J. Am. Chem. Soc., 1951, 73, 5920 CrossRef CAS.
- U. Schmidt, P. Grafen and H. W. Goedde, Angew. Chem., Int. Ed. Engl., 1965, 4, 846–856 CrossRef CAS PubMed.
- (a) Q. Zhang, C. Y. Shi, D. H. Qu, Y. T. Long, B. L. Feringa and H. Tian, Sci. Adv., 2018, 4, eaat8192 CrossRef CAS PubMed; (b) Q. Zhang, Y. X. Deng, H. X. Luo, C. Y. Shi, G. M. Geise, B. L. Feringa, H. Tian and D. H. Qu, J. Am. Chem. Soc., 2019, 141, 12804–12814 CrossRef CAS PubMed; (c) Y. Deng, Q. Zhang, B. L. Feringa, H. Tian and D. H. Qu, Angew. Chem., Int. Ed., 2020, 59, 5278–5283 CrossRef CAS PubMed; (d) Q. Zhang, Y. Deng, C. Y. Shi, B. L. Feringa, H. Tian and D. H. Qu, Matter, 2021, 4, 1352–1364 CrossRef CAS.
- (a) M. Calvin, J. Chem. Phys., 1955, 23, 1750 CrossRef; (b) M. Calvin, Proc. Natl. Acad. Sci. U. S. A., 1955, 41, 563 CrossRef PubMed.
- (a) G. M. Scheutz, J. L. Rowell, S. T. Ellison, J. B. Garrison, T. E. Angelini and B. S. Sumerlin, Macromolecules, 2020, 53, 4038–4046 CrossRef CAS; (b) C. Choi, J. L. Self, Y. Okayama, A. E. Levi, M. Gerst, J. C. Speros, C. J. Hawker, J. R. de Alaniz and C. M. Bates, J. Am. Chem. Soc., 2021, 143, 9866–9871 CrossRef CAS PubMed.
- (a) B. H. Jo, L. M. Van Lerberghe, K. M. Motsegood and D. J. Beebe, J. Microelectromech. Syst., 2000, 9, 76–81 CAS; (b) T. Fujii, Microelectron. Eng., 2002, 61, 907–914 CrossRef.
- F. Denes, M. Pichowicz, G. Povie and P. Renaud, Chem. Rev., 2014, 114, 2587–2693 CrossRef CAS PubMed.
- (a) Y.-M. Tseng, A. Narayanan, K. Mishra, X. Liu and A. Joy, ACS Appl. Mater. Interfaces, 2021, 13, 29048–29057 CrossRef CAS PubMed; (b) L. Kortekaas, J. Simke, D. W. Kurka and B. J. Ravoo, ACS Appl. Mater. Interfaces, 2020, 12, 32054–32060 CrossRef CAS PubMed; (c) C. Y. Shi, Q. Zhang, H. Tian and D. H. Qu, SmartMat, 2020, 1, e1012 CrossRef.
- (a) X. Zhang and R. M. Waymouth, J. Am. Chem. Soc., 2017, 139, 3822–3833 CrossRef CAS PubMed; (b) G. A. Barcan, X. Zhang and R. M. Waymouth, J. Am. Chem. Soc., 2015, 137, 5650–5653 CrossRef CAS PubMed; (c) Y. Liu, Y. Jia, Q. Wu and J. S. Moore, J. Am. Chem. Soc., 2019, 141, 17075–17080 CrossRef CAS PubMed; (d) E. K. Bang, M. Lista, G. Sforazzini, N. Sakai and S. Matile, Chem. Sci., 2012, 3, 1752–1763 RSC; (e) E. K. Bang, G. Gasparini, G. Molinard, A. Roux, N. Sakai and S. Matile, J. Am. Chem. Soc., 2013, 135, 2088–2091 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cc03648c |
| This journal is © The Royal Society of Chemistry 2021 |