 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Amidine-based ionic liquid analogues with AlCl3: a credible new electrolyte for rechargeable Al batteries†
Anthony J.
Lucio
 *a,
Igor
Efimov
a,
Oleg N.
Efimov
b,
Christopher J.
Zaleski
*a,
Igor
Efimov
a,
Oleg N.
Efimov
b,
Christopher J.
Zaleski
 c,
Stephen
Viles
a,
Beata B.
Ignatiuk
a,
Andrew P.
Abbott
c,
Stephen
Viles
a,
Beata B.
Ignatiuk
a,
Andrew P.
Abbott
 a,
A. Robert
Hillman
a,
A. Robert
Hillman
 a and
Karl S.
Ryder
a and
Karl S.
Ryder
 a
a
aMaterials Centre, School of Chemistry, University of Leicester, Leicester LE1 7RH, UK. E-mail: ajl71@le.ac.uk
bRussian Acad. Sci., Inst. Prob. Chem. Phys., 1 Acad. Semenov Ave, Chernogolovka 142432, Moscow Region, Russia
cWolfson School of Mechanical Electrical & Manufacturing Engineering, Loughborough University, Loughborough LE11 3TU, UK
First published on 18th August 2021
Abstract
Here we demonstrate the generation of novel ionic liquid analogue (ILA) electrolytes for aluminium (Al) electrodeposition that are based on salts of amidine Lewis bases. The electrolytes exhibit reversible voltammetric plating/stripping of Al, good ionic conductivities (10–14 mS cm−1), and relatively low viscosities (50–80 cP). The rheological properties are an improvement on analogous amide-based ILAs and make these liquids credible alternatives to ILAs based on urea or acetamide, or conventional chloroaluminate ionic liquids (IL) for Al battery applications.
The focus of this communication is on the exploration of new and novel electrolytes based on the HCl salts of a range of amidine species. Amidines are a class of organic species related to amides, where the O atom has been replaced with an sp2 N atom. Recent studies have demonstrated that in the case of amide-based ILAs (e.g. those based on acetamide or urea), the interaction between the AlCl3 Lewis acid and the amide base likely occurs through an Al–O bond,1,2 consistent with the fact that AlCl3 is highly oxophilic. However, O-based ligands to Al are strongly bound, resulting in slow exchange kinetics that limit the electrochemical reduction and deposition of Al metal. While this is not a kinetic study, our strategy here has been to replace the O atom of the amides with an N atom giving a structurally similar environment where only N-coordination is possible. In addition, unlike amides, amidines can be easily protonated facilitating the delocalisation of electron density across the N-centres. In the case of the guanidinium cation, the three N-centres become equivalent by virtue of their resonance forms (ESI† 2, Fig. S1). These cationic amidinium species are consequentially softer Lewis bases than the analogous amides and therefore, we propose, ligands that are more facile. Additionally, they contribute to the conductivity of the liquids through their positive charge. Specifically, this is the first report to our knowledge of ILAs made from Lewis basic salts of guanidinium chloride (Guan-Cl), acetamidinium chloride (Acet-Cl) and formamidinium chloride (Form-Cl). For comparative purposes, we have included data for the IL, 1-butly-1-methylpyrrolidinium chloride (BMP-Cl).
The rapidly increasing demand for rechargeable battery systems in order to power stationary energy storage, mobile electronics and electric vehicles has generated acute technical and social challenges. Significant research and development has been reported on lithium-, sodium-, and magnesium-based battery technologies, with lithium-ion batteries (LIB) dominating the market share. Although LIBs are widely used in everyday technology they possess drawbacks that need to be addressed e.g. diminishing supply of source materials, difficulty in recycling used battery systems,3 and safety concerns (i.e. thermal runaway and leakage of hazardous components).4 As a result, there are major efforts to develop battery systems to compete with and replace current technology. Even if new battery chemistries cannot match LIB performance metrics, the possibility of cheaper, safer and more environmentally friendly options motivate research in this field.
A very encouraging option are Al-based batteries.5 Al is inexpensive, highly abundant, and possesses an energy density close to Li.6 Like Li, however, Al systems require non-aqueous chemistries, as the reduction potential of Al is more negative than that for hydrogen evolution in aqueous-based electrolytes. The development of non-aqueous electrolytes for Al battery systems has subsequently received significant attention, although much of this has only occurred relatively recently.
The electrolyte component of a battery is fundamental to its performance, and developing electrolytes with low viscosity, high ionic conductivity, a large polarisable potential window and good thermal stability are all important characteristics. To date, chloroaluminate electrolytes from imidazolium-7–9 and pyrrolidinium-based10–12 salts have been studied but they are often costly. ILAs also known as deep eutectic solvents (DES), which are formed by mixing a Lewis acid (e.g. AlCl3) and a Lewis base (e.g. urea) at a desired mole ratio, have been recently explored as they are inexpensive, very easy to synthesize and can be made from abundant often non-toxic materials. In a manner similar to ILs, the Lewis base can be selected to generate a range of different ILAs with AlCl3, thus facilitating control of the physiochemical properties of the electrolyte. At present urea13–16 and acetamide17–22 (and their derivatives) have been explored, however, there is still significant room for improvement with electrolyte development.
The chemical structures of the different amidine salts studied here (Guan-Cl, Acet-Cl, Form-Cl, and BMP-Cl) are shown in Table 1 along with the stoichiometric ratios and molar concentrations of Al in each liquid. The structures represent a sequential substitution of the parent amidine cation, R–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2+)–NH2 where R = NH2, CH3 and H. The variants where R′ = CH3 and NH2 in R′–(C
NH2+)–NH2 where R = NH2, CH3 and H. The variants where R′ = CH3 and NH2 in R′–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–NH2 are acetamide and urea, respectively. The liquid formulations shown were all stable at room temperature (291 K); of particular note is the high concentration of Al achieved with Guan-AlCl3 that is facilitated by the presence of three N-based coordination sites. 27Al NMR spectra of these electrolytes indicate that a mixture of anionic and cationic Al species are present (ESI† 3, Fig. S2). Whilst we were able to make liquid formulations of the Acet-AlCl3 and Form-AlCl3 based liquids with higher AlCl3 content (e.g. at a mole ratio of 2
O)–NH2 are acetamide and urea, respectively. The liquid formulations shown were all stable at room temperature (291 K); of particular note is the high concentration of Al achieved with Guan-AlCl3 that is facilitated by the presence of three N-based coordination sites. 27Al NMR spectra of these electrolytes indicate that a mixture of anionic and cationic Al species are present (ESI† 3, Fig. S2). Whilst we were able to make liquid formulations of the Acet-AlCl3 and Form-AlCl3 based liquids with higher AlCl3 content (e.g. at a mole ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), some of these formed glassy solids on cooling under ambient conditions.
1), some of these formed glassy solids on cooling under ambient conditions.
The cyclic voltammograms (CV) of the different electrolytes were recorded at 18 ± 1 °C with the Pt coated face of a 10 MHz AT-cut quartz crystal, Fig. 1(a–d). In all cases, these show cathodic current associated with Al3+ reduction, an anodic peak corresponding to the dissolution of Al metal, evidence of a nucleation loop, and are commensurate with the chemically reversible plating and stripping of Al. The CVs show the second cycle and we find little difference in the voltammograms of successive CV cycles (ESI† 4, Fig. S3). The i(E) traces also show striking linearity in the region of cathodic current and in the first part of the anodic stripping peak. This characteristic response in the stripping peak has also been seen for pyrrolidinium-11,23 and urea-based22 electrolytes. In addition, no cathodic reduction peak is observed for these CVs in the potential region shown. Taken together, these observations indicate that the reduction of Al3+ (and subsequent deposition of Al metal), is not mass-transport limited in any of these liquids. Numerical integration of the cathodic (Qred) and anodic stripping (Qox) portions of the voltammograms (Fig. 1) shows that for the amidine liquids, the processes are equivalent with a ratio, Qred/Qox, of unity. The corresponding value for the BMP-AlCl3 liquid is lower, at 0.89.
 | ||
| Fig. 1 CVs (2nd cycle) for (a) Guan-AlCl3, (b) Acet-AlCl3, (c) Form-AlCl3, and (d) BMP-AlCl3 liquids (electrode area = 0.21 cm2, scan rate = 20 mV s−1, T = 18 ± 1 °C). EQCM plots are shown in (e–h). | ||
For the three amidine liquids, the onset potential for Al3+ reduction is close to −0.75 V, whereas that for BMP-AlCl3 is closer to −1.0 V, hence the modified potential range examined. Correspondingly, the anodic peaks for the amidine liquids are both sharper in appearance and larger in magnitude than that of the BMP-AlCl3 liquid. These observations are in part related to the relative concentrations of Al3+ in the liquids (Table 1) and in part due to the differences in rheological properties (discussed below) of the liquids. However, we can conclude from these data that under equivalent conditions of time (potential scan rate) and temperature, all of the amidine liquids perform better than the BMP-AlCl3 system where Guan-AlCl3 exhibits the largest anodic and cathodic responses.
The plating and stripping of Al species on the Pt electrode can also be visualised from electrochemical quartz crystal microbalance (EQCM) curves. The EQCM data, plotted as the relative change in mass (Δm) as a function of charge (Q), are presented in Fig. 1(e–h). For a faradaic process we would expect these plots to be linear, with a theoretical slope, Δm/Q, defined by the Sauerbrey equation to be 0.093 μg mC−1. Here, a range of deviations from linear behaviour (not predictable on the basis of i(E) responses) are observed. Average Δm/Q ratios for selected charge ranges within the full voltammetric cycle are indicated by annotated values colour coded to corresponding data ranges in Fig. 1(e–h). The Guan-AlCl3 electrolyte (Fig. 1e) most closely follows the faradaic response with Δm(Q) traces having values close to the theoretical slope; 0.073 μg mC−1 and 0.096 μg mC−1 (plating), and 0.103 μg mC−1 (stripping). Similarly, Acet-AlCl3 (Fig. 1f) shows 0.031 μg mC−1 and 0.110 μg mC−1 (plating), and 0.161 μg mC−1 (stripping). Small, negative deviations from the theoretical slope usually indicate that less mass is deposited on the electrode crystal than is expected. Based on charge this can be due to minor side-reactions or impurities, but as with any surface-sensitive measurement, one cannot exclude them without further quantitative study. However, the larger deviations and non-linear responses for Acet-AlCl3 (0.031 μg mC−1), Form-AlCl3 (Fig. 1g), and BMP-AlCl3 (Fig. 1h) liquids suggest non-gravimetric, local rheological changes occurring at the electrode/electrolyte interface commensurate with changes in Al ion concentration during the redox cycle. In the case of the Form-AlCl3 liquid (Fig. 1g) the apparent mass changes throughout the electrochemical plating/stripping process are very large, and indicates that a phase transformation (e.g. electrolyte gelification) may be occurring locally within the diffusion layer during the electrodeposition process at negative potentials.
The deviation of the Form-AlCl3 data can also be observed in the acoustic loss data gathered from EQCM. The real component of the acoustic impedance (loss) response, which is related to the resistance, Rq, can be estimated from the width of the resonance frequency peak at half height, W, using eqn (1):
| Rq = 2πLW | (1) |
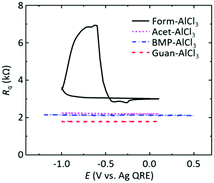 | ||
| Fig. 2 Acoustic loss data (calculated from eqn (1)) as a function of potential for the different electrolytes. | ||
The ionic conductivities (σ) of the electrolytes were also determined via mathematical fitting (see ESI† 5) of the observed currents to eqn (2), as shown previously29 from the stripping (i.e. anodic scan) portion of the i–E curves.
 | (2) |
For comparative purposes, the fitting potential range is truncated to fit data between −1.0 V and −0.53 V, which is characteristic for all four electrolytes. This fitting is operable for a two-component system. Fig. 3 shows the anodic stripping curve for Guan-AlCl3 (black dashed trace) and the corresponding fit (red solid trace) to eqn (2). It can be seen that the mathematical fit matches well to the experimental curve over the selected potential range, and this method allows estimation of the electrolyte conductivity. Using this approach we find conductivities on the order of 10–14 mS cm−1 for the amidine electrolytes and 6.5 mS cm−1 for BMP-AlCl3 (refer to Table 2). Other aluminium-based ILA electrolytes have shown room temperature conductivities of 1–1.5 mS cm−1 (urea),13 0.8 mS cm−1 (acetamide),22 5 mS cm−1 (pyrrolidinium),10 and 10–20 mS cm−1 (imidazolium).7,8,10 The conductivities of the amidine electrolytes are performing as good as state-of-the-art imidazolium-based ILAs and at a fraction of the economic cost. For reference, the current prices of the different Lewis basic salts are provided in ESI† 1, and highlight that the Guan-Cl salt is highly cost-effective which is advantageous for scale-up.
 | ||
| Fig. 3 Experimental voltammetric (anodic) stripping curve (black dashed trace) for Guan-AlCl3 and the corresponding fit (red solid trace) to eqn (2). Refer to ESI† 5 for further details. | ||
| Electrolyte | σ/ms cm−1 | η/cp | E a(η)/kJ mol−1 |
|---|---|---|---|
| Guan-AlCl3 | 14.2 | 51 | 8.9 |
| Acet-AlCl3 | 9.7 | 77 | 4.8 |
| Form-AlCl3 | 11.2 | 80 | — |
| BMP-AlCl3 | 6.5 | 60 | 9.7 |
The QCM set-up also allows the simultaneous estimation the electrolyte viscosity. Using the relationship in eqn (3):
| Rq = [(ωηρ)/2]1/2 | (3) |
In conclusion, the best performance was achieved with Guan-AlCl3, which has the highest concentration of Al3+ species, the highest ionic conductivity, and the lowest viscosity of the electrolytes explored here. In addition, the high Al concentration and low viscosity with Guan-AlCl3 accounts for the facile electrochemical response and may contribute to the early onset reduction potential. Importantly, the Guan-AlCl3 liquid is not only the best performing electrolyte of this series but it can also compete with current state-of-the-art imidazolium- and pyrrolidinium-based electrolytes at a significantly lower economic cost of the starting materials. Overall, this work represents a marked improvement on electrolyte development for Al battery chemistries. Future work will examine the compositional make-up of the amidine electrolytes, in addition to studying their plating characteristics.
This work was funded by the EU H2020-FETOPEN-1-2016-2017 G.A. 766581 SALBAGE and FETPROACT-EIC-06-2019 G.A. 951902 AMAPOLA projects.
Conflicts of interest
There are no conflicts to declare.References
- D. Carrasco-Busturia, S. Lysgaard, P. Jankowski, T. Vegge, A. Bhowmik and J. M. García-Lastra, ChemSusChem, 2021, 14, 2034–2041 CrossRef CAS PubMed.
- Á. Miguel, R. P. Fornari, N. García, A. Bhowmik, D. Carrasco-Busturia, J. M. García-Lastra and P. Tiemblo, ChemSusChem, 2020, 13, 5523–5530 CrossRef PubMed.
- G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater, R. Stolkin, A. Walton, P. Christensen, O. Heidrich, S. Lambert, A. Abbott, K. Ryder, L. Gaines and P. Anderson, Nature, 2019, 575, 75–86 CrossRef CAS.
- G. A. Elia, K. V. Kravchyk, M. V. Kovalenko, J. Chacón, A. Holland and R. G. A. Wills, J. Power Sources, 2021, 481, 228870 CrossRef CAS.
- K. V. Kravchyk and M. V. Kovalenko, Commun. Chem., 2020, 3, 120 CrossRef.
- B. Craig, T. Schoetz, A. Cruden and C. Ponce de Leon, Renewable Sustainable Energy Rev., 2020, 133, 110100 CrossRef CAS.
- C. Ferrara, V. Dall’Asta, V. Berbenni, E. Quartarone and P. Mustarelli, J. Phys. Chem. C, 2017, 121, 26607–26614 CrossRef CAS.
- H. Wang, S. Gu, Y. Bai, S. Chen, N. Zhu, C. Wu and F. Wu, J. Mater. Chem. A, 2015, 3, 22677–22686 RSC.
- M.-C. Lin, M. Gong, B. Lu, Y. Wu, D.-Y. Wang, M. Guan, M. Angell, C. Chen, J. Yang, B.-J. Hwang and H. Dai, Nature, 2015, 520, 324–328 CrossRef CAS.
- G. Zhu, M. Angell, C.-J. Pan, M.-C. Lin, H. Chen, C.-J. Huang, J. Lin, A. J. Achazi, P. Kaghazchi, B.-J. Hwang and H. Dai, RSC Adv., 2019, 9, 11322–11330 RSC.
- G. Pulletikurthi, B. Bödecker, A. Borodin, B. Weidenfeller and F. Endres, Prog. Nat. Sci.: Mater. Int., 2015, 25, 603–611 CrossRef CAS.
- J.-P. M. Veder, M. D. Horne, T. Rüther, A. M. Bond and T. Rodopoulos, Electrochem. Commun., 2013, 37, 68–70 CrossRef CAS.
- M. Angell, G. Zhu, M.-C. Lin, Y. Rong and H. Dai, Adv. Funct. Mater., 2020, 30, 1901928 CrossRef CAS.
- K. L. Ng, M. Malik, E. Buch, T. Glossmann, A. Hintennach and G. Azimi, Electrochim. Acta, 2019, 327, 135031 CrossRef CAS.
- H. Jiao, C. Wang, J. Tu, D. Tian and S. Jiao, Chem. Commun., 2017, 53, 2331–2334 RSC.
- A. P. Abbott, R. C. Harris, Y.-T. Hsieh, K. S. Ryder and I. W. Sun, Phys. Chem. Chem. Phys., 2014, 16, 14675–14681 RSC.
- D. Paterno, E. Rock, A. Forbes, R. Iqbal, N. Mohammad and S. Suarez, J. Mol. Liq., 2020, 319, 114118 CrossRef CAS.
- W. Chu, X. Zhang, J. Wang, S. Zhao, S. Liu and H. Yu, Energy Storage Mater., 2019, 22, 418–423 CrossRef.
- N. Canever, N. Bertrand and T. Nann, Chem. Commun., 2018, 54, 11725–11728 RSC.
- P. Hu, R. Zhang, X. Meng, H. Liu, C. Xu and Z. Liu, Inorg. Chem., 2016, 55, 2374–2380 CrossRef CAS PubMed.
- M. Li, B. Gao, C. Liu, W. Chen, Z. Shi, X. Hu and Z. Wang, Electrochim. Acta, 2015, 180, 811–814 CrossRef CAS.
- H. M. A. Abood, A. P. Abbott, A. D. Ballantyne and K. S. Ryder, Chem. Commun., 2011, 47, 3523–3525 RSC.
- P. Giridhar, S. Zein El Abedin and F. Endres, Electrochim. Acta, 2012, 70, 210–214 CrossRef CAS.
- D. Johannsmann, The Quartz Crystal Microbalance in Soft Matter Research: Fundamentals and Modeling, Springer, Switzerland, 2015 Search PubMed.
- A. R. Hillman, J. Solid State Electrochem., 2011, 15, 1647–1660 CrossRef CAS.
- F. Endres, S. Zein El Abedin, A. Y. Saad, E. M. Moustafa, N. Borissenko, W. E. Price, G. G. Wallace, D. R. MacFarlane, P. J. Newman and A. Bund, Phys. Chem. Chem. Phys., 2008, 10, 2189–2199 RSC.
- J. Komadina, T. Akiyoshi, Y. Ishibashi, Y. Fukunaka and T. Homma, Electrochim. Acta, 2013, 100, 236–241 CrossRef CAS.
- Y. Katayama, R. Fukui and T. Miura, J. Electrochem. Soc., 2013, 160, D251–D255 CrossRef CAS.
- T. Schoetz, O. M. Leung, I. Efimov, C. Zaleski, Á. M. Ortega, N. G. García, P. Tiemblo Magro and C. Ponce de Leon, J. Electrochem. Soc., 2020, 167, 089006 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section (ESI 1), resonance forms of guanidinium cation (ESI 2), 27Al NMR spectra (ESI 3), CV cycles (ESI 4), i–E curve fitting (ESI 5), Arrhenius Plots (ESI 6), charge–discharge curves (ESI 7), and SI references (ESI 8). See DOI: 10.1039/d1cc02680a |
| This journal is © The Royal Society of Chemistry 2021 |

