Self-assembled nanofibers of perylene diimide for the detection of hypochlorite in water, bio-fluids and solid-state: exogenous and endogenous bioimaging of hypochlorite in cells†
Kapil
Kumar
a,
Sandeep
Kaur
b,
Satwinderjeet
Kaur
b,
Gaurav
Bhargava
c,
Subodh
Kumar
 a and
Prabhpreet
Singh
a and
Prabhpreet
Singh
 *a
*a
aDepartment of Chemistry, UGC Centre for Advanced Studies-II, Guru Nanak Dev University, Amritsar, (Pb) 143 005, India. E-mail: prabhpreet.chem@gndu.ac.in; Tel: +91-84271-01534
bDepartment of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar, India
cDepartment of Chemical Sciences, IK Gujral Punjab Technical University, Kapurthala-144601, Punjab, India
First published on 6th November 2019
Abstract
A fluorescent probe PDI–DAMN based on perylenediimide containing diaminomaleonitrile at the bay-position was designed and synthesized for the detection of ClO−. PDI–DAMN self-assembled as nanofibers with diameters in the range of 100–200 nm in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1
CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The addition of ClO− into PDI–DAMN resulted in the disintegration of nanofibers into flake-like aggregates of smaller size (50–80 nm) as supported by SEM and DLS data. The addition of ClO− to HEPES buffer–CH3CN solution (1
1). The addition of ClO− into PDI–DAMN resulted in the disintegration of nanofibers into flake-like aggregates of smaller size (50–80 nm) as supported by SEM and DLS data. The addition of ClO− to HEPES buffer–CH3CN solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) of PDI–DAMN caused a hypochromic effect on the ICT band at 528 nm and ‘turn-on’ fluorescence enhancement at 508/554 nm due to the oxidative cleavage of –C
1, v/v, pH 7.4) of PDI–DAMN caused a hypochromic effect on the ICT band at 528 nm and ‘turn-on’ fluorescence enhancement at 508/554 nm due to the oxidative cleavage of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond. A linear correlation plot between the concentration of ClO−versus fluorescence intensity (R2 = 0.9968)/absorbance (R2 = 0.9988) in the concentration range 0–7 nM (fluorescence)/0–90 nM (absorbance) could determine ClO− with the detection limits of 1 and 10 nM, respectively. Optical studies performed on spiked urine and blood serum samples showed good estimation and recovery of ClO− (100 ± 5%). TLC-based test-strips coated with PDI–DAMN changed colour upon the addition of ClO− with detection as low as 7.44 ng cm−2. The application of PDI–DAMN for the bio-imaging of both exogenous and endogenous ClO− in MG-63 cells with good biocompatibility has also been demonstrated. The detailed mechanism of the interactions of ClO− with PDI–DAMN using 1H NMR titration, DFT studies and response mechanism of pH are also discussed.
N– bond. A linear correlation plot between the concentration of ClO−versus fluorescence intensity (R2 = 0.9968)/absorbance (R2 = 0.9988) in the concentration range 0–7 nM (fluorescence)/0–90 nM (absorbance) could determine ClO− with the detection limits of 1 and 10 nM, respectively. Optical studies performed on spiked urine and blood serum samples showed good estimation and recovery of ClO− (100 ± 5%). TLC-based test-strips coated with PDI–DAMN changed colour upon the addition of ClO− with detection as low as 7.44 ng cm−2. The application of PDI–DAMN for the bio-imaging of both exogenous and endogenous ClO− in MG-63 cells with good biocompatibility has also been demonstrated. The detailed mechanism of the interactions of ClO− with PDI–DAMN using 1H NMR titration, DFT studies and response mechanism of pH are also discussed.
1. Introduction
Hypochlorous (HOCl) or hypochlorite (ClO−; conjugate base), besides being a bioactive molecule, is an important reactive oxygen species (ROS), which can be endogenously generated by the reaction of H2O2 and chloride ion catalyzed by myeloperoxidase (MPO) enzyme in leukocytes.1 Hypochlorous acid helps the human immune system by killing the invading bacteria and pathogens.2 At physiological pH, HOCl could partially dissociate to hypochlorite (ClO−) in water and can be used as a bleaching agent and as a disinfectant for drinking water.3 The uncontrolled level of hypochlorous acid (oxidative agent) can lead to the oxidation of proteins, cholesterol, DNA and RNA in living cells, resulting in pathological diseases such as atherosclerosis, cardiovascular disease, osteoarthritis, cystic fibrosis cancers and pulmonary lesions.4 Therefore, real-time direct visualization or in situ cellular imaging of ClO− in a living system is highly desirable to better understand its role in the biological system.Many detection techniques such as electrochemical, electron paramagnetic resonance (EPR),5 chromatographic methods,6 and chemiluminescence,7 are available in the literature. However, fluorescence and colorimetry-based methods offer several advantages in comparison to other analytical techniques such as non-destructive nature, sensitivity, selectivity, chemical modification and spatiotemporal resolution in living cells. In recent years, many fluorescent probes have been designed for the detection of ClO− with an underlying mechanism involving the oxidation of oxime,8 acyl nitroso group,9p-methoxyphenol,10 selenide,11 arylboronate,12 thioester/thioether13 and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond.14 In this context, fluorescent probes based on a red-to-NIR dye such as cyanine,15 rhodamine,16 BODIPY,17 porphyrin18 have been reported. However, their use is often limited due to fast photobleaching, low quantum yield, interference from other ROS, incompetency in detecting of ClO− in real samples such as biofluids and live cells, slow reaction time and less sensitivity. These limitations inspired us to design a PDI-based probe for the detection of ClO− with excellent fluorescence, absorbance colour changes in solution and solid-state as well as biocompatibility, low cytotoxicity and bio-imaging in live cells.
N– bond.14 In this context, fluorescent probes based on a red-to-NIR dye such as cyanine,15 rhodamine,16 BODIPY,17 porphyrin18 have been reported. However, their use is often limited due to fast photobleaching, low quantum yield, interference from other ROS, incompetency in detecting of ClO− in real samples such as biofluids and live cells, slow reaction time and less sensitivity. These limitations inspired us to design a PDI-based probe for the detection of ClO− with excellent fluorescence, absorbance colour changes in solution and solid-state as well as biocompatibility, low cytotoxicity and bio-imaging in live cells.
Perylenediimides (PDIs) and their derivatives are well established and a prominent class of intensively coloured fluorescent dyes and pigments historically discovered by Kardos in 1913. In recent years, various research groups have chosen PDIs as fluorophore moieties in probe design because they possess high chemical, thermal and photo stabilities, excellent electronic, optical, and redox properties, strong absorption combined with a high fluorescence quantum yield in organic solvents, low reduction potential, good electron acceptor properties, broad colour range properties, and easy chemical functionalization; also it is used as a precursor for higher rylene diimides. Conventionally, PDIs have been exploited to a large extent in the design and fabrication of semiconductor devices, photographic plates and organic field-effect transistors. Moreover, only a few reports are available in the literature regarding the use of bay-functionalized PDIs as chemical sensors in mixed aqueous-organic media and these reports are limited to some of the transition metals, including Hg2+, Pd2+, Zn2+/Cd2+, Fe3+ and Al3+.19–23 This could be attributed to the poor solubility of PDI in an aqueous medium. Considering this, our research group is continuously devoting efforts in the design and self-assembly of bay-functionalized PDI derivatives as fluorescent probes for the detection of different metal ions,24 anions25 and biomolecules26 in aqueous or mixed organic-aqueous media, environmental and clinical samples and in live cells. Our approach to use PDIs in molecular recognition involves (1) the design and synthesis of dissymmetric mono-substituted bay-functionalized PDIs to increase the water solubility; (2) making functional supramolecular aggregates of PDIs in aqueous media to increase the sensitivity for the target analyte and (3) explore molecular recognition properties of the self-assembled PDIs in aqueous media, biofluids and in live cells and elaborating the changes in the morphology of these aggregates during the recognition process.
In this study, we report a novel water-soluble perylenediimide (PDI)–diaminomaleonitrile (DAMN) conjugate (PDI–DAMN), where the PDI fluorophore connected with a DAMN moiety through a Schiff-base (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) linker acts as a chromo-fluorescent probe for the specific (no interference from other bio-analyte) and sensitive (the detection limit of 1–10 nM) detection of ClO− with a quick response time in an aqueous medium, urine and blood serum samples and in solid-state. The confocal microscopy imaging of the MG-63 cells showed that PDI–DAMN was internalized by MG-63 cells and could be used for imaging both exogenous and endogenous ClO− in live cells associated with the appearance of green fluorescence. The distinct colour changes and ‘turn-on’ fluorescence response under UV illumination could be directly seen by naked eye.
N–) linker acts as a chromo-fluorescent probe for the specific (no interference from other bio-analyte) and sensitive (the detection limit of 1–10 nM) detection of ClO− with a quick response time in an aqueous medium, urine and blood serum samples and in solid-state. The confocal microscopy imaging of the MG-63 cells showed that PDI–DAMN was internalized by MG-63 cells and could be used for imaging both exogenous and endogenous ClO− in live cells associated with the appearance of green fluorescence. The distinct colour changes and ‘turn-on’ fluorescence response under UV illumination could be directly seen by naked eye.
2. Experimental section
2.1 Materials and characterization
All chemicals and solvents purchased were of reagent grade and used without further purification. All reactions were performed under N2 atmosphere. CH3CN, DMSO and other solvents were of HPLC grade. Deionized water was obtained from the ULTRA UV/UF Rions Lab Water System Ultra 370 series device. Chromatographic purification was done with silica gel 60–120 mesh. TLC was performed on aluminium sheets coated using silica gel 60 F254 (Merck, Darmstadt). NMR spectra were recorded using a Bruker AVANCE-II FT-NMR AL 400 spectrometer at 400 MHz for 1H; 100 MHz for 13C NMR using CDCl3 as a solvent. The peak values were obtained as ppm (δ) and referenced to the TMS as a reference in 1H NMR and deuterated solvent in 13C NMR spectra. The chemical shift (δ) values are reported in ppm, coupling constants (J values) in hertz (Hz) and abbreviations used for splitting patterns are s = singlet, t = triplet, q = quartet, and m = multiplet.The absorption spectra were recorded using quartz cells of 1 cm in length on a Shimadzu-2450 spectrophotometer (Shimadzu, Japan) equipped with a Peltier system to control the temperature. The spectral bandwidth and the scan rate were fixed at 2 nm and 140 nm min−1, respectively. The fluorescence titrations were performed on a PerkinElmer LS-55 fluorescence spectrophotometer at an excitation of 490 nm, unless otherwise stated. Quartz cells of 1 cm in length were used for sample measurements. FE-SEM measurements were performed on a ZEISS SUPRA™55 microscope operating at an acceleration voltage of 10 kV using a tungsten filament as the electron source. DLS measurements were performed at (25.0 ± 0.1) °C using a light-scattering apparatus (Zetasizer Nano ZS Malvern Instrument Ltd, UK). Solutions were filtered through a Millipore membrane filter (Acrodisc syringe filter, 0.45 μm Supor membrane) before measurements. The samples were thermally equilibrated for 10 min before each measurement, and an average of 10 measurement runs was considered to be data. The temperature was controlled to an accuracy of ±0.1 °C using an in-built Peltier device. Data were analyzed by using standard algorithms. All the theoretical calculations were carried out using density functional theory (DFT) with the B3LYP/6-31G* basis set in the Gaussian 09 package. The HEPES buffer (10 mM) was prepared using deionized water. Stock solutions for various measurements of PDI–DAMN were prepared in CH3CN and the dilutions of these stock solutions were used for the photophysical measurements. Stock solutions (0.1 M) of H2O2, ONOO−, Zn2+, Ca2+, Fe2+, Co2+, Cu2+, Mg2+, Pb2+, Li+, Hg2+, Ni2+, Ba2+, HSO4−, SO42−, Br−, I−, F−, Cl−, AcO−, CO32− and S2O5− ions were prepared in deionized Millipore water and were diluted as required. PDI–DAMN was added in various 10 mL volumetric flasks and subsequently different concentrations of anions were added. The solutions were diluted with HEPES buffer–CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH 7.4) up to the 10 mL mark.
1 v/v, pH 7.4) up to the 10 mL mark.
2.2 Synthesis of PDI–DAMN
Diaminomaleonitrile (41 mg, 0.37 mmol), previously dissolved in ethanol (20 mL), was slowly added into the solution of PDI 2 (200 mg, 0.3 mmol) in dry CH2Cl2 (10 mL). Subsequently, acetic acid (50 μL) was added to the above mixture. The resulted reaction solution was stirred at 80 °C for 10 h under N2 and progress of the reaction was monitored using TLC. The solvent was then evaporated and the crude product was purified using column chromatography (SiO2, MeOH/CHCl3, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v) as eluent to afford PDI–DAMN as a dark reddish-brown solid (224 mg, 0.31 mmol, yield 97.6%), Rf = 0.1; 1H NMR (400 MHz, CDCl3, 25 °C): δ 8.71–8.61 (m, 4H, perylene-ArH), 8.56 (s, 1H, perylene-ArH), 8.51 (s, 1H, –CH
8, v/v) as eluent to afford PDI–DAMN as a dark reddish-brown solid (224 mg, 0.31 mmol, yield 97.6%), Rf = 0.1; 1H NMR (400 MHz, CDCl3, 25 °C): δ 8.71–8.61 (m, 4H, perylene-ArH), 8.56 (s, 1H, perylene-ArH), 8.51 (s, 1H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), 8.11 (d, 1H, J = 8.4 Hz, perylene-ArH), 7.98 (d, 2H, J = 8.4 Hz, Ar-H), 7.81 (d, 1H, J = 8.0 Hz, perylene-ArH), 7.60 (d, 2H, J = 8.4 Hz, Ar-H), 5.53 (s, 2H, –NH2), 5.02–5.07 (m, 2H, ethylpropyl-CH), 2.20–2.28 (m, 4H, ethylpropyl CH2), 1.86–197 (m, 4H, ethylpropyl CH2), 0.87–0.94 (m, 12H, ethylpropyl CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 11.44, 25.09, 25.12, 57.80, 57.94, 107.91, 112.41, 113.56, 123.08, 123.86, 125.37, 127.56, 128.16, 128.81, 129.28, 129.56, 130.34, 131.07, 132.84, 134.34, 134.93, 135.08, 140.38, 146.58, 157.67 ppm; IR (ATR) νmax [cm−1] = 3298, 2965, 2933, 2875, 2236, 2208, 1697, 1653, 1589, 1459, 1405, 1328, 1243, 1200, 1161, 1082, 965; UV-Vis: λmax = 498, 529 nm (CH3CN/water with fw = 50 vol%); Fluorescence: λem = 508, 555 nm; (CH3CN/water with fw = 50 vol%).
N–), 8.11 (d, 1H, J = 8.4 Hz, perylene-ArH), 7.98 (d, 2H, J = 8.4 Hz, Ar-H), 7.81 (d, 1H, J = 8.0 Hz, perylene-ArH), 7.60 (d, 2H, J = 8.4 Hz, Ar-H), 5.53 (s, 2H, –NH2), 5.02–5.07 (m, 2H, ethylpropyl-CH), 2.20–2.28 (m, 4H, ethylpropyl CH2), 1.86–197 (m, 4H, ethylpropyl CH2), 0.87–0.94 (m, 12H, ethylpropyl CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 11.44, 25.09, 25.12, 57.80, 57.94, 107.91, 112.41, 113.56, 123.08, 123.86, 125.37, 127.56, 128.16, 128.81, 129.28, 129.56, 130.34, 131.07, 132.84, 134.34, 134.93, 135.08, 140.38, 146.58, 157.67 ppm; IR (ATR) νmax [cm−1] = 3298, 2965, 2933, 2875, 2236, 2208, 1697, 1653, 1589, 1459, 1405, 1328, 1243, 1200, 1161, 1082, 965; UV-Vis: λmax = 498, 529 nm (CH3CN/water with fw = 50 vol%); Fluorescence: λem = 508, 555 nm; (CH3CN/water with fw = 50 vol%).
2.3 Procedure for NMR titration
The 1H NMR titration of PDI–DAMN against NaOCl was performed in DMSO-d6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9
H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using a Bruker-AVANCE-II FT-NMR AL400 spectrophotometer. All the data were then processed in the Top Spin software to draw the stacking spectra of PDI–DAMN and PDI–DAMN + NaOCl mixture at different concentrations.
1) using a Bruker-AVANCE-II FT-NMR AL400 spectrophotometer. All the data were then processed in the Top Spin software to draw the stacking spectra of PDI–DAMN and PDI–DAMN + NaOCl mixture at different concentrations.
2.4 MTT assay and live cell measurements
The human osteosarcoma (MG-63) cell line used in the study was purchased from the National Centre for Cell Science (NCCS, Pune, India). The cells were cultured in a humidified atmosphere at 37 °C under 95% air mixture and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with an antibiotic–antimycotic solution and 10% fetal bovine serum (Biological Industries). The cells were washed with 1× PBS (pH 7.4) and trypsinized at 37 °C, followed by centrifugation at 1000 rpm for 5 min. The cell pellet collected was suspended in a fresh culture medium and stained by using the trypan blue dye exclusion method for counting the number of viable cells.The cytotoxic potential of the PDI–DAMN was determined in the Human Osteosarcoma MG-63 cell line using the MTT assay. The cytotoxicity study was performed when the confluency level of the cells reached up to 70–80%. The cells suspended in 100 μL of medium were seeded in 96-well microplates and incubated to allow cell adherence. After 24 hours incubation, 100 μL of the fresh medium containing different concentrations of the PDI–DAMN was added in each well. After completion of another 24 hours, 20 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye solution was added to each well and incubated for 2–3 hours for measuring the capability of viable cells to reduce it into purple coloured formazan. Subsequently, the MTT solution was removed and 100 μL of dimethyl sulfoxide (DMSO) was added to dissolve the intracellular insoluble formazan. The absorbance at 570 nm was measured using a multi-well plate reader (BioTek Synergy HT).
| % Cell viability = Mean absorbance in test sample wells/Mean absorbance in control wells × 100 |
Cells of two wells were treated each with PDI–DAMN (10 μM prepared in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) media; 2
media; 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98); four wells were treated with 10 μM of PDI–DAMN for 30 minutes, followed by NaOCl (two wells each with 20 and 40 μM); four wells were treated with 10 μM of PDI–DAMN for 30 minutes, followed by lipopolysaccharides (LPS) + phorbol myristate (PMA) (two wells each with 2 and 5 μg mL−1); NaOCl treatment (40 μM prepared in CH3CN
98); four wells were treated with 10 μM of PDI–DAMN for 30 minutes, followed by NaOCl (two wells each with 20 and 40 μM); four wells were treated with 10 μM of PDI–DAMN for 30 minutes, followed by lipopolysaccharides (LPS) + phorbol myristate (PMA) (two wells each with 2 and 5 μg mL−1); NaOCl treatment (40 μM prepared in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) media; 2
media; 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98) was given to cells of two wells, while two wells served as control. The cells were seeded onto 24-well plates at a density of 1.5 × 104 cells per well and the coverslip (12 mm) was placed in each well. On attaining the adherence and 70–80% confluency, cells were treated with various concentrations of the test samples. After treatment, the cells were washed with 1× PBS, and then fixed with 4% paraformaldehyde for 15 minutes. After incubation, the cells were washed three times with 1× PBS and then the coverslips containing cells were mounted on the glass slides having a drop of an anti-fading reagent (Fluoromount; Sigma). Subsequently, cells images were captured with the help of a Nikon A1R Laser scanning confocal microscope system. Imaging was performed using a 20× or 40× oil-emersion objective lens. The picture obtained under the confocal microscope at an excitation of 490 nm and emission of 508 nm was further analyzed using the software version 4.11.00 of NIS Elements AR analysis (Nikon Corporation, Japan).
98) was given to cells of two wells, while two wells served as control. The cells were seeded onto 24-well plates at a density of 1.5 × 104 cells per well and the coverslip (12 mm) was placed in each well. On attaining the adherence and 70–80% confluency, cells were treated with various concentrations of the test samples. After treatment, the cells were washed with 1× PBS, and then fixed with 4% paraformaldehyde for 15 minutes. After incubation, the cells were washed three times with 1× PBS and then the coverslips containing cells were mounted on the glass slides having a drop of an anti-fading reagent (Fluoromount; Sigma). Subsequently, cells images were captured with the help of a Nikon A1R Laser scanning confocal microscope system. Imaging was performed using a 20× or 40× oil-emersion objective lens. The picture obtained under the confocal microscope at an excitation of 490 nm and emission of 508 nm was further analyzed using the software version 4.11.00 of NIS Elements AR analysis (Nikon Corporation, Japan).
2.5 Detection limit
The detection limit was calculated based on the absorbance or fluorescence titrations. To determine the S/N ratio, the emission intensity of PDI–DAMN (10 μM) without analyte was measured three times and the standard deviation of blank solution (without the addition of analyte) measurements was determined. The detection limit was then calculated using the equation, detection limit = 3σbi/m, where σbi is the standard deviation of the blank solution (without the addition of analyte) measurements, and m is the slope between intensity versus sample concentration.2.6 Preparation of TLC strips
TLC strips were made by dipping silica gel coated aluminium plate into the CH3CN solution of PDI–DAMN, followed by drying under vacuum at room temperature. In the TLC strip method, the different concentrations of ClO− were prepared in an aqueous solution, followed by the addition of an aliquot of 6 μL of each solution on the TLC strips previously coated with PDI–DAMN (10 μM). For the control experiment, a drop of water alone was also added on the TLC strip coated with PDI–DAMN. The TLC strips were then visualized under 365 nm UV light.3. Results and discussion
3.1 Rational design and synthesis of PDI–DAMN
The diaminomaleonitrile (DAMN)-based Schiff-bases are a promising strategy for the detection of ClO− ion.27–34 ClO− behaves as an oxidizing agent to attack the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, followed by the addition of water to recover the DAMN and fluorophore units. Encouraged by this strategy, we have synthesized PDI–DAMN by the reaction of PDI 2 with DAMN using a mixture of C2H5OH/CH2Cl2 (7
N bond, followed by the addition of water to recover the DAMN and fluorophore units. Encouraged by this strategy, we have synthesized PDI–DAMN by the reaction of PDI 2 with DAMN using a mixture of C2H5OH/CH2Cl2 (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) as solvent at 80 °C catalyzed by acetic acid. For the synthesis of PDI 2, we have performed the Suzuki coupling of PDI 1 using 4-formylboronic acid and Pd(PPh3)4 in toluene (Scheme 1). The formation of PDI–DAMN was characterized by 1H, and 13C NMR spectroscopic techniques and HRMS data [m/z for C47H39N7O4 [PDI–DAMN + CH3CN + H]+ calcd 765.3064, found 766.5378] (Fig. S1, ESI†). In PDI–DAMN, perylene diimide (a fluorophore, which possesses a high quantum yield and molar extinction coefficient) is directly linked to DAMN moiety through the C
3, v/v) as solvent at 80 °C catalyzed by acetic acid. For the synthesis of PDI 2, we have performed the Suzuki coupling of PDI 1 using 4-formylboronic acid and Pd(PPh3)4 in toluene (Scheme 1). The formation of PDI–DAMN was characterized by 1H, and 13C NMR spectroscopic techniques and HRMS data [m/z for C47H39N7O4 [PDI–DAMN + CH3CN + H]+ calcd 765.3064, found 766.5378] (Fig. S1, ESI†). In PDI–DAMN, perylene diimide (a fluorophore, which possesses a high quantum yield and molar extinction coefficient) is directly linked to DAMN moiety through the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (Schiff-base).
N bond (Schiff-base).
Consequently, we hypothesized that PDI–DAMN might possess weak fluorescence and charge transfer absorbance band owing to (1) C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N rotation and isomerization phenomena that lead to the fast decay of the excited state and (2) photoinduced electron transfer (PET) and intramolecular charge transfer (ICT) phenomena from nitrogen atom of the DAMN to electron-deficient PDI core. The addition of ClO− would cause the cleavage of the DAMN group (via hydrolysis reaction) and PDI–DAMN would show fluorescence enhancement or colour change. This ClO−-mediated hydrolysis reaction was first monitored by 1H NMR titration.
N rotation and isomerization phenomena that lead to the fast decay of the excited state and (2) photoinduced electron transfer (PET) and intramolecular charge transfer (ICT) phenomena from nitrogen atom of the DAMN to electron-deficient PDI core. The addition of ClO− would cause the cleavage of the DAMN group (via hydrolysis reaction) and PDI–DAMN would show fluorescence enhancement or colour change. This ClO−-mediated hydrolysis reaction was first monitored by 1H NMR titration.
The NMR spectra for the reaction mixture of PDI–DAMN with NaOCl show the appearance of the peak at δ 10.02 ppm, which likely originates from a proton of the aldehyde (PDI 2) and concomitant disappearance of the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– proton peak at δ 8.05 ppm (Fig. 1). The doublets of the phenyl ring showed upfield shifts from 7.60 to 7.37 ppm and 7.52 to 7.07 ppm. The 13C NMR spectra of PDI–DAMN + ClO− showed the appearance of a new peak at 191.42 ppm, which confirmed the generation of the CHO group. The formation of PDI 2 was further confirmed by the HRMS data [m/z for C41H34N2O5 [PDI 2]+ calcd 634.2468, found 634.4560] (Fig. S2, ESI†).
N– proton peak at δ 8.05 ppm (Fig. 1). The doublets of the phenyl ring showed upfield shifts from 7.60 to 7.37 ppm and 7.52 to 7.07 ppm. The 13C NMR spectra of PDI–DAMN + ClO− showed the appearance of a new peak at 191.42 ppm, which confirmed the generation of the CHO group. The formation of PDI 2 was further confirmed by the HRMS data [m/z for C41H34N2O5 [PDI 2]+ calcd 634.2468, found 634.4560] (Fig. S2, ESI†).
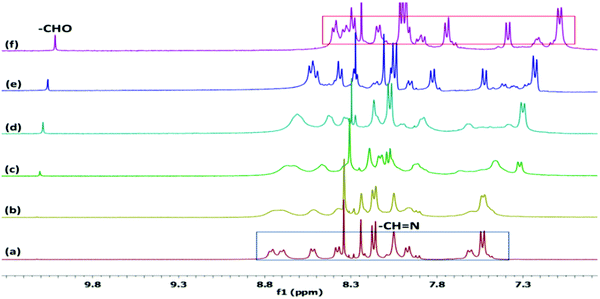 | ||
Fig. 1
1H NMR spectra of PDI–DAMN with a gradual addition of NaOCl in DMSO-d6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9 H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v); (a) PDI–DAMN alone and after addition of (b) 0.1, (c) 0.2, (d) 0.5, (e) 1.0 and (f) 2.0 equiv. of NaOCl. 1, v/v); (a) PDI–DAMN alone and after addition of (b) 0.1, (c) 0.2, (d) 0.5, (e) 1.0 and (f) 2.0 equiv. of NaOCl. | ||
3.2 Effect of pH and specificity of PDI–DAMN towards ClO−
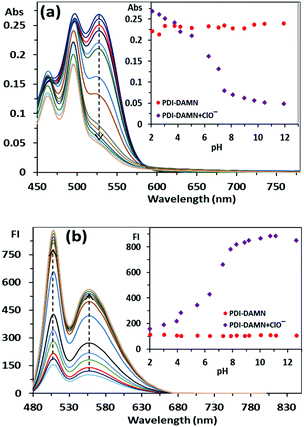
The pH-dependent absorption and fluorescence response of PDI–DAMN alone and PDI–DAMN + ClO− complex were also recorded. The PDI–DAMN did not show any observable change in both absorbance and fluorescence spectral profiles within the wide pH range from 2 to 12 (Fig. S3, ESI†). However, the interactions of ClO− with PDI–DAMN are highly influenced by the pH of the solution (Fig. 2). At pH 2–4, the addition of HOCl into the PDI–DAMN solution caused a minor decrease in the CT absorbance band at 528 nm and minor increase in the fluorescence intensity at 508 and 554 nm, which could be attributed to the presence of an excess of non-ionized HOCl.However, above pH 4, the complete disappearance of the CT absorbance band at 528 nm and a dramatic increase in the fluorescence intensity up to ca. pH = 9, was observed and then plateau was achieved. For this reason, we have chosen pH 7.4 for all experiments where HOCl (pKa = 7.5) exists mostly in ClO− ion.
We further investigated the specificity of PDI–DAMN in the HEPES buffer–CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) solution towards ClO− over other substances including H2O2, ONOO−, Zn2+, Ca2+, Fe2+, Co2+, Cu2+, Mg2+, Pb2+, Li+, K+, Hg2+, Ni2+, Ba2+, Cr3+, HSO4−, S2−, SO32−, PO43−, SO42−, Br−, I−, F−, Cl−, AcO−, CO32− and S2O5− ions. The addition of all the above substances in the solution of PDI–DAMN neither produces any appreciable visible colour change (naked eye) nor fluorescence enhancement (Fig. S4–S6, ESI†). However, only the addition of ClO− resulted in the fluorescence enhancement at 508, 554 nm and an obvious decrease in the charge transfer band at 528 nm, indicating that PDI–DAMN possessed the outstanding selectivity towards ClO− (Fig. S4–S6, ESI†). The selectivity of PDI–DAMN towards the detection of ClO− over other substances can also be determined from the photographs (naked eye and under UV light 365 nm) in the well plates (Fig. 3).
1, v/v, pH 7.4) solution towards ClO− over other substances including H2O2, ONOO−, Zn2+, Ca2+, Fe2+, Co2+, Cu2+, Mg2+, Pb2+, Li+, K+, Hg2+, Ni2+, Ba2+, Cr3+, HSO4−, S2−, SO32−, PO43−, SO42−, Br−, I−, F−, Cl−, AcO−, CO32− and S2O5− ions. The addition of all the above substances in the solution of PDI–DAMN neither produces any appreciable visible colour change (naked eye) nor fluorescence enhancement (Fig. S4–S6, ESI†). However, only the addition of ClO− resulted in the fluorescence enhancement at 508, 554 nm and an obvious decrease in the charge transfer band at 528 nm, indicating that PDI–DAMN possessed the outstanding selectivity towards ClO− (Fig. S4–S6, ESI†). The selectivity of PDI–DAMN towards the detection of ClO− over other substances can also be determined from the photographs (naked eye and under UV light 365 nm) in the well plates (Fig. 3).
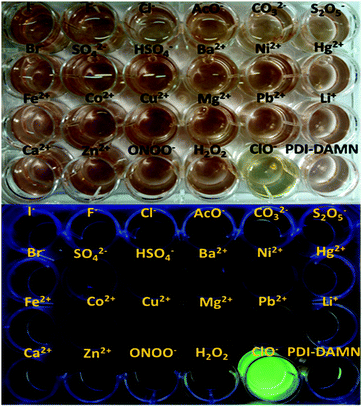 | ||
| Fig. 3 (upper) Colour (naked eye) and (lower) fluorescence images of well plate containing PDI–DAMN alone and PDI–DAMN + different substances. | ||
3.3 Detection of ClO− in solid-state
Based on the above results, we hypothesized that the HEPES buffer–CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) solution of PDI–DAMN in the presence of ClO− underwent hydrolysis reaction to produce PDI 2. So, we anticipated that PDI–DAMN on the addition of ClO− would also undergo morphological changes in the solid-state.
1, v/v, pH 7.4) solution of PDI–DAMN in the presence of ClO− underwent hydrolysis reaction to produce PDI 2. So, we anticipated that PDI–DAMN on the addition of ClO− would also undergo morphological changes in the solid-state.
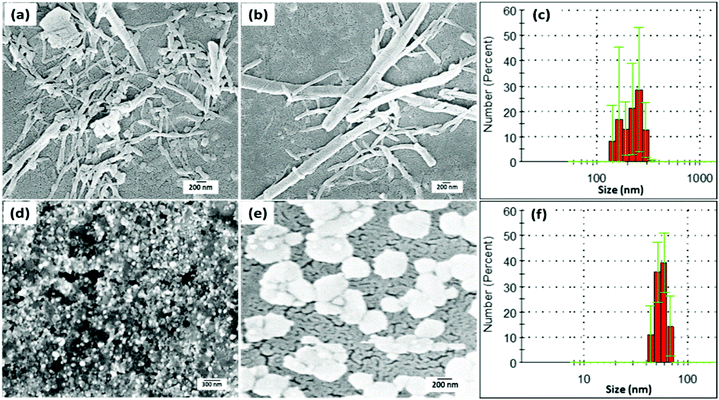
To decipher any such effect on PDI–DAMN in the solid-state due to the addition of ClO−, the self-assemblies of the PDI–DAMN and PDI–DAMN + ClO− mixture in CH3CN: H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) were investigated using a microscopic technique. Field-emission scanning electron microscopy (FE-SEM) images of thin films on a glass plate prepared by the drop cast technique from the solution of PDI–DAMN in CH3CN
1, v/v) were investigated using a microscopic technique. Field-emission scanning electron microscopy (FE-SEM) images of thin films on a glass plate prepared by the drop cast technique from the solution of PDI–DAMN in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed fibre-like aggregates all over the surface (Fig. 4a and b). These fibre-like aggregates showed an average width of 200–300 nm and length up to micrometres. In contrast the FE-SEM images of thin films of PDI–DAMN + ClO− mixture deposited from the CH3CN
1) showed fibre-like aggregates all over the surface (Fig. 4a and b). These fibre-like aggregates showed an average width of 200–300 nm and length up to micrometres. In contrast the FE-SEM images of thin films of PDI–DAMN + ClO− mixture deposited from the CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution revealed the complete loss of fibre-like morphology/assembly and instead we observed the formation of flake-like aggregates with diameters of 100–150 nm (Fig. 4d and e).
1) solution revealed the complete loss of fibre-like morphology/assembly and instead we observed the formation of flake-like aggregates with diameters of 100–150 nm (Fig. 4d and e).
The aggregation-disaggregation process in PDI–DAMN alone and PDI–DAMN + ClO− mixture, respectively, was further supported by dynamic light scattering (DLS) measurements. The particle size analysis of PDI–DAMN (10 μM) in CH3CN:H2O reveals that the size of the fibre-like aggregates is in the range of 150–300 nm (Fig. 4c). In contrast, the particle size analysis of the PDI–DAMN + ClO− mixture in CH3CN:H2O revealed the aggregates of diameter between 50–80 nm (Fig. 4f).
3.4 UV-Vis and fluorescence response of PDI–DAMN towards ClO−
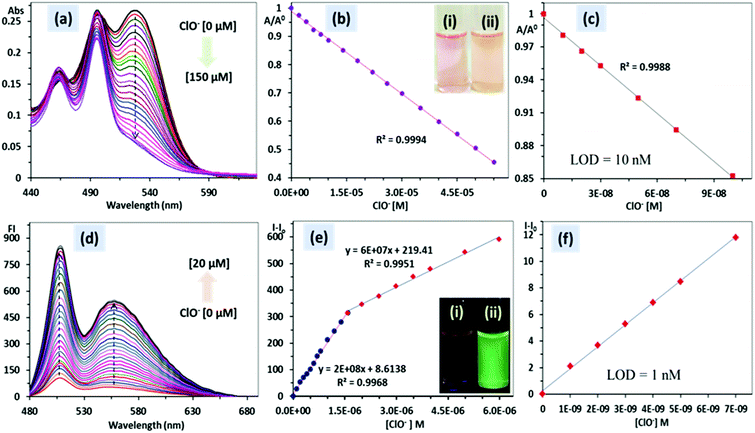
The optical titrations of PDI–DAMN for monitoring ClO− were studied in the HEPES buffer–CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) solution. As shown in Fig. 5a, the absorbance spectrum of PDI–DAMN showed a broad charge transfer (CT) band at 528 nm and normal absorbance band due to the π–π* transition at 498 nm. With the addition of ClO−, the CT absorbance band at 528 nm gradually decreased and completely vanished with the addition of 150 μM concentration of ClO−, whereas the normal absorbance band at 498 nm did not show any observable change. Two linear ranges were observed from 0–55 μM and 55–150 μM. The plot of A/A0versus concentrations of ClO− [M] demonstrates good linear correlation between 0–55 μM ClO− and detection limit was 10 nM (R2 = 0.9994) (3σ/slope). The addition of ClO− to the solution of PDI–DAMN caused a colour change from purple to light yellowish-purple observable by the naked eye (Fig. 5b and c). In the fluorescence spectrum, PDI–DAMN showed weak fluorescence (ϕ = 0.08), with the emission maxima at 508 and 554 nm.
1, v/v, pH 7.4) solution. As shown in Fig. 5a, the absorbance spectrum of PDI–DAMN showed a broad charge transfer (CT) band at 528 nm and normal absorbance band due to the π–π* transition at 498 nm. With the addition of ClO−, the CT absorbance band at 528 nm gradually decreased and completely vanished with the addition of 150 μM concentration of ClO−, whereas the normal absorbance band at 498 nm did not show any observable change. Two linear ranges were observed from 0–55 μM and 55–150 μM. The plot of A/A0versus concentrations of ClO− [M] demonstrates good linear correlation between 0–55 μM ClO− and detection limit was 10 nM (R2 = 0.9994) (3σ/slope). The addition of ClO− to the solution of PDI–DAMN caused a colour change from purple to light yellowish-purple observable by the naked eye (Fig. 5b and c). In the fluorescence spectrum, PDI–DAMN showed weak fluorescence (ϕ = 0.08), with the emission maxima at 508 and 554 nm.
On incremental addition of ClO− (0–10 μM) into the HEPES buffer–CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) solution of PDI–DAMN (1 μM), significant fluorescence enhancement at 508 and 554 nm was observed. The fluorescence intensity achieved a plateau with the addition of 8 μM of ClO−. The plot of the fluorescence intensity (I − I0) versus concentrations of ClO− showed a linear relationship between 0–1.6 μM (R2 = 0.9968) and 1.6–6 μM (R2 = 0.9951). The detection limit based on linear correlation (0–1.6 μM) was calculated to be 1 nM. During the titration of PDI–DAMN with ClO−, fluorescence under UV lamp illumination (365 nm) changed from dark to fluorescent green (ϕ = 0.91) (Fig. 5d–f).
1, v/v, pH 7.4) solution of PDI–DAMN (1 μM), significant fluorescence enhancement at 508 and 554 nm was observed. The fluorescence intensity achieved a plateau with the addition of 8 μM of ClO−. The plot of the fluorescence intensity (I − I0) versus concentrations of ClO− showed a linear relationship between 0–1.6 μM (R2 = 0.9968) and 1.6–6 μM (R2 = 0.9951). The detection limit based on linear correlation (0–1.6 μM) was calculated to be 1 nM. During the titration of PDI–DAMN with ClO−, fluorescence under UV lamp illumination (365 nm) changed from dark to fluorescent green (ϕ = 0.91) (Fig. 5d–f).
As a control experiment, the photophysical studies of PDI 2 in the presence of ClO− were also performed. The PDI 2 showed absorption bands at 495, 463 and 436 nm, corresponding to the A0–0, A0–1 and A0–2 transitions and emission maxima at 510 nm with shoulder bands at 548 and 592 nm in the HEPES buffer–CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) solution.
1, v/v, pH 7.4) solution.
Upon the addition of 20 equivalents of ClO− to the solution of PDI 2, no noticeable change both in the absorbance and emission spectra of PDI 2 was observed (Fig. S7, ESI†). It indicates that –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, which connected the DAMN moiety with PDI, is a reactive site for ClO−.
N–, which connected the DAMN moiety with PDI, is a reactive site for ClO−.
3.5 Bioimaging of exogenous and endogenous ClO− in living cells
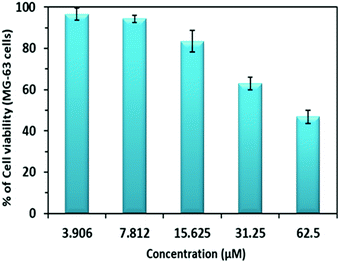
Encouraged by the excellent and sensitive detection of ClO− by PDI–DAMN in aqueous media, further, we investigated the potential of PDI–DAMN for the detection of ClO− in living systems.For this purpose, we first evaluated the cytotoxicity of PDI–DAMN by the MTT assay in MG 63 cells treated with different concentrations of PDI–DAMN for 24 h (Fig. 6). Our results showed that >95% of the MG-63 cells remained viable after 24 h incubation with the 10 μM concentrations of PDI–DAMN. The IC50 value calculated for PDI–DAMN is 54 μM. These data indicated that PDI–DAMN exhibits good biocompatibility and low cytotoxicity, ensuring its application in cellular imaging.
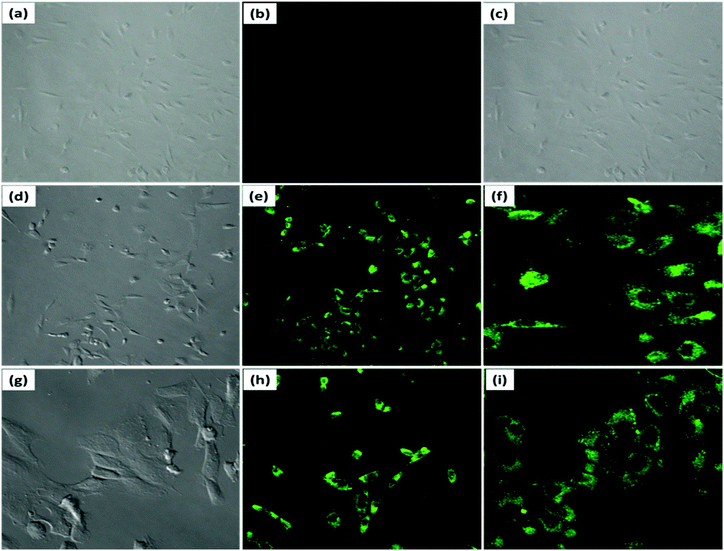
We studied the concentration-dependent fluorescence intensity changes in MG-63 cells, previously treated with PDI–DAMN (10 μM) for 30 min with different concentrations of ClO− (20 and 40 μM). The MG-63 cells incubated with PDI–DAMN (10 μM) for 30 min have neither revealed any morphological changes (see brightfield image, Fig. 7) nor any fluorescence when imaged under confocal microscopy (λex = 488 nm, Fig. 7b). Moreover, when PDI–DAMN-treated MG-63 cells were further incubated with ClO− (20 and 40 μM, respectively), the cells showed a concentration-dependent increase in green fluorescence in the cytoplasmic region (Fig. 7e, f and h, i). Therefore, PDI–DAMN shows permeability toward MG-63 cells and can be used for the detection of exogenous ClO− in living cells.
We also explored the potential of PDI–DAMN for the detection of endogenous ClO− in living cells. It was known from the literature that stimulating cells with lipopolysaccharides (LPS) and phorbol myristate (PMA) can endogenously increase the ClO− level in cells.
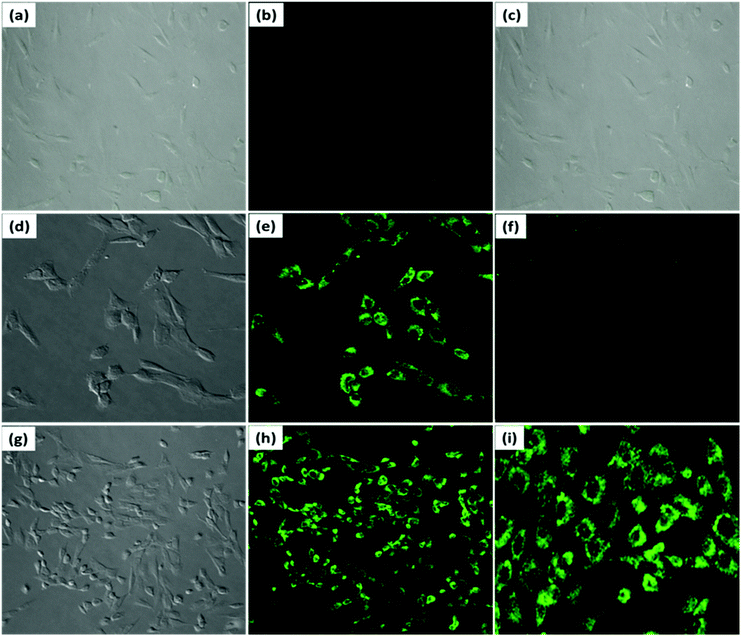
When MG-63 cells were incubated with PDI–DAMN (10 μM) for 30 min at 37 °C, no fluorescence was observed in the cytoplasmic region of the MG-63 cells (Fig. 8b). However, when MG-63 cells were pre-incubated with a LPS (5 μg mL−1) and PMA (5 μg mL−1) reagents (for endogenously generating ClO−) for 30 min and then were incubated with PDI–DAMN for 30 min, an obvious significantly enhanced green fluorescence was observed inside the cells in the cytoplasmic region (Fig. 8e, f and h, i). These results indicated the potential of PDI–DAMN to image endogenous ClO− in living cells (Fig. S8–S9, ESI†).
3.6 Detection of ClO− in biofluid samples
PDI–DAMN was also evaluated for the optical detection of ClO− in the bio-fluid samples of human urine and blood serum collected from the university health centre.
PDI–DAMN showed a decrease in the absorption intensity at 528 nm and fluorescence enhancement at 508 and 554 nm with the addition of ClO− in HEPES buffer–CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4), containing 10% human urine and blood serum. To quantify the concentration of ClO−, the solutions of PDI–DAMN were prepared in HEPES buffer–CH3CN 1
1, v/v, pH 7.4), containing 10% human urine and blood serum. To quantify the concentration of ClO−, the solutions of PDI–DAMN were prepared in HEPES buffer–CH3CN 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4, containing 10% human urine and blood serum and spiked with the known concentration of ClO− (Fig. S10 and S11, ESI†). The concentrations of ClO− in spiked samples were estimated from the standard calibration graphs of UV-Vis and emission data. PDI–DAMN showed an excellent percentage recovery of ClO− (in nM range) from urine and blood serum samples (Table 1). Furthermore, to explore that PDI–DAMN effectively serves to detect hypochlorite in urine or blood serum samples, another experiment was performed where different concentrations of hypochlorite is added in HEPES buffer–CH3CN 1
1, v/v, pH 7.4, containing 10% human urine and blood serum and spiked with the known concentration of ClO− (Fig. S10 and S11, ESI†). The concentrations of ClO− in spiked samples were estimated from the standard calibration graphs of UV-Vis and emission data. PDI–DAMN showed an excellent percentage recovery of ClO− (in nM range) from urine and blood serum samples (Table 1). Furthermore, to explore that PDI–DAMN effectively serves to detect hypochlorite in urine or blood serum samples, another experiment was performed where different concentrations of hypochlorite is added in HEPES buffer–CH3CN 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4, containing 10 or 50% human urine and blood serum solutions, and then PDI–DAMN was added to detect the presence of hypochlorite. Again, PDI–DAMN showed excellent percentage recovery of ClO− (Fig. S12–S19, ESI†).
1, v/v, pH 7.4, containing 10 or 50% human urine and blood serum solutions, and then PDI–DAMN was added to detect the presence of hypochlorite. Again, PDI–DAMN showed excellent percentage recovery of ClO− (Fig. S12–S19, ESI†).
| Techniques | Samples | ClO− added (nM) | ClO− found (nM) | % Age recovery |
|---|---|---|---|---|
| UV-Vis | Blood serum | 30 | 30 | 100 |
| 74 | 70 | 94.59 | ||
| 150 | 150 | 100 | ||
| 210 | 200 | 95.23 | ||
| Urine | 28.5 | 30 | 105.26 | |
| 70.5 | 70 | 93.33 | ||
| 100 | 100 | 100 | ||
| 149 | 150 | 100.67 | ||
| Fluorescence | Blood serum | 10 | 10 | 100 |
| 95 | 100 | 105.26 | ||
| 300 | 300 | 100 | ||
| Urine | 38 | 40 | 105 | |
| 61 | 60 | 98.36 | ||
| 200 | 200 | 100 |
3.7 PDI–DAMN-coated TLC strips for the detection of ClO− in the solid-state through the contact mode method
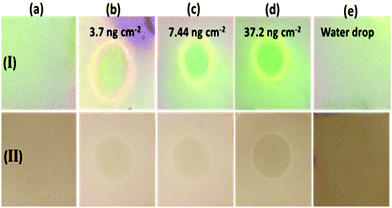
The application of PDI–DAMN for the sensing of ClO− in the solid-state was also evaluated by employing PDI–DAMN coated TLC strips (Fig. 9). These PDI–DAMN-coated TLC strips were prepared using a dipping silica gel coated aluminium plate into a solution of PDI–DAMN (1 × 10−5 M) in CH3CN, followed by air drying.PDI–DAMN-coated TLC strips appeared weakly fluorescent under illumination at 365 nm light and light brown colour visible to naked eye. Different concentrations of ClO− were added in the range of 1 × 10−6 M to 1 × 10−4 M, which indicated the appearance of green ‘turn-on’ fluorescence and bleaching of colour (naked eye). In the solid-state minimum detection of ClO− was estimated to be 7.44 ng cm−2. It should be noted that the addition of water alone does not cause any bleaching of colour and change in fluorescence.
3.8 DFT calculations
The geometry of PDI–DAMN and PDI 2 (reaction product of PDI–DAMN + ClO−) was also optimized using DFT calculations with B3LYP and 6-31G* basis set under the Gaussian 09 software. The HOMO of the PDI–DAMN and PDI 2 extended across the whole framework of PDI along with the localization of electron density on the DAMN moiety in PDI–DAMN, whereas LUMO is mainly located at the perylene core both in PDI–DAMN and PDI 2 (Fig. 10).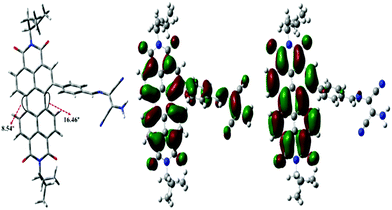 | ||
| Fig. 10 The optimized structure and HOMO, LUMO structures of PDI–DAMN at B3LYP/6-31G* using Gaussian 09 package. | ||
PDI–DAMN shows twisting between two naphthalene rings with a torsion angle of 8.54° on the unsubstituted side and 16.46° on the DAMN-substituted side. The calculated energy values of HOMO and LUMO for PDI–DAMN are −5.98 eV and −3.59 eV, respectively.
4. Conclusions
In conclusion, we have reported the chemodosimetric probe PDI–DAMN for the detection of hypochlorite (ClO−) inducing a one-step de-diaminomaleonitrile reaction with high sensitivity and selectivity. The limit of detection (LOD) was 1 and 10 nM in solution and at the nanogram level by employing solid-state detection techniques. The SEM studies revealed the transformation in morphology from nanofibers to spherical aggregates with the addition of ClO− to the solution of PDI–DAMN. Hypochlorite was also detected in biological samples, including human blood serum and human urine, with satisfactory recoveries. PDI–DAMN also found applications for imaging both exogenous and endogenous ClO− in live cells.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by DST-SERB grant EMR/2016/002239 and CSIR grant 02(0267)/16/EMR-II. Kapil Kumar is thankful to the DST-SERB for fellowship.Notes and references
- (a) Y. W. Yap, M. Whiteman and N. S. Cheung, Cell Signaling, 2007, 19, 219–228 CrossRef CAS; (b) H. B. Dunford, Heme Peroxidases, Wiley, New York, 1999 Search PubMed; (c) B. D. Autreaux and M. B. Toledano, Nat. Rev. Mol. Cell Biol., 2007, 8, 813–824 CrossRef.
- (a) M. B. Hampton, A. J. Kettle and C. C. Winterbourn, Blood, 1998, 92, 3007–3017 CrossRef CAS; (b) C. C. Winterbourn, M. B. Hampton, J. H. Livesey and A. J. Kettle, J. Biol. Chem., 2006, 281, 39860–39869 CrossRef CAS.
- T. Aoki and M. Munemori, Anal. Chem., 1983, 55, 209–212 CrossRef CAS.
- (a) L. J. Hazell, L. Arnold, D. Flowers, G. Waeg, E. Malle and R. Stocker, J. Clin. Invest., 1996, 97, 1535–1544 CrossRef CAS; (b) D. I. Pattison and M. J. Davies, Chem. Res. Toxicol., 2001, 14, 1453–1464 Search PubMed; (c) X. Chen, C. Guo and J. Kong, Neural Regener. Res., 2012, 7, 376–385 Search PubMed; (d) F. Giacco and M. Brownlee, Circ. Res., 2010, 107, 1058–1070 CrossRef CAS; (e) S. A. Weitzman and L. I. Gordon, Blood, 1990, 76, 655–663 CrossRef CAS.
- Z. Takats, K. J. Koch and R. G. Cooks, Anal. Chem., 2001, 73, 4522–4529 CrossRef CAS.
- T. Watanabe, T. Idehara, Y. Yoshimura and H. Nakazawa, J. Chromatogr. A, 1998, 796, 397–400 CrossRef CAS.
- J. B. Claver, M. V. Miron and L. Capitán-Vallvey, Anal. Chim. Acta, 2004, 522, 267–273 CrossRef.
- (a) S. Yu, C. Hsu, W. Chen, L. Wei and S. Wu, Sens. Actuators, B, 2014, 196, 203–207 CrossRef CAS; (b) B. Guo, H. Nie, W. Yang, Y. Tian, J. Jing and X. Zhang, Sens. Actuators, B, 2016, 236, 459–465 CrossRef CAS.
- X. Q. Zhan, J. H. Yan, J. H. Su, Y. C. Wang, J. He, S. Y. Wang, H. Zheng and J. G. Xu, Sens. Actuators, B, 2010, 150, 774–780 CrossRef CAS.
- (a) W. J. Zhang, C. Guo, L. H. Liu, J. G. Qin and C. L. Yang, Org. Biomol. Chem., 2011, 9, 5560–5563 RSC; (b) K. Cui, D. Q. Zhang, G. X. Zhang and D. B. Zhu, Tetrahedron Lett., 2010, 51, 6052–6055 CrossRef CAS.
- (a) S. R. Liu and S. P. Wu, Org. Lett., 2013, 15, 878–881 CrossRef CAS; (b) Z. Qu, J. Ding, M. Zhao and P. Li, J. Photochem. Photobiol., A, 2015, 299, 1–8 CrossRef CAS.
- L. Shi, X. Li, M. Zhou, F. Muhammad, Y. Ding and H. Wei, Analyst, 2017, 142, 2104–2108 RSC.
- (a) S. Kenmoku, Y. Urano, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 7313–7318 CrossRef CAS; (b) Q. Xu, K.-A. Lee, S. Lee, K. M. Lee, W.-J. Lee and J. Yoon, J. Am. Chem. Soc., 2013, 135, 9944–9949 CrossRef CAS; (c) Y. Koide, Y. Urano, K. Hanaoka, T. Terai and T. Nagano, J. Am. Chem. Soc., 2011, 133, 5680–5682 CrossRef CAS.
- (a) X. Wang, X. Wang, Y. Feng, M. Zhu, H. Yin, Q. X. Guo and X. Meng, Dalton Trans., 2015, 44, 6613–6619 RSC; (b) L. Qiao, H. Nie, Y. Wu, F. Xin, C. Gao, J. Jing and X. Zhang, J. Mater. Chem. B, 2017, 5, 525–530 RSC; (c) K. Wang, P. Sun, X. Chao, D. Cao, Z. Mao and Z. Liu, RSC Adv., 2018, 8, 6904–6909 RSC.
- (a) Z. Lou, P. Li, P. Song and K. Han, Analyst, 2013, 138, 6291–6295 RSC; (b) G. Cheng, J. Fan, W. Sun, J. Cao, C. Hu and X. Peng, Chem. Commun., 2014, 50, 1018–1020 RSC.
- (a) K. Xiong, F. Huo, C. Yin, Y. Chu, Y. Yang, J. Chao and A. Zheng, Sens. Actuators, B, 2016, 224, 307–314 CrossRef CAS; (b) J. X. Ong, V. T. Pang, L. M. Tng and W. H. Ang, Chem. – Eur. J., 2018, 24, 1870–1876 CrossRef CAS.
- (a) D. Soni, N. Duvva, D. Badgurjar, T. K. Roy, S. Nimesh, G. Arya, L. Giribabu and R. Chitta, Chem. – Asian J., 2018, 13, 1594–1608 CrossRef CAS; (b) C. Xu, Y. Qian, Z.-Q. Qi, C.-G. Lu and Y.-P. Cui, New J. Chem., 2018, 42, 6910–6917 RSC; (c) G. Li, D. Ji, S. Zhang, J. Li, C. Li and R. Qiao, Sens. Actuators, B, 2017, 252, 127–133 CrossRef CAS; (d) X. Xu and Y. Qian, New J. Chem., 2017, 41, 9607–9612 RSC.
- (a) X. Wang, J. Min, W. Wang, Y. Wang, G. Yin and R. Wang, Analyst, 2018, 143, 2641–2647 RSC; (b) J. Zha, B. Fu, C. Qin, L. Zeng and X. Hu, RSC Adv., 2014, 4, 43110–43113 RSC.
- K. Liu, Z. Xu, M. Yin, W. Yang, B. He, W. Wei and J. Shen, J. Mater. Chem. B, 2014, 2, 2093–2096 RSC.
- L. Zhang, Y. Wang, J. Yu, G. Zhang, X. Cai, Y. Wu and L. Wang, Tetrahedron Lett., 2013, 54, 4019–4022 CrossRef CAS.
- Y. Zhao, J. Sun, Z. Shi, C. Pan and M. Xu, Luminescence, 2011, 26, 214–217 CrossRef CAS.
- X. Guo, D. Zhang and D. Zhu, Adv. Mater., 2004, 16, 125–130 CrossRef CAS.
- A. Kundu, J. Pitchaimani, V. Madhu, P. Sakthivel, R. Ganesamoorthy and S. P. Anthony, J. Fluoresc., 2017, 27, 491–500 CrossRef CAS.
- (a) K. Kumar, G. Bhargava, S. Kumar and P. Singh, New J. Chem., 2018, 42, 1010–1020 RSC; (b) P. Singh, K. Kumar, G. Bhargava and S. Kumar, J. Mater. Chem. C, 2016, 4, 2488–2497 RSC; (c) P. Singh, L. S. Mittal, S. Kumar, G. Bhargava and S. Kumar, J. Fluoresc., 2014, 24, 909–915 CrossRef CAS; (d) P. Singh, L. S. Mittal, V. Vanita, R. Kumar, G. Bhargava, A. Walia and S. Kumar, J. Mater. Chem. B, 2016, 4, 3750–3759 RSC.
- (a) P. Sharma, K. Kumar, S. Kaur, S. Kaur, G. Bhargava, S. Kumar and P. Singh, J. Photochem. Photobiol., A, 2020, 388, 112151 CrossRef CAS; (b) P. Singh, L. S. Mittal, H. Singh, G. Bhargava and S. Kumar, New J. Chem., 2017, 41, 10281–10290 RSC; (c) P. Singh, L. S. Mittal, V. Vanita, R. Kumar, G. Bhargava, A. Walia and S. Kumar, Chem. Commun., 2014, 50, 13994–13997 RSC; (d) L. S. Mittal, P. Sharma, N. Kaur and P. Singh, Anal. Methods, 2019, 11, 5320–5327 RSC.
- (a) K. Kumar, S. Kaur, S. Kaur, G. Bhargava, S. Kumar and P. Singh, J. Mater. Chem. B, 2019, 7, 7218–7227 RSC; (b) P. Singh, L. S. Mittal, S. Kaur, S. Kaur, G. Bhargava and S. Kumar, Sens. Actuators, B, 2018, 255, 478–489 CrossRef CAS; (c) P. Singh, L. S. Mittal, G. Bhargava and S. Kumar, Chem. – Asian J., 2017, 12, 890–899 CrossRef CAS; (d) P. Singh, L. S. Mittal, K. Kumar, P. Sharma, G. Bhargava and S. Kumar, Chem. Commun., 2018, 54, 9482–9485 RSC.
- X. Q. Chen, K. A. Lee, E. M. Ha, K. M. Lee, Y. Y. Seo, H. K. Choi, H. N. Kim, M. J. Kim, C. S. Cho, S. Y. Lee, W. J. Lee and J. Yoon, Chem. Commun., 2011, 47, 4373–4375 RSC.
- S. R. Liu and S. P. Wu, Org. Lett., 2013, 15, 878–881 CrossRef CAS.
- Y. K. Yang, H. J. Cho, J. Lee, I. Shin and J. Tae, Org. Lett., 2009, 11, 859–861 CrossRef CAS.
- Y. Chen, Q. Lv, Z. Liu and Q. Fang, Inorg. Chem. Commun., 2015, 52, 38–40 CrossRef CAS.
- S. Goswami, S. Maity, A. C. Maity and A. K. Das, Sens. Actuators, B, 2014, 204, 741–745 CrossRef CAS.
- Y. Zhao, H. Li, Y. Xue, Y. Ren and T. Han, Sens. Actuators, B, 2017, 241, 335–341 CrossRef CAS.
- Y. Feng, S. Li, D. Li, Q. Wang, P. Ning, M. Chen, X. Tian and X. Wang, Sens. Actuators, B, 2018, 254, 282–290 CrossRef CAS.
- Y. Jiang., S. Wu, C. Jin, B. Wang and J. Shen, Sens. Actuators, B, 2018, 265, 365–370 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9tb01902b |
| This journal is © The Royal Society of Chemistry 2020 |