Shuttle confinement of lithium polysulfides in borocarbonitride nanotubes with enhanced performance for lithium–sulfur batteries†
Mingzhi
Yang‡
,
Dong
Shi‡
,
Xiucai
Sun
,
Yanlu
Li
 ,
Zhenyan
Liang
,
Lei
Zhang
,
Zhenyan
Liang
,
Lei
Zhang
 ,
Yongliang
Shao
,
Yongliang
Shao
 *,
Yongzhong
Wu
*,
Yongzhong
Wu
 * and
Xiaopeng
Hao
* and
Xiaopeng
Hao

State Key Laboratory of Crystal Materials, Shandong University, 27# Shanda South Road, Jinan, Shandong 250100, P. R. China. E-mail: ylshao@sdu.edu.cn; wuyz@sdu.edu.cn
First published on 27th November 2019
Abstract
The sluggish redox kinetics and severe shuttle effect remain the key issues for lithium–sulfur (Li–S) batteries, and result in their inferior electrochemical properties. In this contribution, metal-free borocarbonitride nanotubes (BCNNTs) are readily fabricated by pyrolysis of an organic precursor and used as the sulfur host material in Li–S batteries for the first time. The unique nanotube structure and the existence of B and N endow BCNNTs with high surface area, excellent stability, improved electrical conductivity, and they show efficient physical and chemical anchoring and conversion of lithium polysulfides (LiPSs). By combining the fast Li+ and electron transportation, a Li–S battery with superior rate and cycling performance is achieved, which is consistent with the results of the density functional theory (DFT) calculations. Typically, BCNNT based Li–S batteries show prominent cycling life with a capacity degradation of 0.041% per cycle (1000 cycles) and a high sulfur loading of 5.3 mg cm−2. This work defines an efficacious strategy to restrain the shuttle effect of LiPSs and shed light on the great potential of metal-free BCNNTs in Li–S batteries.
Introduction
Lithium–sulfur (Li–S) batteries are considered as promising next-generation green secondary batteries due to the ultrahigh theoretical capacity (∼1672 mA h g−1) and energy density (∼2600 W h kg−1) of sulfur electrodes, environmental friendliness, and cost effectiveness.1–3 However, the practical performance of Li–S batteries has been largely limited by the low conductivity of sulfur, large volume variation (∼80%) during the charging/discharging process, and shuttle effect of lithium polysulfides (LiPSs, Li2Sx, 2 < x ≤ 8), especially the last issue.4 The redox shuttling of LiPSs causes the loss of electrical contact between active materials and the cathode surface and triggers slow redox reactions.5–8 These issues apparently result in rapid sulfur loss, which further leads to poor cycling stability, rate capability and anode corrosion of Li–S batteries.To overcome the aforementioned hurdles, various carbon materials, such as micro/mesoporous carbon,9,10 carbon nanotubes,11,12 carbon nanofibers,13,14 hollow carbon spheres,15,16 and graphene,17,18 have been explored toward the development of advanced host materials that can not only improve the overall electronic conductivity but also localize the soluble LiPSs. Among them, the nanotube structure has received much research interest by virtue of its several advantages: (1) the unique tubular structure for hosting sulfur; (2) the large interior void space fraction for accommodating the volume variation; (3) sufficient contact area with the electrolyte for rapid Li+ and electron transport.19 However, because of the intrinsic nonpolarity of pristine carbon-based materials they only serve as a physical barrier while adsorbing polar LiPSs, which makes them incapable of effectively suppressing the dissolution of LiPSs.20–22 Therefore, host materials with a larger specific surface area, higher conductivity, and stronger polysulfide adsorption ability are always pursued.
Among the metal-free materials, borocarbonitride, BCN, is a low-cost material with excellent surface and catalytic properties. The existence of C–N, B–N, C–C, and B–C bonds is beneficial to electron transfer.23 Moreover, the electronegative N atoms in the BCN framework exhibit strong chemisorption for LiPSs and long cycling performance via SxLi⋯N interactions. B atoms, as an electron-deficient alternative, are electropositive, thus leading to the chemisorption of polysulfide anions (Sx2−).22,24 Thus, BCN shows great promise for boosting the performance of Li–S batteries because electron-rich BCN can polarize the host carbon atoms and strengthen the chemical affinity for LiPSs, and catalyze the redox reactions of sulfur species to reduce electrochemical polarization and enhance the Li+ and electron conductivity.25
Here, we designed a BCN nanotube (BCNNT) structure as a highly efficient sulfur host for Li–S batteries. Benefiting from the unique nanotube structure, the BCNNTs/S electrodes exhibit high electrical conductivity, high sulfur loading and improve the electrochemical reaction kinetics of Li–S batteries. The experimental and density functional theory (DFT) calculation results show that BCNNTs not only provide physical confinement for the diffusion of LiPSs, but also serve as polysulfide-trapping centers through the strong chemical interaction with LiPSs. Our work puts forward a simple but straightforward strategy to design a fascinating nanotube structure with highly efficient LiPS trapping for practical Li–S batteries.
Results and discussion
The synthetic routes to the BCNNTs and the BCNNTs/S composites are schematically illustrated in Fig. 1a. First, boric acid, polyethylene glycol (PEG), and urea, serving as the boron, carbon and nitrogen source, respectively, are dispersed in deionized water to form a suspension. Second, the precursor is obtained after drying. Third, the BCNNTs are synthesized through thermal-induced polymerization at 900 °C in a tube furnace system. After introducing B, C, and N elements, the BCN is encapsulated to form a nanotube structure, further connected into a 3D network. Then, sulfur is impregnated into the as-prepared BCNNTs by a melt-infiltration method,26 and the obtained BCNNTs/S composites still retain the original morphology of BCNNTs, as shown in Fig. 1e and f.Scanning electron microscopy (SEM) images reveal that BCNNTs exhibit a hollow tubular interior with an average diameter of about 100 nm (Fig. 1b) and the length is several hundred nanometers (Fig. 1c). The high magnification transmission electron microscopy (TEM) image (Fig. 1d) also indicates the hollow nanotube structure with an average diameter of about 100 nm. The B, C and N elements are uniformly distributed in BCNNTs, with B and N contents of 18.51% and 16.14%, respectively, as shown in Fig. S1 (ESI†). Benefiting from the unique structural features, elemental sulfur is successfully incorporated into BCNNTs (Fig. 1e). TEM images (Fig. 1f and g) confirm the effective limitation of sulfur by BCNNTs more clearly, and the sulfur content is ∼68% (Fig. S2, ESI†). The elemental mapping analysis (Fig. 1h and S2, ESI†) shows that the S, B, C and N elements are uniformly distributed in the BCNNTs/S composites, indicating the successful loading of sulfur into BCNNTs.
The structures of the BCNNTs before and after sulfur loading were examined by XRD measurements. As shown in Fig. 2a, pristine BCNNTs exhibit two characteristic peaks at 2θ values of ∼26° and ∼43°. After loading sulfur, the XRD pattern of BCNNTs/S composites shows that sulfur exists as orthorhombic S8 in the composites. The porous structures of BCNNTs and BCNNTs/S were investigated by N2 adsorption–desorption measurements. As shown in Fig. 2b, both BCNNTs and BCNNTs/S manifest similar behavior with combined type II isotherms. The pronounced hysteresis loop at P/P0 above 0.4 and rapid adsorption at high relative pressure are typical characteristics of micropores.27,28 The hierarchical porosity is verified using their corresponding pore size distribution calculated with the nonlocal density functional theory model (inset of Fig. 2b). In comparison with BCNNTs (249.3 m2 g−1), the Brunauer–Emmett–Teller (BET) specific surface area of BCNNTs/S shows a relatively small value (43.6 m2 g−1). This result indicates that the pipelines of BCNNTs are already full of sulfur, which is consistent with the SEM and TEM measurements. The existence of a large number of micropores on the surface of BCNNTs is favorable for LiPS trapping and accelerates the Li+ and electron transport, which is favorable for the redox kinetics of LiPSs.29 Thus, the optimal porous properties and suitable nanotube structure allow BCNNTs to serve as an optimal reservoir to effectively suppress the shuttle effect of LiPSs.
To further confirm the BCNNT state, Raman spectra were recorded (Fig. 2c). Apparently, both BCNNTs and BCNNTs/S exhibit two distinct peaks at 1358 cm−1 (D band) and 1589 cm−1 (G band), which represent sp3-type disordered carbon and sp2-type graphitic carbon, respectively.30,31 For the BCNNTs/S, the ID/IG value decreases, meaning that the reaction of sulfur with BCNNTs lowers the disorder degree of BCNNTs.32 To confirm the accurate sulfur content in BCNNTs/S, thermogravimetric analysis (TGA) was conducted under an N2 atmosphere (Fig. 2d). Clearly, the sulfur almost evaporates completely when the temperature is elevated to 350 °C. As reflected from the weight loss between 150 and 350 °C, after the filtration of sulfur, the sulfur loading in the BCNNTs is determined to be approximately 64 wt% (Fig. 2d). Besides, BCNNTs show a negligible mass change until 600 °C, indicating the high thermostability.
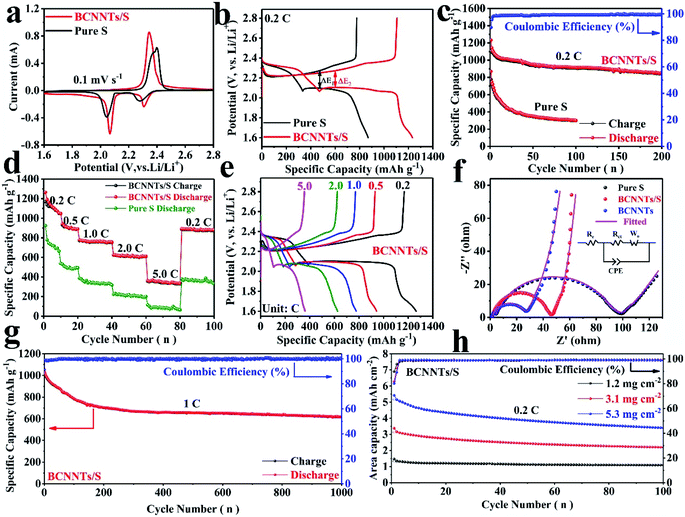
The cyclic voltammetry (CV) curves at 0.1 mV s−1 of BCNNTs/S and pure sulfur electrodes are displayed in Fig. 3a, which show the typical electrochemical behavior of sulfur electrodes. A pair of prominent peaks at around 2.30 V and 2.06 V is related to the restoration process from cyclo-sulfur to long-chain Li2Sx (2 < x ≤ 8) and further conversion to Li2S2/Li2S during the discharging process.33,34 In the subsequent anodic scan, the oxidation peak around 2.35 V arises from the oxidation of Li2S2/Li2S to Li2Sx (2 < x ≤ 8) and finally to sulfur.35,36 Notably, compared to the CV curve of the pure sulfur electrode, the cathodic peak of the BCNNTs/S electrode shows a positive shift and the anodic peak shows a negative shift, indicating a smaller polarization. This phenomenon mainly arises from the increased ion and electron conductivity of the BCNNTs/S electrode, and the LiPS adsorption capability of BCNNTs.37,38 This critically blocks the shuttling of the LiPSs into the electrolyte, accelerates the transformation of soluble Li2Sx (x > 2) to final insoluble products (Li2S) during the discharging process, reduces the activation energy for the formation of Li2Sx (x > 2) during the charging process and enhances the reaction kinetics.33,39,40 Being consistent with the reversible redox peaks in CV curves, the galvanostatic charge/discharge profile (Fig. 3b) of the BCNNTs/S electrode exhibits a lower charge/discharge platform potential difference and a longer charge/discharge platform than that of the pure sulfur electrode, suggesting high sulfur utilization and improved redox kinetics in the BCNNTs/S electrode.40 Besides, the subsequent CV profiles of the BCNNTs/S electrode almost overlapped after the second cycle, indicating high reversibility and good electrochemical stability (Fig. S3, ESI†).
The cycling stability of the BCNNTs/S electrode (with 1.2 mg cm−2 sulfur loading) is shown in Fig. 3c. The BCNNTs/S electrode exhibits a high initial discharge capacity of 1233.0 mA h g−1 at 0.2C (1C = 1672 mA h g−1) and the capacity is maintained at 852.5 mA h g−1 after 200 cycles with a coulombic efficiency of ∼99.8%. In contrast, the pure sulfur electrode delivers an initial discharge capacity of 870.3 mA h g−1 at 0.2C, and the capacity rapidly fades to only 300.9 mA h g−1 after 100 cycles. The outstanding cycling stability is further displayed at a higher current density of 1.0C (Fig. 3g). The discharge capacity of the BCNNTs/S electrode after 1000 cycles is retained at 619.6 mA h g−1. The corresponding average coulombic efficiency and capacity decay per cycle are 99.5% and 0.041%, respectively. Therefore, the excellent electrochemical performance of BCNNTs/S electrodes is mainly attributed to the high surface area of BCNNTs, which cooperatively alleviates the shuttle effect of LiPSs through synergistic interaction of physical confinement and chemisorption. Meanwhile, the overall conductivity and perfect interfusion of the hollow nanotube structure are favorable for reaction kinetics. These advantages cooperatively lead to high sulfur utilization and high cycling stability, even at high current densities.
The rate performance of the BCNNTs/S electrode was also evaluated at various current densities from 0.2 to 5.0C (Fig. 3d). It can be seen that the BCNNTs/S electrode delivers a capacity of 1048.5 mA h g−1 at the rate of 0.2C, which then slowly decreases to 890.5, 756.7, and 611.7 mA h g−1 at the rate of 0.5C, 1.0C, and 2.0C, respectively. Even at a high rate of 5.0C, a reversible capacity of 333.6 mA h g−1 can still be achieved. Moreover, when the rate returns to 0.2C, the capacity of the BCNNTs/S electrode recovers to ∼881.0 mA h g−1 after 100 cycles. Compared with the BCNNTs/S electrode, the pure sulfur electrode suffers from a more serious decay in the first several cycles and inferior rate performance under the same conditions. Notably, because of the rapid dissolution of LiPSs into the organic electrolyte, the discharge capacities of pure sulfur are 662.7, 492.9, 317.2, 200.1 and 64.7 mA h g−1 at the rate of 0.2C, 0.5C, 1.0C, 2.0C and 5.0C, respectively. These results indicate that the hollow BCNNTs have great adsorption capability for LiPSs and enhance the performance of Li–S batteries. Fig. 3e displays the corresponding voltage profiles of the BCNNTs/S electrode at different rates ranging from 0.2 to 5.0C. It is worth noting that although the charge/discharge capacities gradually decrease with the increase of the rates, apparent charge/discharge platforms are still discerned in these electrodes. Two typical discharge plateaus of sulfur electrodes can be observed in the voltage profiles at all current rates which are associated with the reduction from the cyclic S8 to the short-chain lithium sulfide. The excellent rate performance is attributed to both the enhancement of conductivity by the highly conductive BCNNTs and the strong physical confinement and chemisorption of LiPSs. In order to evaluate the effect of BCNNTs (without sulfur loading) on the specific capacity, the electrochemical performance of the BCNNT electrodes was investigated. It shows that Li+ insertion of BCNNTs has little contribution to the overall capacity of the Li–S batteries under the same voltage window (Fig. S4, ESI†). In addition, the nanotube structure of the BCNNTs/S electrode (Fig. S5, ESI†) is well preserved after cycling, which is favorable to the long-term cycling and high-rate performances.
For the purpose of achieving high energy density, it is a common method to increase the loading capacity of the active material to achieve high area capacity. As shown in Fig. 3h, the BCNNTs/S electrodes with a high sulfur loading of 3.1 and 5.3 mg cm−2 are assessed at 0.2C. The BCNNTs/S electrode with a sulfur loading up to 5.3 mg cm−2 still has an initial discharge capacity of 1024.5 mA h g−1, corresponding to an area capacity up to 5.43 mA h cm−2. After 100 cycles, discharge capacities (areal capacities) of 709.7 mA h g−1 (2.20 mA h cm−2) and 643.4 mA h g−1 (3.41 mA h cm−2) are maintained for the BCNNTs/S electrodes with a sulfur loading of 3.1, and 5.3 mg cm−2, respectively. Such positive features indicate that the BCNNTs/S electrode has large discharge and area capacities as well as good cycling performance in practical application. To investigate the Li+ diffusion dynamics in the BCNNTs/S and pure sulfur electrodes, electrochemical impedance spectroscopy (EIS) measurements were conducted (Fig. 3f). The EIS spectra for the BCNNTs/S and pure sulfur electrodes consist of a semicircle in the high-frequency region (Rct, the charge transfer resistance) and an inclined line in the low-frequency region (Wo, the Warburg impedance). Moreover, the equivalent circuit (inset of Fig. 3f) was used to model the Nyquist plot of each electrode, including Rct, Wo, Re (the resistance of electrolyte) and CPE (the constant phase element).41–44 The fitted Re and Rct values of the cells according to the equivalent circuit model are shown in Table S1 (ESI†). It is found that the Rct of the electrode without BCNNTs (94.8 Ω) is much higher than that of the electrode with BCNNTs (43.4 Ω), which can be ascribed to the enhanced conductivity after introducing BCNNTs in the electrodes. This result further illustrates the enhanced redox kinetics of the BCNNTs/S electrode, which is responsible for the excellent electrochemical performance.
To investigate the effect of BCNNTs on the redox kinetics of LiPSs, CV curves at various scan rates (0.1–1.0 mV s−1) were used to explore the kinetic behavior and Li+ diffusion properties of BCNNTs/S and pure sulfur electrodes, as shown in Fig. 4a and S6 (ESI†). The CV curves show similar shapes, but with a slight peak shift as the scan rate increases. Fig. 4b–d show that the redox peak currents of the two materials are linear with the square root of the scan rate, indicating that the rate-determining step is dominated by the diffusion process of LiPSs.38,45 Therefore, the Li+ diffusion process in the electrode can be described using the classical Randles–Sevcik equation:46
| Ip = (2.69 × 105)n1.5ADLi0.5CLiν0.5 |
To investigate the electrochemical mechanism of BCNNTs, XPS analyses were executed (Fig. 5 and S7–S9, ESI†). The appearance of characteristics peaks of B 1s, C 1s and N 1s in BCNNTs and BCNNTs/S proves the successful synthesis of BCNNTs. As shown in Fig. 5a, the B 1s XPS spectrum of BCNNTs can be resolved into B–C (189.6 eV), B–N (191.8 eV), and B–O (192.6 eV),25,47,48 which are also found in BCNNTs/S and the cycled electrode. Besides, two new peaks located at 186.1 and 191.1 eV can be observed in BCNNTs/S and the cycled electrode, which can be assigned to the S loss energy and B–S bonds.25,49 In particular, the presence of the B–S bond illustrates the chemical interaction of BCNNTs with LiPSs. For the C 1s spectra of BCNNTs, BCNNTs/S and the cycled electrode, the peaks at 284.3 eV, 284.7 eV, 285.8 eV and 288.5 eV are attributed to the C–B, sp2 C, C–N and C–O bonds (Fig. 5b),47,48 respectively. Furthermore, for BCNNTs/S and the cycled electrode, the C–S bond located at ∼285.8 eV confirms the strong chemical affinity for LiPSs and fast LiPS interconversion.25 Besides, comparing the C 1s XPS results of BCNNTs and BCNNTs/S, two distinct peaks are observed at 288.8 and 292.8 eV in the cycled electrode, which can be assigned to the C–F bond due to partial electrolyte decomposition.38,50 The N 1s spectra of BCNNTs and BCNNTs/S in Fig. 5c can be resolved into five peaks. The peaks at 398.6 and 399.6 eV are attributed to pyridinic-type and pyrrolic-type N atoms, respectively, which create numerous extrinsic defects and active sites and thus improve the electronic conductivity.30,31,51 The N–B bonds at 398.1 eV also confirm the BCN structure, which can chemically bind sulfur.25 In the cycled electrode, the binding energy peaks of N 1s are slightly shifted to high energy, and these results are indicative of the interaction between Li+ and N atoms in the BCNNTs with maximizes the reduction of the LiPS shuttle effect during the charge and discharge process.52–54 As shown in Fig. S9b (ESI†), the Li 1s XPS spectrum after discharge (1.6 V) can be fitted with two peaks at 55.7 and 54.7 eV, corresponding to Li–N and Li–S bonds, respectively, demonstrating the strong interaction between LiPSs and BCNNTs.48,49 The S 2p XPS peak of BCNNTs/S (Fig. S8b, ESI†) can be resolved into three major components for S 2p3/2 (163.8 eV), S 2p1/2 (164.9 eV), and sulphate species (167.9 eV), and this further exhibits that the sulfur is successfully introduced into the BCNNTs/S nanocomposites.25,53 After the first discharge process, lithium sulfide peaks are observed at 160.2 and 161.9 eV, revealing the discharge of sulfur (Fig. S9c, ESI†).38 Other peaks can be assigned to thiosulfate (166.9 eV), a polythionate complex (168.4 eV), and sulfate (169/170.1 eV).34,55
To better understand the enhanced interaction between LiPSs and BCNNTs, the adsorption of LiPSs on pristine graphene and BCNNTs was studied by DFT calculations. We first constructed a p(8 × 8) monolayer graphene as the model for pristine graphene and constructed the BCN substrate via partially replacing the C atoms of the above graphene with B and N atoms, as shown in Fig. S10 (ESI†). Then we placed the typical LiPS intermediates – Li2S4 and Li2S6 – at different sites of pristine graphene and BCN for structural relaxation, to explore their adsorption behavior and evaluate their adsorption activities via comparing the calculated adsorption energies. The obtained most stable adsorption configurations and energies of Li2S4 and Li2S6 are illustrated in Fig. 5d. It can be clearly seen that BCN possesses more negative Eads (−0.889 eV for Li2S6 and −1.032 eV for Li2S4) than pristine graphene (−0.102 eV for Li2S6 and −0.097 eV for Li2S4), indicating stronger adsorption of LiPSs on BCN compared to pristine graphene. In order to understand the bonding properties between LiPSs and B atoms in BCN, we simplified the theoretical model by substituting the BCN structure with the edge hydrogen-passivated C5B structure (C5BH5) and calculated the differential charge density to describe the interaction between LiPSs and B atoms. As presented in Fig. 5e, a net gain of charge between the Li2S4 molecule and the C5BH5 can be observed; an electron is transferred from the B atom to its nearest adsorbed S42− ion, and thus the charge accumulates between the S42− and B atoms. The strong bonding interaction between B and S is consistent with the XPS result in Fig. 5a. Besides, the bond interaction between N and Li has been reported,56 in which the result is also consistent with our result shown in Fig. 5c. Overall, it can be concluded that the BCN structure shows superior chemical absorbability for LiPS intermediates (Li2S4 and Li2S6) to pristine graphene, which is beneficial for enhancing the electrochemical performance of sulfur electrodes, and prevents the dissolution of Li2S4 and Li2S6 into the electrolyte most effectively.
Furthermore, a conventional adsorption experiment was performed to illustrate the adsorptive ability of the BCNNTs for LiPSs. A typical LiPS solution of Li2S6 dissolved in a solvent mixture (1,2-dimethoxyethane and 1,3-dioxolane with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio) was prepared. After soaking BCNNTs into Li2S6 solution, as Fig. 5f demonstrates, the dark brown color of pristine Li2S6 solution progressively fades. And after 12 hours, the solution soaking BCNNTs is finally almost transparent. This obvious distinction adequately confirms the excellent adsorptive ability of BCNNTs for LiPSs. The digital photos of the separators before and after cycling are shown in Fig. 5g. A small amount of LiPSs on the separator after cycling can be observed, indicating that the BCNNTs/S electrode has a weak shuttle effect after cycling.
1 volume ratio) was prepared. After soaking BCNNTs into Li2S6 solution, as Fig. 5f demonstrates, the dark brown color of pristine Li2S6 solution progressively fades. And after 12 hours, the solution soaking BCNNTs is finally almost transparent. This obvious distinction adequately confirms the excellent adsorptive ability of BCNNTs for LiPSs. The digital photos of the separators before and after cycling are shown in Fig. 5g. A small amount of LiPSs on the separator after cycling can be observed, indicating that the BCNNTs/S electrode has a weak shuttle effect after cycling.
The BCNNTs/S cathode exhibits surprising electrochemical performance in terms of reversible capacity and capacity retention, and these electrochemical properties are also superior to previously reported related carbon-based materials (Table S2, ESI†). The exceptional electrochemical performance unambiguously demonstrates the unique advantages of the BCNNT electrodes, which can be ascribed to the following factors (Fig. 5h): first, the unique 1D nanotube structure of the BCNNTs favorably interconnects as a superior electron conductive network and enables the physical adsorption of the LiPSs. Second, the robust hollow structure provides sufficient void space for loading a large amount of sulfur and alleviates the volume variation during the discharge and charge process. Third, B atoms in the BCNNT framework have a lower electronegativity than both N and C, and they are positively polarized, leading to chemisorption of negative species on the surface of BCNNTs and suppression of the shuttle effect of LiPSs. Finally, the pyrrolic N and pyridinic N functional groups can chemically adsorb the Li+ cations in LiPSs and supply an effective Li+ diffusion pathway, which is critical to the promotion of rate performance.
Conclusions
In summary, we have successfully developed novel BCNNTs as a sulfur host material in Li–S batteries. The Li–S batteries with the BCNNTs/S electrodes exhibit excellent electrochemical performance, with a dramatically improved initial capacity from 870.3 mA h g−1 (pure sulfur electrode) to 1233.0 mA h g−1 at 0.2C. When increasing the current density to 1C, the battery capacity is still maintained at 619.6 mA h g−1 after 1000 cycles, exhibiting a capacity decay of only 0.041% per cycle. The superior performance can be attributed to the synergy of factors including the high conductivity accelerating the Li+ and electron diffusion, unique 1D nanotube structure accommodating the large volume change and the physical and chemical anchoring of the LiPSs, which is confirmed by both visual discrimination and DFT calculations. This opens a new path departing from using traditional carbon hosts in sulfur electrode design for Li–S batteries and should inspire further studies on the nature of BCNNTs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NSFC (Contract 51602177, 51872162, 11890700) and the Major Basic Program of the Natural Science Foundation of Shandong Province (Contract ZR2017ZB0317, ZR2018MEM013).References
- Q. Pang, X. Liang, C. Y. Kwok and L. F. Nazar, Nat. Energy, 2016, 1, 16132 CrossRef CAS.
- Y.-X. Yin, S. Xin, Y.-G. Guo and L.-J. Wan, Angew. Chem., Int. Ed., 2013, 52, 13186–13200 CrossRef CAS PubMed.
- A. Manthiram, Y. Fu and Y.-S. Su, Acc. Chem. Res., 2013, 46, 1125–1134 CrossRef CAS PubMed.
- G. Li, S. Wang, Y. Zhang, M. Li, Z. Chen and J. Lu, Adv. Mater., 2018, 30, 1705590 CrossRef PubMed.
- J.-Y. Hwang, H. M. Kim, S. Shin and Y.-K. Sun, Adv. Funct. Mater., 2018, 28, 1704294 CrossRef.
- L. Kong, B.-Q. Li, H.-J. Peng, R. Zhang, J. Xie, J.-Q. Huang and Q. Zhang, Adv. Energy Mater., 2018, 8, 1800849 CrossRef.
- Z. Xiao, Z. Yang, L. Zhang, H. Pan and R. Wang, ACS Nano, 2017, 11, 8488–8498 CrossRef CAS PubMed.
- C.-Y. Fan, Y.-P. Zheng, X.-H. Zhang, Y.-H. Shi, S.-Y. Liu, H.-C. Wang, X.-L. Wu, H.-Z. Sun and J.-P. Zhang, Adv. Energy Mater., 2018, 8, 1703638 CrossRef.
- J. Schuster, G. He, B. Mandlmeier, T. Yim, K. T. Lee, T. Bein and L. F. Nazar, Angew. Chem., Int. Ed., 2012, 51, 3591–3595 CrossRef CAS PubMed.
- S. Chen, B. Sun, X. Xie, A. K. Mondal, X. Huang and G. Wang, Nano Energy, 2015, 16, 268–280 CrossRef CAS.
- S. Dörfler, M. Hagen, H. Althues, J. Tübke, S. Kaskel and M. J. Hoffmann, Chem. Commun., 2012, 48, 4097–4099 RSC.
- R. Fang, G. Li, S. Zhao, L. Yin, K. Du, P. Hou, S. Wang, H.-M. Cheng, C. Liu and F. Li, Nano Energy, 2017, 42, 205–214 CrossRef CAS.
- N. Jayaprakash, J. Shen, S. S. Moganty, A. Corona and L. A. Archer, Angew. Chem., Int. Ed., 2011, 50, 5904–5908 CrossRef CAS PubMed.
- S. Peng, L. Li, J. Kong Yoong Lee, L. Tian, M. Srinivasan, S. Adams and S. Ramakrishna, Nano Energy, 2016, 22, 361–395 CrossRef CAS.
- G. Zheng, Y. Yang, J. J. Cha, S. S. Hong and Y. Cui, Nano Lett., 2011, 11, 4462–4467 CrossRef CAS PubMed.
- S. Liu, T. Zhao, X. Tan, L. Guo, J. Wu, X. Kang, H. Wang, L. Sun and W. Chu, Nano Energy, 2019, 63, 103894 CrossRef CAS.
- S. Evers and L. F. Nazar, Chem. Commun., 2012, 48, 1233–1235 RSC.
- S. Wu, R. Ge, M. Lu, R. Xu and Z. Zhang, Nano Energy, 2015, 15, 379–405 CrossRef CAS.
- J. Wang, H. Yang, Z. Chen, L. Zhang, J. Liu, P. Liang, H. Yang, X. Shen and Z. X. Shen, Adv. Sci., 2018, 5, 1800621 CrossRef PubMed.
- X. Jia, C. Zhang, J. Liu, W. Lv, D.-W. Wang, Y. Tao, Z. Li, X. Zheng, J.-S. Yu and Q.-H. Yang, Nanoscale, 2016, 8, 4447–4451 RSC.
- Y.-L. Ding, P. Kopold, K. Hahn, P. A. van Aken, J. Maier and Y. Yu, Adv. Funct. Mater., 2016, 26, 1112–1119 CrossRef CAS.
- J. Song, M. L. Gordin, T. Xu, S. Chen, Z. Yu, H. Sohn, J. Lu, Y. Ren, Y. Duan and D. Wang, Angew. Chem., Int. Ed., 2015, 54, 4325–4329 CrossRef CAS PubMed.
- M. Chhetri, S. Maitra, H. Chakraborty, U. V. Waghmare and C. N. R. Rao, Energy Environ. Sci., 2016, 9, 95–101 RSC.
- Y. Qiu, W. Li, W. Zhao, G. Li, Y. Hou, M. Liu, L. Zhou, F. Ye, H. Li, Z. Wei, S. Yang, W. Duan, Y. Ye, J. Guo and Y. Zhang, Nano Lett., 2014, 14, 4821–4827 CrossRef CAS PubMed.
- S. Yuan, J. L. Bao, L. Wang, Y. Xia, D. G. Truhlar and Y. Wang, Adv. Energy Mater., 2016, 6, 1501733 CrossRef.
- X. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 8, 500–506 CrossRef CAS PubMed.
- M. Yang, X. Hu, Z. Fang, L. Sun, Z. Yuan, S. Wang, W. Hong, X. Chen and D. Yu, Adv. Funct. Mater., 2017, 27, 1701971 CrossRef.
- Y. Li, J. Fan, J. Zhang, J. Yang, R. Yuan, J. Chang, M. Zheng and Q. Dong, ACS Nano, 2017, 11, 11417–11424 CrossRef CAS PubMed.
- Z. Li, Z. Xiao, S. Wang, Z. Cheng, P. Li and R. Wang, Adv. Funct. Mater., 2019, 29, 1902322 CrossRef.
- J. Guo, X. Du, X. Zhang, F. Zhang and J. Liu, Adv. Mater., 2017, 29, 1700273 CrossRef PubMed.
- C. Wang, K. Su, W. Wan, H. Guo, H. Zhou, J. Chen, X. Zhang and Y. Huang, J. Mater. Chem. A, 2014, 2, 5018–5023 RSC.
- G. Zhou, Y. Zhao and A. Manthiram, Adv. Energy Mater., 2015, 5, 1402263 CrossRef.
- X. Yang, N. Yan, W. Zhou, H. Zhang, X. Li and H. Zhang, J. Mater. Chem. A, 2015, 3, 15314–15323 RSC.
- X. Liang, C. Hart, Q. Pang, A. Garsuch, T. Weiss and L. F. Nazar, Nat. Commun., 2015, 6, 5682 CrossRef PubMed.
- P. Strubel, S. Thieme, T. Biemelt, A. Helmer, M. Oschatz, J. Brückner, H. Althues and S. Kaskel, Adv. Funct. Mater., 2015, 25, 287–297 CrossRef CAS.
- Z. Li, J. Zhang and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 12886–12890 CrossRef CAS PubMed.
- C.-P. Yang, Y.-X. Yin, H. Ye, K.-C. Jiang, J. Zhang and Y.-G. Guo, ACS Appl. Mater. Interfaces, 2014, 6, 8789–8795 CrossRef CAS PubMed.
- X. Chen, Y. Xu, F.-H. Du and Y. Wang, Small Methods, 2019, 3, 1900338 CrossRef.
- M. Wang, Q. Liang, J. Han, Y. Tao, D. Liu, C. Zhang, W. Lv and Q.-H. Yang, Nano Res., 2018, 11, 3480–3489 CrossRef CAS.
- R. Fang, S. Zhao, Z. Sun, D.-W. Wang, H.-M. Cheng and F. Li, Adv. Mater., 2017, 29, 1606823 CrossRef PubMed.
- S. Lin, M. K. Shafique, Z. Cai, J. Xiao, Y. Chen, Y. Wang and X. Hu, ACS Nano, 2019, 13, 13037–13046 CrossRef CAS PubMed.
- X. Gu, C.-j. Tong, C. Lai, J. Qiu, X. Huang, W. Yang, B. Wen, L.-m. Liu, Y. Hou and S. Zhang, J. Mater. Chem. A, 2015, 3, 16670–16678 RSC.
- X. Gu, Y. Wang, C. Lai, J. Qiu, S. Li, Y. Hou, W. Martens, N. Mahmood and S. Zhang, Nano Res., 2015, 8, 129–139 CrossRef CAS.
- F. Pei, L. Lin, D. Ou, Z. Zheng, S. Mo, X. Fang and N. Zheng, Nat. Commun., 2017, 8, 482 CrossRef PubMed.
- Y.-T. Liu, D.-D. Han, L. Wang, G.-R. Li, S. Liu and X.-P. Gao, Adv. Energy Mater., 2019, 9, 1803477 CrossRef.
- G. Zhou, H. Tian, Y. Jin, X. Tao, B. Liu, R. Zhang, Z. W. Seh, D. Zhuo, Y. Liu, J. Sun, J. Zhao, C. Zu, D. S. Wu, Q. Zhang and Y. Cui, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 840 CrossRef CAS PubMed.
- H. Tabassum, R. Zou, A. Mahmood, Z. Liang and S. Guo, J. Mater. Chem. A, 2016, 4, 16469–16475 RSC.
- H. Tabassum, W. Guo, W. Meng, A. Mahmood, R. Zhao, Q. Wang and R. Zou, Adv. Energy Mater., 2017, 7, 1601671 CrossRef.
- W. Cai, J. Zhou, G. Li, K. Zhang, X. Liu, C. Wang, H. Zhou, Y. Zhu and Y. Qian, ACS Appl. Mater. Interfaces, 2016, 8, 27679–27687 CrossRef CAS PubMed.
- J. Xu, S. Bi, W. Tang, Q. Kang, D. Niu, S. Hu, S. Zhao, L. Wang, Z. Xin and X. Zhang, J. Mater. Chem. A, 2019, 7, 18100–18108 RSC.
- Q. Pang, J. Tang, H. Huang, X. Liang, C. Hart, K. C. Tam and L. F. Nazar, Adv. Mater., 2015, 27, 6021–6028 CrossRef CAS PubMed.
- L. Ma, H. L. Zhuang, S. Wei, K. E. Hendrickson, M. S. Kim, G. Cohn, R. G. Hennig and L. A. Archer, ACS Nano, 2016, 10, 1050–1059 CrossRef CAS PubMed.
- Z. Wang, Y. Dong, H. Li, Z. Zhao, H. Bin Wu, C. Hao, S. Liu, J. Qiu and X. W. Lou, Nat. Commun., 2014, 5, 5002 CrossRef CAS PubMed.
- H.-J. Peng, Z.-W. Zhang, J.-Q. Huang, G. Zhang, J. Xie, W.-T. Xu, J.-L. Shi, X. Chen, X.-B. Cheng and Q. Zhang, Adv. Mater., 2016, 28, 9551–9558 CrossRef CAS PubMed.
- W. Chen, T. Lei, T. Qian, W. Lv, W. He, C. Wu, X. Liu, J. Liu, B. Chen, C. Yan and J. Xiong, Adv. Energy Mater., 2018, 8, 1702889 CrossRef.
- T.-Z. Hou, W.-T. Xu, X. Chen, H.-J. Peng, J.-Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2017, 56, 8178–8182 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta11500e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |