Three-dimensional assemblies of carbon nitride tubes as nanoreactors for enhanced photocatalytic hydrogen production†
Chen
Zhao
a,
Qi
Li
a,
Ying
Xie
 a,
Liping
Zhang
a,
Liping
Zhang
 c,
Xudong
Xiao
a,
Dan
Wang
a,
Yanqing
Jiao
a,
Cameron Alexander
Hurd Price
c,
Xudong
Xiao
a,
Dan
Wang
a,
Yanqing
Jiao
a,
Cameron Alexander
Hurd Price
 bd,
Baojiang
Jiang
bd,
Baojiang
Jiang
 *a and
Jian
Liu
*a and
Jian
Liu
 *bd
*bd
aKey Laboratory of Functional Inorganic Material Chemistry, Ministry of Education of the People's Republic of China, School of Chemistry and Materials Science, Heilongjiang University, Harbin 150080, China. E-mail: jbj@hlju.edu.cn
bState Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023, China
cDepartment of Chemistry and Biochemistry, Kent State University, Kent, OH 44242, USA
dDICP-Surrey Joint Centre for Future Materials, Department of Chemical and Process Engineering, University of Surrey, Guildford, Surrey, UK. E-mail: jian.liu@surrey.ac.uk
First published on 25th November 2019
Abstract
The three-dimensional character of carbon nitride can endow it with a larger specific surface area and more catalytic active sites, which are beneficial for charge and mass transfer to enhance photocatalytic hydrogen production reactions. Herein, we reported the direct assembly of three-dimensional structured carbon nitride from the thermal polymerization of 3-amino-1,2,4-triazole (AT) and melamine molecules. The 3D C3N4 consisted of one-dimensional thick tubes with numbers of thin tubes grown uniformly on their surfaces. It was noted that the assembly was decorated with triazole ring groups that generated a special nitrogen-rich structure, further broadening the visible-light response range. Furthermore, the nitrogen-rich carbon nitride had a large surface area of 71 m2 g−1, leading to a significant increase in the number of catalytic active sites. Thus, the photocatalytic hydrogen production rate reached 7.1 mmol g−1 h−1, 11.2 times higher than that of bulk C3N4. This work may provide a new strategy to controllably synthesize three-dimensional carbon nitride assemblies for photocatalytic applications.
1. Introduction
Graphitic carbon nitride (g-C3N4), as a metal-free semiconductor photocatalyst, has attracted increasing attention in the past ten years due to its excellent physicochemical stability and unique electronic structure,1,2 resulting in many promising applications, such as water splitting,3,4 the reduction of carbon dioxide to hydrocarbon fuels,5,6 the degradation of organic pollutants,7,8 bacterial disinfection,9,10 and the selective synthesis of organic compounds.11,12 However, bulk g-C3N4 still faces some serious shortcomings, including its small specific surface area, which causes a deficiency of active sites on the surface, and high levels of photogenerated charge carrier recombination,13–15 greatly inhibiting its application as an efficient photocatalyst.One of the solutions is to prepare g-C3N4 with specially designed structures that are able to provide large specific surface areas, such as nanoparticles, nanorods, nanosheets, and pores.16–22 Among these, 1D carbon nitride tubes have received extensive attention, because the 1D structure offers favourable transport properties for photogenerated electrons through inhibited charge recombination due to the large aspect ratio.23 Meanwhile, a porous structure is beneficial for mass diffusion during a catalytic reaction.24 Commonly, these materials are obtained using template- and surfactant-assisted hydrothermal methods, though these strategies are expensive and complex.25,26 Therefore, using more rationally designed strategies, such as molecular self-assembly, could possibly make the preparation simpler and more controllable.
According to our previous work,27 melamine can be hydrolyzed in situ into cyanuric acid at the proper pH, and it then coordinates with melamine to form a supramolecular precursor for carbon nitride. Polymerization of the precursor induced via heat treatment gives a hexagonal tubular structure, which showed enhanced photocatalytic activity. Furthermore, the decoration of g-C3N4 structures has become an effective method to promote photogenerated charge separation. Nowadays, many functional groups have been introduced onto g-C3N4, such as –COOH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –NH2.28,29 However, in previous reports, these groups were generally introduced via post-synthesis treatment, while the controllable formation of modified groups involving the carbon nitride precursors has rarely been reported. Interestingly, 3-amino-1,2,4-triazole (AT), as a raw material with high nitrogen content, has been used previously to prepare C3N5 with a narrow band gap of 2.2 eV, which has shown an extended visible-light response range.30 Considering that the triazole ring is an electron acceptor, its introduction into the structure of g-C3N4 could produce a built-in electric field that promotes the anisotropic character, allowing for more efficient photogenerated charge carrier separation.
O and –NH2.28,29 However, in previous reports, these groups were generally introduced via post-synthesis treatment, while the controllable formation of modified groups involving the carbon nitride precursors has rarely been reported. Interestingly, 3-amino-1,2,4-triazole (AT), as a raw material with high nitrogen content, has been used previously to prepare C3N5 with a narrow band gap of 2.2 eV, which has shown an extended visible-light response range.30 Considering that the triazole ring is an electron acceptor, its introduction into the structure of g-C3N4 could produce a built-in electric field that promotes the anisotropic character, allowing for more efficient photogenerated charge carrier separation.
In this work, we prepared a carbon nitride precursor via the self-assembly of AT and melamine molecules. After calcination, a 3D assembled carbon nitride structure was obtained, which is composed of 1D tubes with numerous thinner 1D tubes grown on their surfaces (Scheme 1). The 3D assembly structure and triazole ring group decoration not only successfully amplified the light response range but also enhanced the specific surface area and charge carrier separation efficiency. Thus, the resultant 3D C3N4 nanoreactor showed excellent photocatalytic hydrogen production performance compared to that of bulk C3N4. This synthetic strategy is considered a simple and effective way to synthesize 3D porous carbon nitride networks.
2. Experimental section
2.1 Preparation of bulk graphitic carbon nitride (GCN)
GCN was prepared via subjecting melamine to pyrolysis according to a previous method.31 In a typical synthesis, 3 g of melamine was added into a porcelain crucible and heated to 550 °C at a ramp rate of 2.5 °C min−1 and calcined at this temperature for 2 h under a nitrogen (N2) atmosphere.2.2 Preparation of three-dimensional tubular CN
First, 1.26 g of melamine and different amounts of AT (0.21, 0.42, 0.84 and 1.68 g) were dissolved in 100 mL of deionized (DI) water at 80 °C. The resultant mixture was stirred rapidly for 30 min, followed by the addition of 1.44 g of phosphorous acid. The stirring was continued for another 30 min and then the mixture was transferred into a Teflon-lined autoclave and heated at 180 °C for 18 hours. The precipitate formed was collected via centrifugation, washed with DI water several times, and dried at 60 °C for 24 h. Finally, the solid sample was used as the precursor for preparing CN via calcination under the same conditions as described above. The products are designated as A-CN-0.25, A-CN-0.5, A-CN-1 and A-CN-2, where “A” represents AT and 0.25, 0.5, 1, and 2 correspond to the molar ratio of AT to melamine.2.3 Characterization
X-ray powder diffraction (XRD) data were collected using a Rigaku D/max-IIIB diffractometer, which uses a copper anode as the X-ray source (Kα, λ = 1.5406 Å); the working voltage and current were 40 kV and 40 mA, respectively. The surface morphologies and structures of the samples were investigated using a Hitachi S-4800 field-emission scanning electron microscope (SEM) and a JEOL JEM-2100 transmission electron microscope (TEM) with acceleration voltages of 20 and 200 kV, respectively. HRTEM images were obtained using a JEOL JEM-2100 transmission electron microscope equipped with a Gatan-776 electron energy loss spectrometer (EELS). Fourier transform infrared (FT-IR) spectra were recorded with a PerkinElmer Spectrum One spectrometer using KBr pellets. Photoluminescence spectra (PL) were recorded at room temperature using a Hitachi F-4600 fluorescence spectrophotometer. Gas adsorption–desorption experiments were carried out using a Tristar II 3020 surface area and porosity analyzer (Micromeritics) and specific surface areas were determined using the Brunauer–Emmett–Teller (BET) model. UV-vis absorption spectroscopy was conducted using a UV-vis spectrophotometer (Shimadzu UV-2550). X-ray photoelectron spectroscopy (XPS) was carried out using a VG ESCALAB MK II spectrometer with a magnesium Kα (1253.6 eV) achromatic X-ray source; the binding energy was calibrated via referencing the C 1s peak to 284.6 eV.2.4 Photocatalytic hydrogen production experiments
The experiments were conducted using an online photocatalytic hydrogen evolution system (AuLight, Beijing, CEL-SPH2N), which was connected to an online gas chromatograph (SP7800, TCD, molecular sieve: 5 Å, Beijing Keruida Limited) with nitrogen at 20 °C as the carrier gas. Firstly, 0.01 g of a synthesized photocatalyst was added into a solvent mixture made up of 20 mL of methanol and 80 mL of DI water in a specialized quartz container with a magnetic stirrer. Subsequently, a certain amount of H2PtCl6 was added to the suspension to generate Pt with a weight corresponding to 1% of the weight of the photocatalyst. In preparation for the reaction, the mixture was ultrasonicated for 10 min to get a homogeneous suspension, which was kept under vacuum to remove CO2 and O2 dissolved in water. A 300 W Xe lamp (Oriel, USA) equipped with a filter that gives an AM1.5G optical spectrum was used as the light source. The produced gas was collected and analyzed at two-hour intervals. The determination of the apparent quantum efficiency (AQE) for hydrogen generation was carried out using the same closed circulating system under 300 W Xe lamp illumination with a bandpass filter (365, 420, 450 and 520 nm) system. The AQE was calculated via the following equation:2.5 Photoelectrochemical characterization
Electrochemical impedance spectroscopy (EIS) and photocurrent measurements were carried out using an electrochemical workstation with a three-electrode cell (Princeton Versa STAT). The working electrodes were prepared via depositing the photocatalysts on fluorine-doped tin oxide (FTO) glass (20 Ω per square, Nippon sheet glass, Japan) during the calcination process; Ag/AgCl and Pt foil (3 × 2 cm) were used as the reference and counter electrodes, respectively, with 0.2 M Na2SO4 used as the electrolyte. All working electrodes were subsequently calcined at 350 °C for 120 min under a flow of nitrogen (heating rate: 3 °C min−1). The same light source as was used for the photocatalytic hydrogen production experiments was used for the photoelectrochemical measurements.2.6 Density functional theory (DFT) calculations
To investigate the electronic structures and properties, we performed density functional theory (DFT) calculations using the Cambridge Sequential Total Energy Package (CASTEP). The Perdew–Burke–Ernzerhof (PBE) generalized gradient approximation (GGA) was employed to describe the exchange-correlation functional in this work. The kinetic energy cut-off and self-consistent field tolerance values were set as 340 eV and 1.0 × 10−6 atom per eV, respectively. A (3 × 3 × 1) k-point mesh was used to treat the integration over the Brillouin zone during the geometry optimization process. In this work, we used the HSE06 functional with a smearing width of 0.05 eV to describe the electronic structure. The core electrons were treated with the ultrasoft-pseudopotentials and the force tolerance was set as 0.01 eV Å−1. A tetragonal cell was adopted for the model with lattice constants of a = 13.2 Å and b = 17.2 Å. A vacuum slab of 15 Å was constructed in the perpendicular direction to the 2D A-CN slabs in order to avoid interactions from neighbouring slabs.3. Results and discussion
3.1 Structural characterization
Fig. 1a and b show SEM images of the hydrothermally induced precursor of A-CN-1. As shown, the supramolecular precursor displays a 3D assembly structure made up of thick 1D rods with many thin rods uniformly grown on their surfaces. The formation of this precursor was driven by the assembly of the molecules used, namely, melamine and AT. Specifically, some of the former compound first underwent hydrolysis and gave cyanuric acid, which then interacted with the remaining melamine, resulting in the larger microrods. Subsequently, AT connected with melamine and cyanuric acid through hydrogen bonding.32 The formation of the precursor was proved via XRD (Fig. S1, ESI†) and FI-IR spectroscopy (Fig. S2, ESI†) studies. As shown in the XRD patterns, the characteristic peaks of the precursor corresponded to those of melamine, cyanuric acid and AT. During the synthesis process, the presence of AT prompts cyanuric acid to convert into melamine, and then melamine bonds with cyanuric acid and AT via intermolecular hydrogen bonding. Agreeing with this, the results obtained via FT-IR further clearly demonstrate that the precursor was composed of melamine, cyanuric acid and AT. The structure of the precursor was also characterized via13C MAS NMR and the results are shown in Fig. S3.† The NMR spectrum of the precursor exhibits four distinct peaks at chemical shift values of 166.8, 160.7, 155.0 and 148.9 ppm, which are assigned to melamine, the C(1) atoms in AT, cyanuric acid, and the C(2) atoms in AT. Compared with melamine and cyanuric acid, the peaks at 166.8 and 155.0 ppm from the precursor reveal obvious differences due to the formation of hydrogen bonds between melamine and cyanuric acid. Interestingly, compared to AT, the C(2) peak of AT at 148.9 ppm in the precursor does not move, but the C(1) peak shifts to a lower field value, indicating that the electron cloud density around the C(1) atoms decreases, which can be attributed to the N atoms around the C(1) atoms being connected to melamine and cyanuric acid via intermolecular hydrogen bonds. After thermal treatment of the precursor, the three-dimensional assembly structure of the carbon nitride tubes could be obtained, consisting of heptazine rings with modified triazole ring groups. Interestingly, following the calcination procedure, interior collapse of the precursor occurred, which induced the 1D rods to transform into hollow 1D tubes, forming the 3D assembly (Fig. 1c and d). The 1D tube structure offers an enlarged surface area and accelerated electron transport, and provides more active sites on the surface. Then, nitrogen adsorption–desorption measurements of A-CN-1 demonstrated a type IV isotherm with a typical H3-type hysteresis loop (Fig. S4, ESI†), indicating the formation of mesopores.33 The BET surface area was calculated to be 71 m2 g−1, which is about 11 times larger than that of GCN (6.4 m2 g−1). In addition, the pore size distribution was calculated via the BJH (Barrett–Joyner–Halenda) method using the desorption branch of the isotherm, as shown in Fig. S5 ESI†.34 The pore volume of GCN is much smaller than that of A-CN-1. The mesoporous structure of A-CN-1 can provide a large surface, resulting in more active sites and improvements in the photocatalytic activity. | ||
| Fig. 1 (a) and (b) SEM images of the self-assembled supramolecular precursor for A-CN-1 and (c) and (d) SEM images of A-CN-1. | ||
The crystallographic properties of GCN and A-CN samples were studied via XRD (Fig. 2a). The XRD patterns of all samples exhibit two typical diffraction peaks. The weaker peak at 2θ = 13.3° is assigned to the (100) crystal plane, arising from in-plane structural ordering.35 The stronger peak at 2θ = 27.3° is attributable to the (002) plane and corresponds to the interlayer stacking of carbon nitride layers.36 In comparison with the (002) peak of GCN, that of A-CN-1 is seen to shift toward a smaller diffraction angle, indicating an increase in the interlayer spacing. Furthermore, as more AT was added, this peak shift became more pronounced due to the incorporation of triazole groups into the interlayer structure (Fig. S6, ESI†). Fig. S7 ESI† shows solid-state 13C NMR spectra of GCN and A-CN-1, which confirm the presence of heptazine units (δC1 ≈ 157 ppm and δC2 ≈ 165 ppm).37 Additionally, the molecular structures of GCN and A-CN-1 were further studied via FT-IR spectroscopy. The typical sharp peaks at 806 cm−1 in the spectra shown in Fig. 2b can be ascribed to breathing vibrations of the heptazine units. Several strong peaks located in the range of 1100–1800 cm−1 are assigned to the vibrations of N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the heterocycles of the heptazine units.38 For the A-CN-1 sample, most peaks between 1100 and 1800 cm−1 exhibit shifts of 3–10 cm−1 compared with those of GCN (Fig. 2c) due to changes in the chemical environment of the heterocycles caused by the introduction of triazole rings. In addition, the presence of triazole rings is also evidenced by the peak in the FT-IR spectrum of A-CN-1 at 740 cm−1, which corresponds to heterocyclic N–N bonds.39,40 It is worth mentioning that the peak at 1460 cm−1 indicates a networked structure of triazine rings linked by the –NH end groups.41 And the peak intensity of A-CN-1 at 1460 cm−1 is clearly weaker than that of GCN due to AT condensation with the terminal amino groups. A wide peak between 2900 and 3400 cm−1, attributed to N–H stretching vibrations, could be clearly observed, showing a distinct decrease in N–H stretching vibrations in A-CN-1, which further demonstrates the condensation of triazole ring groups with amino groups (Fig. 2b).42
N in the heterocycles of the heptazine units.38 For the A-CN-1 sample, most peaks between 1100 and 1800 cm−1 exhibit shifts of 3–10 cm−1 compared with those of GCN (Fig. 2c) due to changes in the chemical environment of the heterocycles caused by the introduction of triazole rings. In addition, the presence of triazole rings is also evidenced by the peak in the FT-IR spectrum of A-CN-1 at 740 cm−1, which corresponds to heterocyclic N–N bonds.39,40 It is worth mentioning that the peak at 1460 cm−1 indicates a networked structure of triazine rings linked by the –NH end groups.41 And the peak intensity of A-CN-1 at 1460 cm−1 is clearly weaker than that of GCN due to AT condensation with the terminal amino groups. A wide peak between 2900 and 3400 cm−1, attributed to N–H stretching vibrations, could be clearly observed, showing a distinct decrease in N–H stretching vibrations in A-CN-1, which further demonstrates the condensation of triazole ring groups with amino groups (Fig. 2b).42
In order to further study the surface electronic structure and elemental composition of A-CN-1, X-ray photoelectron spectroscopy (XPS) and elemental analysis (EA) measurements were performed. The surface N/C atomic ratio rises from 1.34 for GCN to 1.46 for A-CN-1 based on XPS analysis (Table S1, ESI†). This is consistent with the EA results (Table S2, ESI†), indicating the formation of a nitrogen-rich structure in the case of A-CN-1. In addition, the presence of triazole rings in the structure is also confirmed from the C 1s and N 1s XPS spectra of GCN and A-CN-1 (Fig. S8, ESI,† and Fig. 2d). In the C 1s spectra of A-CN-1, four peaks are observed at binding energies of 293.5, 288.1, 286.3 and 284.6 eV, corresponding to π electron delocalization in CN heterocycles, N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N coordination, C–NHx (x = 1 or 2) at the edges of heptazine units, and graphitic carbon (C–C), respectively.43 Compared with the area of the C 1s XPS peak at 286.3 eV for GCN, that for A-CN-1 is significantly reduced, which can also be taken as evidence for the thermal polymerization of triazole ring groups through the edge amino groups of the heptazine units. The N 1s spectrum (Fig. 2d) shows a peak located at 404.1 eV, which is also assigned to π electron delocalization in CN heterocycles, confirming the existence of graphitic stacking layers in A-CN-1. The N 1s XPS spectrum over the range from 402.6 to 396.2 eV may be deconvoluted into three peaks at 401.1, 399.8, and 398.5 eV, corresponding to amino group nitrogen (C–N–H), tertiary nitrogen N–(C3), and sp2-hybridized nitrogen (C–N
N coordination, C–NHx (x = 1 or 2) at the edges of heptazine units, and graphitic carbon (C–C), respectively.43 Compared with the area of the C 1s XPS peak at 286.3 eV for GCN, that for A-CN-1 is significantly reduced, which can also be taken as evidence for the thermal polymerization of triazole ring groups through the edge amino groups of the heptazine units. The N 1s spectrum (Fig. 2d) shows a peak located at 404.1 eV, which is also assigned to π electron delocalization in CN heterocycles, confirming the existence of graphitic stacking layers in A-CN-1. The N 1s XPS spectrum over the range from 402.6 to 396.2 eV may be deconvoluted into three peaks at 401.1, 399.8, and 398.5 eV, corresponding to amino group nitrogen (C–N–H), tertiary nitrogen N–(C3), and sp2-hybridized nitrogen (C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), respectively.44 It is apparent that the C
C), respectively.44 It is apparent that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C peak area of A-CN-1 is increased and the peak area of C–N–H is decreased, compared with GCN. This shows that the triazole ring groups connected with the amino groups, bringing more N–C
N–C peak area of A-CN-1 is increased and the peak area of C–N–H is decreased, compared with GCN. This shows that the triazole ring groups connected with the amino groups, bringing more N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups into the CN skeleton and resulting in a decrease in the C–N–H peak area. This result also completely matches with the results from the C 1s spectra. It is worth noting that the position of the N–(C3) peak for A-CN-1 is shifted to a higher binding energy, which can be attributed to changes in the electron cloud density of N–(C3) after the introduction of triazole rings. Moreover, in the O 1s spectrum, one peak is observed at 531.8 eV due to H2O and CO2 being adsorbed on the surface (Fig. S9, ESI†).45 Moreover, no characteristic P element peak is found in the XPS spectrum, indicating that adsorbed phosphorous acid molecules on the precursors have been fully removed (Fig. S10, ESI†).
N groups into the CN skeleton and resulting in a decrease in the C–N–H peak area. This result also completely matches with the results from the C 1s spectra. It is worth noting that the position of the N–(C3) peak for A-CN-1 is shifted to a higher binding energy, which can be attributed to changes in the electron cloud density of N–(C3) after the introduction of triazole rings. Moreover, in the O 1s spectrum, one peak is observed at 531.8 eV due to H2O and CO2 being adsorbed on the surface (Fig. S9, ESI†).45 Moreover, no characteristic P element peak is found in the XPS spectrum, indicating that adsorbed phosphorous acid molecules on the precursors have been fully removed (Fig. S10, ESI†).
To investigate the chemical bonding of GCN and A-CN-1, electron energy loss spectroscopy (EELS) was conducted around the K edges of C and N (Fig. S11, ESI†). The normalized EELS spectra of GCN and A-CN-1 show four major peaks due to the excitation of C and N electrons at the K-edges. Two similar peaks at 284.6 and 293.3 eV in the cases of GCN and A-CN-1 are observed in the C K-edge spectra (Fig. 2e), which are assigned to the 1s-π* and 1s-σ* electronic transitions of sp2 hybridized carbon bonded with nitrogen in the heptazine rings. In the C K-edge spectrum of A-CN-1, the relative intensity of the π* peak and the π* and σ* peak area ratios were higher than those of GCN, suggesting a proportional increase in sp2 hybridized carbon bonds in A-CN-1, which can be attributed to triazole rings grafted into the heptazine skeleton.46 In the N K-edge spectrum (Fig. 2f), the peaks for GCN and A-CN-1 are located at 396.2 and 405.1 eV, corresponding to the 1s-π* and 1s-σ* electronic transitions of sp2 hybridized nitrogen and bridging N. This shows that abundant sp2-hybridized nitrogen exists in the carbon nitride structure. No additional peaks are seen in the N K-edge spectrum of A-CN-1, indicating that N in the compound has a similar electronic environment to N in the N–(C3) groups of GCN.47 In comparison with the σ* signal in the N K-edge spectrum of GCN, that of A-CN-1 is more intense, which may illustrate an enhanced contribution from the triazole rings to the 1s-σ* transition. Furthermore, the total peak areas of A-CN-1 are larger than those of GCN in the N K-edge spectra, which implies the existence of extra nitrogen in the carbon nitride structure.
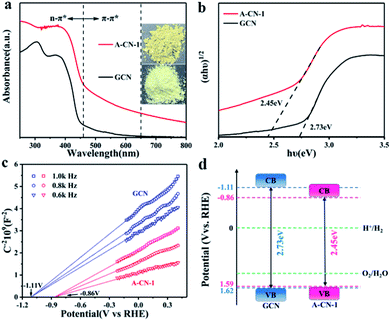
Fig. 3a represents the UV-vis absorption spectra of GCN and A-CN-1, in which enhanced adsorption is observed for both the π–π* and n–π* transition regions in the case of A-CN-1 compared to GCN. Further, strong tailing of the absorbance curve from 500 nm onwards is also found, indicating that the tested sample was able to utilize visible light effectively.48 This enhanced light response is attributed to the 3D assembly structure and nitrogen-rich structure. Therefore, A-CN-1 shows a brown colour (Fig. 3a, inset). Moreover, a red shift in the absorption edge of A-CN-1 is observed, suggesting a reduction in the band gap. From the transformed Kubelka–Munk function, the band gap of A-CN-1 was calculated to be 2.45 eV, which is lower than that of GCN (2.73 eV, Fig. 3b).49 This is possibly because the introduction of triazole ring groups into the CN skeleton will cause molecular modification, reducing the band gap of A-CN-1, which is also beneficial for visible-light harvesting. The conduction band (CB) edges of GCN and A-CN-1 were measured using Mott–Schottky pots at frequencies of 0.6, 0.8 and 1.0 kHz (Fig. 3c). Both GCN and A-CN-1 show positive slopes, consistent with typical n-type semiconductors. The flat band positions of GCN and A-CN-1 are ∼−1.11 V vs. RHE and ∼−0.86 V vs. RHE. The CB potential of an n-type semiconductor is similar to the flat potential,50 so the CB edges of GCN and A-CN-1 are determined to be −1.11 eV and −0.86 eV, respectively. Compared with GCN, the CB of A-CN-1 is altered by 0.25 eV, which is mainly due to the introduction of triazole rings. Then, combining the bandgap and CB position data, the valence band (VB) positions of GCN and A-CN-1 are calculated to be 1.62 eV and 1.59 eV. The corresponding energy band diagrams of GCN and A-CN-1 are depicted in Fig. 3d.
3.2 Photoelectrochemical tests
The introduction of triazole ring groups into the carbon nitride framework is not only able to influence the optical properties and band gap structure, but it can also improve charge carrier transfer and separation in A-CN-1. Photoluminescence (PL) emission spectra were recorded to investigate the recombination of electron–hole pairs in the photocatalysts. As shown in Fig. 4a, the PL intensity of A-CN-1 is dramatically lower than that of GCN, indicating an enhancement in the electron–hole separation efficiency for A-CN-1. In addition, the red shift of the PL emission peak from 466 to 493 nm for A-CN-1 is in agreement with the changes in the band gap. The fluorescence lifetimes of GCN and A-CN-1 were measured via time-resolved transient fluorescence decay spectroscopy (Fig. 4b and Table S3, ESI†). A-CN-1 exhibits faster exponential decay, with an average lifetime of 9.28 ns, which is shorter than that of GCN (9.92 ns). The shorter lifetime of A-CN-1 is attributed to the strong electron withdrawing properties of the triazole rings. When the triazole rings are introduced into the CN structure, an anisotropic built-in electric field is fabricated, which drives charge transport. Photocurrent response curves from GCN and A-CN-1 are shown in Fig. 4c. In the absence of light, as expected, the photocurrent value was zero. With the subsequent introduction of light, the photocurrent value rose rapidly to a constant value of ∼16 μA. For A-CN-1, the photocurrent values are higher than those of GCN, and no obvious weakening of the photocurrent response was detected after six cycles. This indicates that A-CN-1 allows more efficient charge separation and can stably provide more photoinduced charge carriers during light irradiation. In EIS spectra (Fig. 4d), with and without light, A-CN-1 displays a much smaller semicircle than GCN, implying that it has lower resistance, favouring interfacial charge transfer.51,52 The excellent charge separation and electron transport capabilities of A-CN-1 shown above signal the great potential of this material for photocatalytic applications.3.3 Photocatalytic performance
The photocatalytic performances of the samples for hydrogen evolution were examined in aqueous solution containing 20% (v/v) anhydrous methanol and 1% H2PtCl6 under Xe lamp irradiation with an AM1.5G filter. As shown in Fig. 5a, GCN had a low hydrogen production rate of 6.3 μmol h−1 (0.01 g of catalyst), whereas the A-CN photocatalysts gave much higher hydrogen production rates. The highest hydrogen production rate of 71 μmol h−1 was observed in the case of A-CN-1, which is 11.2 times greater than that of GCN, and this hydrogen production rate is higher than most reported C3N4-based photocatalysts, as summarized in Table S4.† This result can be attributed to the tubular structure with enhanced light absorption abilities, a large specific surface area and improved charge separation efficiency. Additionally, an examination of the recyclability revealed the high stability of A-CN-1 during photocatalytic hydrogen evolution (Fig. 5b). After four cycles of a four-hour experiment, there were no significant changes in the rate of hydrogen production. In addition, after photocatalytic reactions, the sample still maintains its original morphology, indicating that the material is very stable and can be reused (Fig. S12, ESI†). Fig. 5c shows the apparent quantum efficiency (AQE) values from A-CN-1 for hydrogen production at 365, 420, 450 and 520 nm, respectively, and the variation tendency of the AQE curve is similar to that of the UV-vis curve. A-CN-1 shows a high AQE value of 7.4% at 420 nm, benefiting from the high N element content and large specific surface area.3.4 DFT calculations
Melon-type graphitic carbon nitride (GCN) was adopted to perform DFT calculations. In order to understand the role of triazole groups, a triazole group was introduced into the unit cell of GCN. The optimized geometry structures of GCN and A-CN are shown in Fig. 6a and c, respectively. The total density of states (TDOS) and partial density of states (PDOS) of GCN and A-CN are shown in Fig. 6b and d, respectively. It can be seen that both the CB and VB are primarily composed of the p orbitals of C and N atoms, which agrees well with previously reported results.53 Moreover, the top of the VB is mainly made up of N 2p orbitals, which indicates that photogenerated holes are likely to accumulate in the 2p orbitals of the N atoms. The PDOS of A-CN shows similar VB and CB compositions (Fig. 6d). Nevertheless, a new peak located at 1.5 eV appears in the PDOS of A-CN, which mainly belongs to N atoms of triazole groups. Herein, the value of the band gap of A-CN is decreased after triazole group addition, which agrees with the UV-vis results (Fig. 3b). The lower band gap is not only beneficial for electron transfer from N 2p orbitals to the CB band, but it also efficiently extends the range of light adsorption. Considering the illustrations of the C 2p and N 2p PDOS, triazole modification was shown to be a prominent strategy for improving the performance of carbon nitride base materials.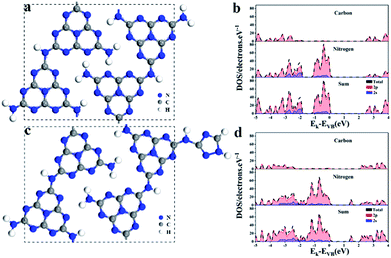 | ||
| Fig. 6 Optimized structure models of (a) GCN and (c) A-CN. The density of states (DOS) of (b) GCN and (d) A-CN. | ||
4. Conclusions
In summary, we have designed a new strategy based on supramolecular self-assembly for preparing highly photoactive carbon nitride photocatalysts, incorporating triazole ring groups into the skeleton. This alters the electrical properties and C/N ratio of the material. The close relationships between the 3D morphology, electronic structure and catalytic properties were surveyed, illustrating that the 3D structure provided a large specific surface area (71 m2 g−1) and increased the number active sites, in addition to allowing excellent charge carrier transfer and separation properties when compared to standard GCN material produced for this study. As a result, all the synthesized samples manifested superior hydrogen production rates compared to standard GCN, achieving a hydrogen production rate of 7.1 mmol h−1 g−1 due to the narrow band gap, existence of greater numbers of active reaction sites, and rapid charge transport. This work details 3D structures that could be beneficial for the generation of novel organic semiconductor materials for hydrogen production and for the larger field of environmentally responsible energy production.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21771061, 21601055), and the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2017118).Notes and references
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- Y. Wang, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- Q. Wang, T. Hisatomi, Q. Jia, H. Tokudome, M. Zhong, C. Wang, Z. Pan, T. Takata, M. Nakabayashi, N. Shibata, Y. Li, I. D. Sharp, A. Kudo, T. Yamada and K. Domen, Nat. Mater., 2016, 15, 611–615 CrossRef CAS PubMed.
- K. Zhang, L. Wang, X. Sheng, M. Ma, M. S. Jung, W. Kim, H. Lee and J. H. Park, Adv. Energy Mater., 2016, 6, 1502352 CrossRef.
- J. Mao, K. Li and T. Peng, Catal. Sci. Technol., 2013, 3, 2481 RSC.
- D. Li, S. Ouyang, H. Xu, D. Lu, M. Zhao, X. Zhang and J. Ye, Chem. Commun., 2016, 52, 5989–5992 RSC.
- D. Chatterjee and S. Dasgupta, J. Photochem. Photobiol., C, 2005, 6, 186–205 CrossRef CAS.
- K.-G. Zhou, D. McManus, E. Prestat, X. Zhong, Y. Shin, H.-L. Zhang, S. J. Haigh and C. Casiraghi, J. Mater. Chem. A, 2016, 4, 11666–11671 RSC.
- Q. Li, S. Mahendra, D. Y. Lyon, L. Brunet, M. V. Liga, D. Li and P. J. Alvarez, Water Res., 2008, 42, 4591–4602 CrossRef CAS PubMed.
- H. Lin, W. Deng, T. Zhou, S. Ning, J. Long and X. Wang, Appl. Catal., B, 2014, 43, 473–486 Search PubMed.
- Y. Shiraishi and T. Hirai, J. Photochem. Photobiol., C, 2008, 9, 157–170 CrossRef CAS.
- X. Lang, X. Chen and J. Zhao, Chem. Soc. Rev., 2014, 43, 473–486 RSC.
- M. Xie, W. Wei, Z. Jiang, Y. Xu and J. Xie, Ceram. Int., 2016, 42, 4158–4170 CrossRef CAS.
- H. Wang, X. Li and J. Yang, ChemPhysChem, 2016, 17, 2100–2104 CrossRef CAS PubMed.
- P. Niu, L. Zhang, G. Liu and H.-M. Cheng, Adv. Funct. Mater., 2012, 22, 4763–4770 CrossRef CAS.
- X. Bai, S. Yan, J. Wang, L. Wang, W. Jiang, S. Wu, C. Sun and Y. Zhu, J. Mater. Chem. A, 2014, 2, 17521–17529 RSC.
- W. Wang, J. C. Yu, Z. Shen, D. K. Chan and T. Gu, Chem. Commun., 2014, 50, 10148–10150 RSC.
- S. Wang, C. Li, T. Wang, P. Zhang, A. Li and J. Gong, J. Mater. Chem. A, 2014, 2, 2885 RSC.
- S. Yang, Y. Gong, J. Zhang, L. Zhan, L. Ma, Z. Fang, R. Vajtai, X. Wang and P. M. Ajayan, Adv. Mater., 2013, 25, 2452–2456 CrossRef CAS PubMed.
- X. She, H. Xu, Y. Xu, J. Yan, J. Xia, L. Xu, Y. Song, Y. Jiang, Q. Zhang and H. Li, J. Mater. Chem. A, 2014, 2, 2563 RSC.
- T. Y. Ma, S. Dai, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2014, 53, 7281–7285 CrossRef CAS PubMed.
- Z. Tong, D. Yang, J. Shi, Y. Nan, Y. Sun and Z. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 25693–25701 CrossRef CAS PubMed.
- S. Zhang, J. Li, X. Wang, Y. Huang, M. Zeng and J. Xu, J. Mater. Chem. A, 2015, 3, 10119–10126 RSC.
- J. Cho, Y. Hong and Y. Kang, ACS Nano, 2015, 4, 4026 CrossRef PubMed.
- Z. Yang, Y. Zhang and Z. Schnepp, J. Mater. Chem. A, 2015, 3, 14081–14092 RSC.
- H. Yan, Chem. Commun., 2012, 48, 3430–3432 RSC.
- S. Guo, Z. Deng, M. Li, B. Jiang, C. Tian, Q. Pan and H. Fu, Angew. Chem., Int. Ed., 2016, 55, 1830–1834 CrossRef CAS PubMed.
- N. Meng, J. Ren, Y. Liu, Y. Huang, T. Petit and B. Zhang, Energy Environ. Sci., 2018, 11, 566–571 RSC.
- Z. Teng, N. Yang, H. Lv, S. Wang, M. Hu, C. Wang, D. Wang and G. Wang, Chem, 2019, 5, 664–680 CAS.
- G. P. Mane, S. N. Talapaneni, K. S. Lakhi, H. Ilbeygi, U. Ravon, K. Al-Bahily, T. Mori, D. H. Park and A. Vinu, Angew. Chem., Int. Ed., 2017, 56, 8481–8485 CrossRef CAS PubMed.
- A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J.-O. Müller, R. Schlögl and J. M. Carlsson, J. Mater. Chem., 2008, 18, 4893 RSC.
- A. Ranganathan, V. R. Pedireddi and C. N. R. Rao, J. Am. Chem. Soc., 1999, 121, 1752–1753 CrossRef CAS.
- J. Pampel, A. Mehmood, M. Antonietti and T. P. Fellinger, Mater. Horiz., 2017, 4, 493–501 RSC.
- X. Zhang, Y. Wang, F. Hou, H. Li, Y. Yang, X. Zhang, Y. Yang and Y. Wang, Appl. Surf. Sci., 2017, 391, 476–483 CrossRef CAS.
- Y. Yu, W. Yan, X. Wang, P. Li, W. Gao, H. Zou, S. Wu and K. Ding, Adv. Mater., 2018, 30, 1705060 CrossRef PubMed.
- J. Zhang, M. Zhang, G. Zhang and X. Wang, ACS Catal., 2012, 2, 940–948 CrossRef CAS.
- X. Li, I. V. Sergeyev, F. Aussenac, A. F. Masters, T. Maschmeyer and J. M. Hook, Angew. Chem., Int. Ed., 2018, 57, 6848–6852 CrossRef CAS PubMed.
- Y. Xu, H. Xu, L. Wang, J. Yan, H. Li, Y. Song, L. Huang and G. Cai, Dalton Trans., 2013, 42, 7604–7613 RSC.
- H. Wang, M. Li, Q. Lu, Y. Cen, Y. Zhang and S. Yao, ACS Sustainable Chem. Eng., 2018, 7, 625–631 CrossRef.
- I. Y. Kim, S. Kim, X. Jin, S. Premkumar, G. Chandra, N. S. Lee, G. P. Mane, S. J. Hwang, S. Umapathy and A. Vinu, Angew. Chem., Int. Ed., 2018, 57, 17135–17140 CrossRef CAS PubMed.
- G. Jiang, C.-H. Zhou, X. Xia, F. Yang, D. Tong, W. Yu and S. Liu, Mater. Lett., 2010, 64, 2718–2721 CrossRef CAS.
- Y. Tang, M. Yuan, B. Jiang, Y. Xiao, Y. Fu, S. Chen, Z. Deng, Q. Pan, C. Tian and H. Fu, J. Mater. Chem. A, 2017, 5, 21979–21985 RSC.
- H. Yu, R. Shi, Y. Zhao, T. Bian, Y. Zhao, C. Zhou, G. I. N. Waterhouse, L. Z. Wu, C. H. Tung and T. Zhang, Adv. Mater., 2017, 29, 1605148 CrossRef PubMed.
- Y. Xiao, G. Tian, W. Li, Y. Xie, B. Jiang, C. Tian, D. Zhao and H. Fu, J. Am. Chem. Soc., 2019, 141, 2508–2515 CrossRef CAS PubMed.
- F. Dong, Z. Zhao, T. Xiong, Z. Ni, W. Zhang, Y. Sun and W. K. Ho, ACS Appl. Mater. Interfaces, 2013, 5, 11392–11401 CrossRef CAS PubMed.
- G. P. Mane, D. S. Dhawale, C. Anand, K. Ariga, Q. Ji, M. A. Wahab, T. Mori and A. Vinu, J. Mater. Chem. A, 2013, 1, 2913 RSC.
- P. Kumar, E. Vahidzadeh, U. K. Thakur, P. Kar, K. M. Alam, A. Goswami, N. Mahdi, K. Cui, G. M. Bernard, V. K. Michaelis and K. Shankar, J. Am. Chem. Soc., 2019, 141, 5415–5436 CrossRef CAS PubMed.
- G. Zhang, G. Li, T. Heil, S. Zafeiratos, F. Lai, A. Savateev, M. Antonietti and X. Wang, Angew. Chem., Int. Ed., 2019, 58, 3433–3437 CrossRef CAS PubMed.
- Y. Li and K. Bjön, J. Opt. Soc. Am. A, 2004, 21, 1993 Search PubMed.
- Y. Wang, Z. Zhang, L. Zhang, Z. Luo, J. Shen, H. Lin, J. Long, J. C. S. Wu, X. Fu, X. Wang and C. Li, J. Am. Chem. Soc., 2018, 140, 14595–14598 CrossRef CAS PubMed.
- C. Yin, L. Cui, T. Pu, X. Fang, H. Shi, S. Kang and X. Zhang, Appl. Surf. Sci., 2018, 456, 464–472 CrossRef CAS.
- X. Zhang, Y. Yang, W. Huang, Y. Yang, Y. Wang, C. He, N. Liu, M. Wu and L. Tang, Mater. Res. Bull., 2018, 99, 349–358 CrossRef CAS.
- G. Dong, K. Zhao and L. Zhang, Chem. Commun., 2012, 48, 6178–6180 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta10688j |
| This journal is © The Royal Society of Chemistry 2020 |