An antimonene/Cp*Rh(phen)Cl/black phosphorus hybrid nanosheet-based Z-scheme artificial photosynthesis for enhanced photo/bio-catalytic CO2 reduction†
Xiaoyuan
Ji
 ac,
Yong
Kang
b,
Taojian
Fan
ac,
Yong
Kang
b,
Taojian
Fan
 d,
Qingqing
Xiong
d,
Qingqing
Xiong
 c,
Songping
Zhang
c,
Songping
Zhang
 *b,
Wei
Tao
*b,
Wei
Tao
 *c and
Han
Zhang
*c and
Han
Zhang
 *d
*d
aSchool of Pharmaceutical Sciences (Shenzhen), Sun Yat-sen University, Guangzhou 510275, China
bState Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China. E-mail: spzhang@ipe.ac.cn
cCenter for Nanomedicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115, USA. E-mail: wtao@bwh.harvard.edu
dShenzhen Engineering Laboratory of Phosphorene and Optoelectronics, SZU-NUS Collaborative Innovation Center for Optoelectronic Science and Technology, Key Laboratory of Optoelectronic Devices and Systems of Ministry of Education and Guangdong Province, Shenzhen University, Shenzhen 518060, China. E-mail: hzhang@szu.edu.cn
First published on 27th November 2019
Abstract
Developing a biocatalyzed artificial photosynthesis system (APS) with a Z-scheme for CO2 conversion has a promising outlook. However, it remains unexplored and challenging. Herein, we present an antimonene (AM) and black phosphorus (BP) hybrid nanosheet (HNSs) based Z-scheme APS for enhanced photo/bio-catalytic CO2 reduction. An “amphipathic” polymer PEI-PEG-C18 with a positively charged PEI-head and hydrophobic C18-tail was synthesized and further modified with an electron mediator (M, Cp*Rh(phen)Cl). Using this PEI-PEG-C18-M as a “double-side tap”, AM/M/BP HNS-based Z-scheme photocatalytic systems were constructed through catenating AM NSs via a hydrophobic interaction with the hydrophobic C18-tail and subsequent BP NSs via electrostatic interactions with the positively charged PEI-head. Thereafter, the biocatalytic system, including NAD(H)+ and redox enzymes, were sandwiched between the AM and BP layers through an electrostatic attraction with the amide groups in the “double-side tap”. Due to the high separation efficiency of the photogenerated electrons and holes and the improved reduction and oxidation potentials, the integrated Z-scheme APS shows excellent performance. In particular, although neither AM nor BP could catalyze NADH regeneration using H2O as an electron donor under visible light, a 35 ± 4% regeneration of NADH and 160 ± 24 μmol formic acid production were achieved by the AM/M/BP HNS-based Z-scheme APS. Moreover, the compatibility and omnipotence of the HNS-based APS for the synthesis of other high value-added products from CO2 have been demonstrated through assembling the corresponding biocatalysts.
Introduction
Converting CO2 into chemical fuel using limitless and renewable solar energy is growing in popularity worldwide by mitigating the greenhouse effect and relieving the deficiency of fossil fuels.1–4 Natural photosynthesis, a process that converts sunlight, water, and CO2 into carbohydrates and oxygen, provides a blueprint for the design of an artificial photosynthesis system (APS) aiming at converting solar energy into chemicals and fuels.5,6 One of the most promising bionic photosynthesis processes is a biocatalyzed APS, which relies on coupling the photo-reduction process of NAD(P)H from NAD(P)+ by photocatalysts with NAD(P)H-dependent redox enzymes to synthesize various chemicals and fuels from CO2.7–10Although some single semiconductor or organic photocatalysts have been widely used for biocatalyzed APS, their performance is greatly limited by the contradiction between sufficient light absorption, fast electron–hole recombination, and high reduction/oxidation potentials.11–14 Generally, a narrow band gap can broaden the absorbance range of sunlight and enhance the separation of charges (electrons and holes). However, a more negative conduction band (CB) potential and a more positive valence band (VB) potential are favored for the reduction and oxidation reactions, respectively. Hence, for a single photocatalyst, it is difficult to simultaneously satisfy the efficient utilization of sunlight and strong redox ability. The construction of heterostructure photocatalysts (type-II heterojunctions) is a conventional and effective method to broaden the light absorption range and facilitate the separation of photo-induced electron–hole pairs.15,16 In typical type-II heterojunctions, electrons photogenerated from the CB of one photocatalyst transfer to the CB of another photocatalyst with a relatively lower band-edge position, while holes accumulate in the VB of the photocatalyst with a higher band edge. Therefore, the oxidation and reduction potentials would be reduced, and the redox ability would be ultimately weakened.17,18 In our previous studies,7–10 although the construction of integrated biocatalyzed APS could improve the electron transfer efficiency and retard the recombination of photogenerated charges through shortening the electron transfer distance, the conversion efficiency of solar energy still needs to be further improved.
In natural photosynthesis, one of the most unique properties is the so-called Z-scheme mechanism of the light reaction within the thylakoid membrane. In this mechanism, PS II and PS I are activated simultaneously and the electrons in the CB of PS II can be transferred to the VB of PS I, leaving stronger reduction/oxidation potentials in the CB of PS I and the VB of PS II for NADPH regeneration and water oxidization, respectively.19,20 The primary advantage of biomimetic Z-scheme systems is not only being able to improve the separation efficiency of the photogenerated electrons and holes, but also promoting the reduction and oxidation potentials. In recent decades, numerous efforts have been devoted to mimicking natural photosynthesis to explore possibilities for future renewable fuel technologies.19,21–24 However, biomimetic Z-scheme systems for biocatalyzed APS still have not been completely explored due to the complexity of kinetically coupling the photocatalytic system with the biocatalytic system, which induces photocatalytic NAD+ reduction and biocatalytic CO2 conversion.
The landmark discovery and successful application of graphene have triggered considerable interest for exploring other atomically thin two-dimensional (2D) materials.25,26 Since 2014, black phosphorus (BP), a mono-elemental 2D layered material, has become a rising star in the post-graphene era with superior photochemical and electrochemical properties such as layer-dependent direct-bandgap, strong optical absorption, and high hole mobility.27–29 More recently, another 2D layered material, antimonene (AM), was successfully exfoliated from its layered bulk counterpart.30–32 Theoretical calculations and geometrical experimental findings have indicated that AM has a bandgap of about 1.2 eV, which is within the range suitable for APS applications.30–32 The tunable and synergic band structure of BP and AM made them perfect candidates for the construction of the Z-scheme system.
Herein, we report our efforts in developing a highly integrated AM and BP ultrathin hybrid nanosheet (HNS) based Z-scheme APS. As shown in Scheme 1a, both the AM and BP NSs were prepared by liquid-phase exfoliation processes. An electron mediator (M, [Cp*Rh(phen)Cl]Cl (Cp* = C5Me5, phen = 1,10-phenanthroline)) functionalized “amphipathic” polymer (PEI-PEG-C18-M) with a positively charged amino group in the head and hydrophobic chains at the end was synthesized and used as a “double-side tap” to catenate the AM and BP NSs in turn via the hydrophobic interaction between AM and the tail of PEI-PEG-C18-M as well as the electrostatic attraction between BP and the head of PEI-PEG-C18-M. According to their electronic band structure, a direct Z-scheme electron transfer could be easily formed in the AM/M/BP HNSs with electrons flowing along “BP-AM-M” (Scheme 1b). Moreover, the integration of NAD(H) and the enzyme between the AM and BP layers was achieved through an electrostatic attraction with PEI, in which the electron transfer from M to NAD+ and the synergy between the photocatalytic and biocatalytic systems were largely enhanced. The AM/M/BP HNS-based Z-scheme system offers the advantages of efficient charge separation and transfer, consequently enhancing the lifetime and density of photoexcited electrons and holes, leading to ∼90% NADH regeneration and >300 μmol formic acid conversion within 2 hours using TEOA as the electron donor. More interestingly, an NADH regeneration of ∼50% and formic acid conversion of ∼250 μmol also could be obtained within 2 hours using H2O as the electron donor, even though neither AM nor BP could catalyze H2O splitting to obtain electrons under visible light. Moreover, the compatibility and omnipotency of the AM/M/BP hybrid nanosheet (HNSs) based APS for the synthesis of other high value-added products from CO2 through a multi-step cascade reaction was also demonstrated by assembling the corresponding biocatalysts. This study provides a new idea and view for designing high-efficiency APS through constructing a 2D NS-based Z-scheme system.
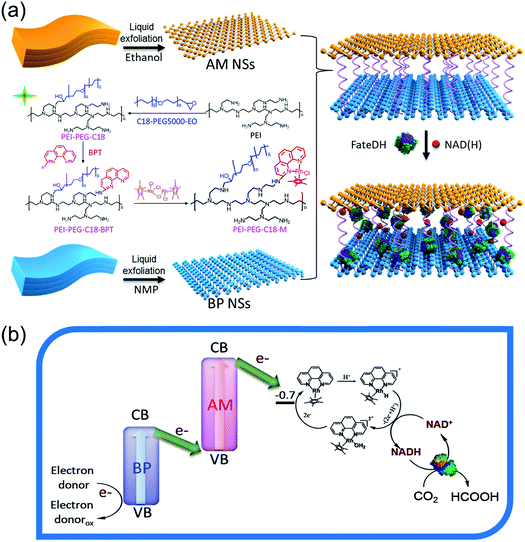 | ||
| Scheme 1 Schematic showing (a) the preparation process for the AM/M/BP HNS-based integrated APS and (b) Z-scheme electron transfer mechanism. | ||
Results and discussion
In the first set of experiments, an AM/M/BP HNS-based Z-scheme APS was prepared through catenating the AM and BP NSs in-turn via an M-functionalized “amphipathic” polymer, PEI-PEG-C18-M. First, both the AM and BP NSs were exfoliated through probe sonication in ethanol and N-methyl pyrrolidone (NMP) solution, separately. Transmission electron microscopy (TEM) and atomic force microscopy (AFM) images confirmed that ultrathin AM and BP NSs were obtained by exfoliation with a planar size of ∼95 and ∼105 nm, respectively, and thicknesses of about 1.2 nm (Fig. 1b, c, f, g, S1a and b†). Almost the same planar size and thickness of the AM and BP NSs endowed them with the same active sites, resulting in a convenient and efficient cooperation with each other. The absorbance curves of the AM and BP NSs were all detected and shown in Fig. S2,† all of which exhibit strong light absorbance from the visible to near-infrared region.AM NSs are hydrophobic with slightly negatively charged surfaces with zeta potential of −0.45 mV, while BP NSs are hydrophilic with rather low zeta potential of −32.18 mV. In order to construct stable Z-scheme AM/BP HNSs and facilitate the electron transfer between AM and BP, an “amphipathic” polymer (PEI-PEG-C18) with positively charged amines in the head and hydrophobic C18 chains at the end was synthesized by a reaction between the amino group of polyethylene imine (PEI) and the epoxy group of C18-PEG-epoxy (Fig. S3†). In order to facilitate an electron transfer from the photocatalyst (AM/BP HNSs) to NAD+, an organometallic compound, M, [Cp*Rh(phen)Cl]Cl (Cp* = C5Me5, phen = 1,10-phenanthroline), which is one of the most popular electron mediators for the specific reduction of NAD(P)H,7–10,33 was covalently crosslinked to PEI-PEG-C18 previously to obtain PEI-PEG-C18-M. Using this PEI-PEG-C18-M as a “double-side tap”, M was sandwiched between the AM and BP layers during the formation of AM/BP HNSs to form AM/M/BP HNSs. Thus, a Z-scheme electron transfer system was constructed. The nuclear magnetic resonance (NMR) analysis shown in Fig. S4† demonstrates the successful synthesis of PEI-PEG-C18 and PEI-PEG-C18-M. Fig. 1d and h show the TEM and AFM images of the as-prepared AM/M/BP HNSs. These images reveal the formation of 2D hierarchical structures with a diameter of ∼130 nm and a thickness of ∼9 nm. To investigate the interfacial interaction between AM and BP in the AM/M/BP HNSs, high-resolution TEM (HRTEM), scanning electron microscopy (SEM), and the corresponding energy dispersive X-ray spectroscopy (EDX) elemental mapping of Sb, P, O, C, N, and Rh elements were performed. Fig. 1e presents a typical HRTEM image of the AM/M/BP HNSs, implying the successful combination between AM and BP. The distinct interface between the two phases can be clearly identified along with the intersection of two different lattice fringes. The lattice fringes of 0.21 and 0.33 nm on AM/M/BP HNSs, corresponding to the planes of AM and BP, respectively, are observed. The SEM image of the AM/M/BP HNSs and the corresponding EDX images of Sb, P, O, C, N, and Rh elements clearly illustrate the homogeneous hybridization of AM and BP. These TEM and EDX images not only show that AM NSs, BP NSs, and electron mediator-functionalized electron transfer chain, PEI-PEG-C18-M, were successfully combined, but also further demonstrate that the AM and BP NSs were almost completely flush-laminated and the electron mediator (M) was sandwiched between them, which will enable a more facile and efficient transfer of electrons and energy.
To further investigate the successful construction of AM/M/BP HNSs, Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, and X-ray diffraction (XRD) analyses were conducted. As shown in Fig. 2a, in the FTIR spectra, the weak peaks located at around 2000 cm−1 belong to AM and BP. The other absorption peaks demonstrate the assembly of PEI-PEG-C18-M with AM and BP. For example, the characteristic stretching vibration at ∼2900 cm−1 is attributable to the CH vibration in the PEG segment. The absorption band at ∼800 cm−1 from the amino group of PEI and the absorption band at ∼1600 and 1500 cm−1 from phenanthroline are also confirmed. The peaks in the Raman spectra of the as-fabricated AM and BP NSs exhibited almost the same peaks as those of bulk AM and BP (Fig. S5†). The slight shift toward a higher wavenumber can be attributed to the ultrathin NS structures compared to their corresponding bulk counterparts. After being catenated by PEI-PEG-C18-M, a slight shift toward a lower wavenumber is observed, which may arise from the slight increase in the thickness after forming the AM/M/BP HNSs (Fig. 2b). As shown in the XRD spectra (Fig. 2c), the AM/M/BP HNSs displayed all of the characteristic peaks of AM and BP NSs, which also demonstrated the successful construction of AM/M/BP HNSs.
X-ray photoelectron spectroscopy (XPS) is the most commonly used method for analyzing interfacial interactions between nanosheets.34 The XPS spectra in Fig. 2d clearly show Sb 3d and 3p in AM and AM/M/BP HNSs and P 2s and 2p in BP and the AM/M/BP HNSs. In addition to Sb and P elements, other elements, including C (1s), N (1s), O (1s), and Rh (3d), originating from PEI-PEG-C18-M, are observed. In the C 1s XPS spectra (Fig. 2e), there are three different peaks at 284.8, 286.1, and 286.5 eV. The peak at 284.8 eV is assigned to the C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, which originated from the framework of PEI-PEG-C18 and phenanthroline of M. The peak at 286.1 eV is attributed to the C–O bond, which originated from C18-PEG-epoxy and hydroxyl after a reaction between PEI and C18-PEG-epoxy. The peaks at 286.5 eV may be assigned to C–N and C
C bonds, which originated from the framework of PEI-PEG-C18 and phenanthroline of M. The peak at 286.1 eV is attributed to the C–O bond, which originated from C18-PEG-epoxy and hydroxyl after a reaction between PEI and C18-PEG-epoxy. The peaks at 286.5 eV may be assigned to C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, which originated from PEI and M. In the N 1s XPS spectra (Fig. 2f), the peaks at 399.8, 400.0, and 401.5 eV are assigned to N–H, N–C, and N
N, which originated from PEI and M. In the N 1s XPS spectra (Fig. 2f), the peaks at 399.8, 400.0, and 401.5 eV are assigned to N–H, N–C, and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, respectively, which originated from PEI and M. In the Sb 3d spectra, there are four different peaks at 527.9, 530.6, 537.2, and 539.5 eV (Fig. 2g). The peaks at 530.6 and 539.5 eV are attributed to the Sb 3d5/2 and Sb 3d3/2, respectively.35 The binding energy peaks at 527.9 and 537.2 eV are assigned to Sb 3d5/2 and Sb 3d3/2 of oxidized antimony (SbxOy), respectively. Interestingly, in the spectrum of AM/M/BP HNSs, although the characteristic peaks of Sb 3d5/2 and Sb 3d3/2 are observed, a slight shift to the lower binding energies of these peaks is shown in Fig. 2g. In order to further clarify the interfacial interaction between AM and BP in the AM/M/BP HNSs, the XPS spectra of P 2p were recorded (Fig. 2h). The P 2p XPS spectra are composed of three bands at 129.1, 130.4, and 133.5 eV, which are attributed to P 2p3/2, P 2p1/2, and oxidized phosphorus (PxOy), respectively. In contrast, these binding energies shifted to higher binding energies in the AM/M/BP HNSs. Generally, the binding energy shifts in the hybrid could indicate that there was a strong interaction in the components. The decrease in the binding energy demonstrates the strengthened electron screening effect due to the increase in the electron concentration, whereas the increase in the binding energy indicates a decrease in the electron concentration. Under X-ray irradiation in the XPS test, both AM and BP were excited by the X-rays. The excited electron at the conduction band of AM and BP transferred to their surface. According to the XPS spectra of the AM/M/BP HNSs, the lower and higher binding energy shifts of Sb 3d and P 2p in the AM/M/BP HNSs were attributed to the increase and decrease in the electron density of AM and BP, respectively, indicating that the excited electron on the surface of BP transferred to the surface of AM due to strong interfacial interactions between AM and the BP NSs.
C, respectively, which originated from PEI and M. In the Sb 3d spectra, there are four different peaks at 527.9, 530.6, 537.2, and 539.5 eV (Fig. 2g). The peaks at 530.6 and 539.5 eV are attributed to the Sb 3d5/2 and Sb 3d3/2, respectively.35 The binding energy peaks at 527.9 and 537.2 eV are assigned to Sb 3d5/2 and Sb 3d3/2 of oxidized antimony (SbxOy), respectively. Interestingly, in the spectrum of AM/M/BP HNSs, although the characteristic peaks of Sb 3d5/2 and Sb 3d3/2 are observed, a slight shift to the lower binding energies of these peaks is shown in Fig. 2g. In order to further clarify the interfacial interaction between AM and BP in the AM/M/BP HNSs, the XPS spectra of P 2p were recorded (Fig. 2h). The P 2p XPS spectra are composed of three bands at 129.1, 130.4, and 133.5 eV, which are attributed to P 2p3/2, P 2p1/2, and oxidized phosphorus (PxOy), respectively. In contrast, these binding energies shifted to higher binding energies in the AM/M/BP HNSs. Generally, the binding energy shifts in the hybrid could indicate that there was a strong interaction in the components. The decrease in the binding energy demonstrates the strengthened electron screening effect due to the increase in the electron concentration, whereas the increase in the binding energy indicates a decrease in the electron concentration. Under X-ray irradiation in the XPS test, both AM and BP were excited by the X-rays. The excited electron at the conduction band of AM and BP transferred to their surface. According to the XPS spectra of the AM/M/BP HNSs, the lower and higher binding energy shifts of Sb 3d and P 2p in the AM/M/BP HNSs were attributed to the increase and decrease in the electron density of AM and BP, respectively, indicating that the excited electron on the surface of BP transferred to the surface of AM due to strong interfacial interactions between AM and the BP NSs.
In natural photosynthesis systems, the elaborate structure of chloroplasts provides an ideal strategy to improve the solar-to-chemical energy conversion efficacy through directly interacting with the biocatalytic system and the photocatalytic reaction system, thus enabling the efficient trafficking of electron species and chemicals between them. To develop an efficient APS, the biocatalytic system, including NAD(H) and redox enzymes, was effectively interfaced with the photocatalytic system directly. Thus, the biocatalytic system could efficiently receive the reduced equivalents generated in the photocatalytic system to catalyze the targeted chemical synthesis. In this study, PEI-PEG-C18-M not only catenated the AM and BP NSs to form the AM/M/BP HNS-based enhanced Z-scheme photocatalytic system, but also provided anchor sites for NAD(H) and the enzymes via an electrostatic attraction between the negatively charged NAD(H) and enzymes and positively charged PEI (Fig. 3a). Confocal laser scanning microscopy (CLSM) was applied to demonstrate the successful assembly and uniform distribution of FateDH and NAD(H) (Fig. 3b and c). Moreover, the amount of FateDH and NAD(H) assembled onto the interface of the AM/M/BP HNSs was precisely controlled and quantitatively determined by adjusting the assembly time and the initial concentration. The final amount and activity recovery of each component, including FateDH, NAD+, and M assembled on the AM/M/BP HNSs, were calculated and listed in Table S1.†
After constructing the AM/M/BP HNS-based APS, the solar-to-chemical energy conversion efficacy of this APS was investigated by the photo-regeneration of NADH and formic acid conversion from CO2. According to the schematic of the AM/M/BP HNS-based APS for the formic acid synthesis from CO2 (Fig. S6†), the AM/M/BP HNSs absorb visible light and supply photoexcited electrons to Mox. After accepting two electrons from HNSs, Mox transforms to its reduced form, Mred,1. Then, Mred,1 recruits one proton from the aqueous solution and transforms to another reduced form, Mred,2. Mred,2 is capable of regenerating active NADH from NAD+ through releasing one proton and two electrons to NAD+. Then, the holes of AM/M/BP HNSs in the VB of BP are supplemented through oxidizing sacrificial electron donors such as TEOA or H2O. For comparison, the photo-reduction of NADH was also performed and analyzed by utilizing AM, BP, physically mixed AM and BP (AM + BP) coupled with free M and AM/M/BP HNSs. Every group was tested under the same conditions with same final concentration of TEOA (15 wt%), NSs (0.2 mg mL−1), M (0.1 mM), and NAD+ (1 mM). As shown in Fig. 3d, the efficiency of NADH regeneration by AM/M/BP HNSs reached 89 ± 1.4%, corresponding to a quantum efficiency (QE) of 18 ± 0.4% and a turnover frequency (TOF) of 0.5 ± 0.1 h−1, which was much higher than that of AM NSs (20 ± 0.8%), BP NSs (26 ± 0.7%), and the system with physically mixed AM and BP NSs (33 ± 1.2%).
With the help of renewable solar energy, converting CO2 into chemical fuel is the best strategy to mitigate the greenhouse effect and relieve the deficiency of fossil fuels.36–41 In this study, in order to improve the specificity of the product and detect the cooperation efficiency in the AM/M/BP HNS-based APS directly, a single enzyme or multi-enzyme-based biocatalytic system was introduced to catalyze the CO2 reduction by solar energy. First, by coupling the AM/M/BP HNS-driven photo-regeneration of NADH with FateDH, 365 ± 23 μmol of formic acid was produced after 4 h irradiation of visible light with the AM/M/BP HNS-based APS, with a TONNADH of 91 ± 0.3 h−1 and TONFateDH of 3.0 × 104 h−1 (Fig. 3e). In contrast, only 6 ± 0.5 and 71 ± 3.4 μmol formic acid were produced on catalysis by the APS when only AM or BP NSs was used, respectively. Even when AM and BP NSs were used in combination, the conversion of formic acid was only 80 ± 4.7 μmol, which was much less than that using AM/M/BP HNS-based APS. Most previous reports on discrete biocatalytic APS or reports from our group on integrated biocatalytic APS needed organic molecules such as TEOA as a sacrificial electron donor, which would lead to an increased reaction cost and difficulty in the target product separation and purification. Therefore, directly using H2O instead of sacrificial materials as the electron donor has always been the research emphasis and challenge for APS. In this study, AM, BP NSs and AM/M, BP/M NSs were employed as photocatalysts for regenerating NADH using H2O as an electron donor. However, due to the high oxidation potential and thermodynamic energy required for catalyzing O2 evolution from H2O, there was nearly no detectable NADH regenerated from NAD+ using AM, BP NSs or AM/M, BP/M NSs as photocatalysts and H2O as the electron donor. Interestingly, 35 ± 4% regeneration of NADH (QE of 6 ± 0.1% and TOF of 0.2 ± 0.1 h−1) and 160 ± 24 μmol conversion of formic acid (TONNADH of 40 ± 0.5 h−1 and TONFateDH of 1 ± 0.4 × 104 h−1) were observed when the Z-scheme AM/M/BP HNS-based APS was used, even on using H2O as an electron donor. Considering the high oxidation potential of our developed AM/M/BP HNSs, there was a possibility of damage on the structure and bioactivity of regenerated NADH. To clarify this suspicion, 1H nuclear magnetic resonance (NMR) spectroscopy was employed to detect the structure of the regenerated NADH. As exhibited in Fig. S7,† the 1H NMR spectrum of the reaction products after removing the AM/M/BP HNSs was consistent with the 1H NMR spectra of fresh NAD+ and NADH. The perfectly matched 1H NMR spectra not only confirmed the photocatalytic activity of the AM/M/BP HNSs, but also indicated that the high oxidation potential of the HNSs did not destroy the structure of the regenerated NADH.
Moreover, in order to produce more valuable chemicals from CO2 and detect the compatibility of these AM/M/BP HNSs with other biocatalysts, a cascade reduction reaction catalyzed by three dehydrogenases (FateDH, formaldehyde dehydrogenase (FaldDH), and alcohol dehydrogenase (ADH)) with NADH as the carrier for the hydride transfer for the methanol conversion from CO2 (Fig. S8†) was employed and assembled into the AM/M/BP HNS-based integrated APS. The synthesis of methanol from CO2 as a function of the reaction time catalyzed by this cascade reaction with photocatalyzed NADH regeneration using TEOA or H2O as an electron donor is shown in Fig. S9.† After a 4 h irradiation of visible light, 1.2 ± 0.5 μmol of methanol was produced using TEOA as the electron donor, which corresponds to a TONNADH of 1 ± 0.1 h−1 and TONFateDH/FaldDH/ADH of 133 ± 1.2 h−1. To our surprise, even after using H2O as the electron donor, 0.2 ± 0.04 μmol of methanol was produced, which corresponds to a TONNADH of 0.2 ± 0.04 h−1 and TONFateDH/FaldDH/ADH of 24 ± 0.5 h−1. Both formic acid and methanol conversion by coupling the AM/M/BP HNS-based photocatalyzed NADH regeneration system with a single enzyme (FateDH) or multi-enzyme (FateDH, FaldDH, and ADH) were much higher than that of previous reports (Table S2†), which directly demonstrates the excellent efficiency of the AM/M/BP HNS-based Z-scheme APS. Moreover, the O2 evolution curves after water oxidation were detected and shown in Fig. S10.† Nearly 45 ± 4.7 μmol of O2 was produced after a 5 h reaction under the irradiation of visible light. Based on the stoichiometry for the CO2 reduction to formic acid, 1 mol of evolved O2 generates 4 electrons, equivalent to 2 mol of formic acid product. In this regard, the stoichiometry between 45 ± 4.7 μmol of the oxidation products (O2) and 160 ± 24 μmol of the reduction products (formic acid) was not met. In the other words, the O2 evolution values detected from the dissolved O2 in the reaction solution were relatively lower than the theoretically calculated values. One possible reason for this inconsistency might be part of the O2 being distributed in the upper air of the reactor, which is hard to detect in the pressurized sealed reaction vessel. In contrast, for the methanol reduction (1.2 ± 0.5 μmol), the O2 evolution values (45 ± 4.7 μmol) detected from the dissolved O2 in the reaction solution were much higher than the theoretically calculated values, considering that 6e− were required for the CO2 reduction to methanol. The production of intermediate products such as formic acid and formaldehyde, which also consumed the electrons from O2 evolution, could have been the main reason. Although the O2 evolution values were not stoichiometrically matched with the reduction products due to the detection method and the complexity of this reaction, the detectable O2 evolution from H2O in the absence of any sacrificial electron donor demonstrated the great capacities of AM/M/BP HNS-based APS for CO2 conversion using H2O as an electron donor.
The operational stability of APS is crucial for its potential applications in industry. In order to test the operational stability of our APS, the reaction was performed for 4 h and the HNSs were centrifuged and extracted from the reaction solution. Then, the HNSs were applied for next reaction cycles by immersing the HNSs into a fresh reaction solution. Fig. 3f presents the residual activity of the AM/M/BP HNS-based APS versus the reuse cycles. After 5 cycles of reuse, above 80% of the original activity of the AM/M/BP HNSs were retained, which confirmed the high operational stability of the AM/M/BP HNS-based APS. In order to further determine the main reasons for the less than 20% activity loss, the structure and size distribution of the AM/M/BP HNSs as well as the leakage of M, NAD(H), and the enzymes were detected. As shown in Fig. S11,† the structure and size distribution (134 nm) of the AM/M/BP HNSs after 5 cycles of reuse remained almost the same as that of the fresh prepared sample. For the leakage of M, NAD(H), and the enzymes, the UV-vis-NIR absorbance of the reaction solution after removing the AM/M/BP HNSs was detected and there was nearly no specific absorbance peak for M and NAD(H), which indicated that M and NAD(H) were assembled tightly in the AM/M/BP HNSs. However, after Coomassie blue staining of the reaction solution, a slight blue color in the solution was observed, demonstrating the mild leakage of the enzyme from the AM/M/BP HNSs might have been the main reason for the activity loss.
We attribute the significant improvement in the NADH photo-regeneration and CO2 conversion efficiency to the following aspects. First, the dual-functionalized polymer (PEI-PEG-C18) not only ensured the tight Z-scheme AM/M/BP HNSs, but also improved the stability and dispersity of AM/M/BP HNSs in water, which had more advantages than that of the other traditional Z-scheme hybrids through van der Waals forces. Second, the precise arrangement of AM/BP HNSs and M through covalently bonding M onto PEI-PEG-C18-M, which was deposited between the AM and BP layers of the AM/BP HNSs, may significantly promote the electron transfer between AM/M/BP HNSs, forming the Z-scheme electron transfer. In the AM/BP HNSs, a charge transfer was also possible through type-II heterojunctions (Fig. S12†), in which the holes in the VB of BP transferred to the VB of AM and the electrons in the CB of AM transferred to the CB of BP. This type-II band alignment would compete with the Z-scheme charge transfer route, which would lead to a reduction in the redox capacity of the systems and the inevitable lowering of the photocatalytic activity of the AM/BP HNSs. In the present study, an efficient electron mediator, M, was introduced for damming the type-II band alignment to effectively facilitate the completion of the Z-scheme charge carrier transfer between BP and AM, thus forming the new Z-scheme electron transfer system. The superiority of the assembly of M into AM/M/BP HNSs was also proven by the NADH regeneration and formic acid conversion, in which only 47.99% of the NADH regeneration and 216 μmol of formic acid conversion were obtained using the AM/BP HNSs with free M (Fig. S13†). Third, the integration of the biocatalyzed system with the photocatalyzed system also improved the electron transfer efficiency from M to NAD+ by shortening the electron transfer distance. In addition, the biocatalyzed system (enzymes and NAD(H)) tethered on the PEI-PEG-C18-M facilitated the shuttling and communication of NAD(H) between the enzymes near the vicinity.42 This well-defined spatial organization of these individual components made the AM/M/BP HNS-based APS present a chloroplast-mimicking structure and function. Therefore, the AM/M/BP HNS catalyst with co-integrated NAD+ and FateDH was kinetically advantageous over the free, mixed, and even composite Z-scheme hybrid through van der Waals forces.
In order to further demonstrate the superiority of the AM/M/BP HNS-based APS, the photochemical and electrochemical properties of the AM/M/BP HNSs were detected. The UV-vis diffuse reflectance spectra were recorded to test the optical properties of AM, BP, and AM/M/BP HNSs. As shown in Fig. 4a, pure AM and BP NSs all exhibited absorption throughout the entire visible region with an absorption edge at 700 and 1000 nm. According to the Kubelka–Munk conversion, the band gaps (Eg) of the AM and BP NSs were calculated to be about 1.9 and 1.7 eV, respectively (Fig. 4a). However, for the AM/M/BP HNSs, the absorption range and density became wider and higher than that of pure AM and BP NSs, which demonstrated the remarkably enhanced light harvesting ability of the AM/M/BP HNSs. Then, the VB value of the AM and BP NSs was determined by XPS spectra. As shown in Fig. 4b, the VB values of the AM and BP NSs are 0.9 eV and 1.3 eV, respectively. The CB of the AM and BP NSs therefore were −1.0 eV and −0.4 eV, respectively, as calculated from the differences between the Eg and VB. Fig. 4c shows electron movement and the energy band of the AM/M/BP HNSs. Since both the AM and BP can be excited by visible light, the CB-electrons of BP flow easily into the VB of AM because the VB of AM is lower than the CB of BP. This electron transfer process was faster than the electron–hole recombination between the VB and CB of BP due to the VB of AM being higher than the VB of BP. Thus, the electrons in the CB of BP and the holes in the VB of AM recombined at the interface contact, leaving high energy electrons and holes in the CB of AM and VB of BP, respectively, to drive the photocatalytic redox processes. Hence, the lifetime of the photogenerated CB-electrons at the AM and VB-holes at BP became longer due to the lower probability of their recombination. Since the oxidization potential of TEOA is about 0.8 eV and the reduction potential of M and NAD+ is about −0.7 and −0.3 eV, there is a match in the energy bands among TEOA, AM/M/BP HNSs, and NAD+. Moreover, when H2O was used as an electron donor, this Z-scheme electron transfer mechanism made the VB of BP have a high oxidizing power to split H2O, although the oxidization potential of H2O is 1.23 eV, which is very close to the VB of BP.
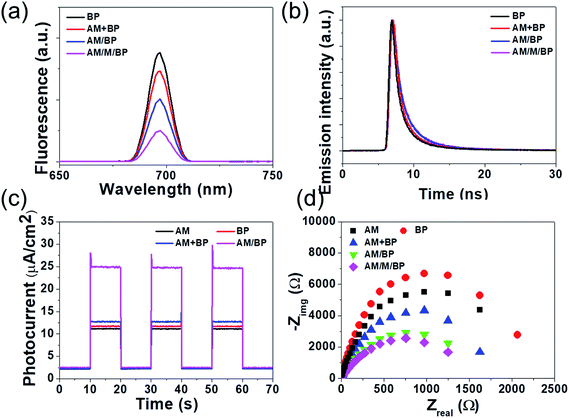
According to the photo-induced electron transfer mechanism involved in the bio-catalyzed APS, the rapid transfer of photoexcited electrons from the photocatalysts to M was crucial for the subsequent regeneration of NAD(P)H by receiving electrons from M. It is well known that the recombination of electron–hole pairs emits a strong fluorescence. Otherwise, the fluorescence decreases if the electrons in the CB of the photocatalyst are transferred to another electron acceptor. The photoluminescence spectra in Fig. 5a shows a strong emission peak for BP, which is ascribed to the recombination of the electron–hole pairs. For the AM + BP NSs, the strong photoluminescence quenched slightly, indicating that some excited electrons on the CB of the BP NSs transfer to the VB of the AM NSs through their random contact. For the AM/BP HNSs without assembly with M, a gradual decrease in the fluorescence intensity was observed compared to that for the AM + BP NSs, which indicated that the construction of the Z-scheme AM/BP HNSs through the dual-functionalized polymer, PEI-PEG-C18, improved the direct contact between AM and BP and accelerated the electron transfer from the CB of BP to the VB of AM. Furthermore, the AM/M/BP HNSs with an assembled M shows the lowest fluorescence intensity, demonstrating that M acted as a new electron acceptor, which accepted an electron from the CB of AM and greatly compressed the energy-consuming charge recombination occurring in the BP NSs. For the purpose of demonstrating the retarded recombination of the electron–hole pairs in the AM/M/BP HNSs and the prolonged lifetime of the photogenerated CB-electrons at the AM and VB-holes at the BP, time-resolved fluorescence decay spectra were recorded (Fig. 5b). The excited state electron radioactive decay lifetime curves show that AM/M/BP HNSs has the longest fluorescence lifetime (8.51 ns) compared with that of AM/BP HNSs without being assembled with M (5.66 ns), AM + BP (4.21 ns), and BP (2.04 ns), indicating that the construction of AM/M/BP HNSs through the dual-functionalized polymer, PEI-PEG-C18-M, increased the photoelectron lifetime of AM/M/BP HNSs. This result further certified that AM/M/BP HNSs has the best coordination between AM and BP for the most efficient photoinduced electron transfer. Finally, transient photocurrent responses for pure AM, BP, AM + BP, and AM/BP HNSs were recorded by switching the irradiation light on and off for several cycles to further investigate the charge separation and transfer process of the AM/BP HNSs. As shown in Fig. 5c, a slight photocurrent response was observed when pure AM and BP NSs were irradiated by simulated solar light, which may be attributed to the fast recombination process of the photogenerated electron–hole pairs. The photocurrent density increased after the physically mixed AM and BP NSs were used. The random contact between AM and BP NSs in their mixture could slightly enhance the photocurrent density due to the Z-scheme electron transfer in their interface, which might restrain the recombination of photogenerated electron–hole pairs. Notably, the introduction of the AM/BP HNSs resulted in the highest photocurrent intensity, indicating the most efficient separation of the photogenerated charges. This result agreed with the above photocatalytic NADH regeneration and formic acid conversion performances. In addition, a good correspondence between the photocurrent response spectra and the absorption spectra ranging from 400 nm to 700 nm is exhibited in Fig. S14,† which suggests that the AM/BP HNSs are the source of the photocurrent. The electrochemical conductivity of the photocatalysts is one of the indispensable properties for guaranteeing an efficient photocatalytic performance. Therefore, an electrochemical impedance spectroscopy (EIS) analysis was performed in the frequency range of 105 to 0.1 Hz with an amplitude of 5 mV at a bias potential of 0 V versus Ag/AgCl. Generally, the semicircle at a high frequency in a Nyquist plot corresponds to the charge transfer limiting process and the diameter of the semicircle is equal to the charge transfer resistance (Rct). Fig. 5d exhibits that AM + BP and AM/BP show much lower Rct than bare AM and BP, suggesting the introduction of the Z-scheme electron transfer system at the interface of the AM and BP NSs. Notably, AM/M/BP HNSs assembled with M showed the smallest Rct value, indicating that the overall charge transfer was further improved when M was assembled between the AM and BP layers, which agreed with its good photoelectric response. Based on the above results, all of the photochemical and electrochemical measurements distinctly demonstrate that the AM/M/BP HNSs exhibit much more efficient generation, separation, and transportation of the photoexcited charges.
Conclusions
In summary, the precise arrangement of the AM/M/BP HNS-based Z-scheme APS was constructed via an “amphipathic” polymer covalent cross-linking M to catenate the AM and BP NSs as NADH regeneration catalysts. The integration of NAD(H) and the enzyme between the AM and BP layers was achieved via an electrostatic attraction with the polymer. The chloroplast-mimicking AM/M/BP HNS-based integrated Z-scheme APS not only decreased the transfer distance of the electron and energy among the light-harvest and enzymatic redox systems, but also enabled an efficient charge separation and facilitated the transportation of photogenerated excitons. The AM/M/BP HNS-based Z-scheme APS shows excellent performance over a free and physically mixed system such that the yield of NADH and formic acid improved from 20% and 56 μmol to 89% and 365 μmol, respectively, by using TEOA as the electron donor. Although neither AM nor BP could catalyze NADH regeneration using H2O as the electron donor under visible light, 35 ± 4% regeneration of NADH and 160 ± 24 μmol conversion of formic acid were observed when the AM/M/BP HNS-based Z-scheme APS was used. The effect of the AM/M/BP HNS-based Z-scheme APS was confirmed by the detection of its optical, photochemical, and electrochemical properties. This is the first study describing the use of 2D ultrathin NSs to construct dual-functionalized polymer-mediated HNSs for the Z-scheme NADH photo-regeneration and CO2 conversion via coupling with enzymes. This brand-new concept of a Z-scheme APS will broaden the horizon of exploiting solar energy for sustainable energy resources.Methods
Synthesis of antimonene/Cp*Rh(phen)Cl/black phosphorus hybrid nanosheets (AM/M/BP HNSs)
The preparation processes for AM NSs, BP NSs, and PEI-PEG-C18-M are shown in the ESI.† For the synthesis of AM/M/BP HNSs, AM NSs (10 mg) were dispersed in 100 mL chloroform with 50 mg pre-dissolved PEI-PEG-C18-M. After ultra-sonication for 10 min in an ice bath, chloroform was removed by vacuum rotary evaporation. The AM-PEI-PEG-C18-M NSs samples were dissolved in DMF, centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, and washed with DMF three times to remove the unbound PEI-PEG-C18-M. The final AM-PEG NS samples were re-suspended in water and added into the BP NS DI water solution (10 mg mL−1, 20 mL). After a mild bath sonication for 30 min and stirring for 4 h, the resulting mixture was centrifuged at 10
000 rpm, and washed with DMF three times to remove the unbound PEI-PEG-C18-M. The final AM-PEG NS samples were re-suspended in water and added into the BP NS DI water solution (10 mg mL−1, 20 mL). After a mild bath sonication for 30 min and stirring for 4 h, the resulting mixture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and washed with water three times using the same method. The resulting AM/M/BP HNS sample was resuspended in buffer for further use and stored at 4 °C.
000 rpm and washed with water three times using the same method. The resulting AM/M/BP HNS sample was resuspended in buffer for further use and stored at 4 °C.
Assembly of NAD+ and FateDH on the synthesized AM/M/BP HNSs
The AM/M/BP HNSs (10 mg) were immersed in a PBS buffer solution (5 mL, 100 mM, pH 7.0) containing 5 mg mL−1 FateDH. After absorption for 12 h at 4 °C, the AM/M/BP HNSs were centrifuged and washed several times until no FateDH was detected. To assemble NAD+ with the AM/M/BP HNSs by electrostatic attraction, 10 mg of HNSs assembled with FateDH were immersed into the PBS buffer solution (5 mL, 50 mM, pH 7.0) containing 50 mg mL−1 NAD+ for 24 h at 4 °C. HNSs with the assembled FateDH and NAD+ were centrifuged and washed several times until no NAD+ was detected. The loading amount of FateDH and NAD+ was calculated via mass balance.Photo-regeneration of NADH
A quartz reactor equipped with a 450 W Xenon lamp was used to process the photochemical regeneration of NADH from NAD+. Briefly, AM NSs, BP NSs, AM + BP NSs, or AM/M/BP HNSs were added to a 10 mL buffer solution (100 mM phosphate, pH 7.0) containing 1 mM NAD+ with the same final concentrations of M (0.10 mM) TEOA (15% w/v). Before being exposed to light, the reaction system was incubated in the dark for 1 h. The concentration of NADH regenerated from NAD+ was monitored by measuring the absorbance at 340 nm.Photo-conversion of formic acid from CO2
A quartz reactor equipped with a 450 W Xenon lamp was used to process conversion of formic acid from CO2. Generally, a 10 mL buffer solution (100 mM phosphate, pH 7.0) was bubbled with CO2 gas for 0.5 h and then, a definite amount of each component involved in the artificial photosynthesis systems was added to reach a final concentration of 15 w/v% TEOA, 0.2 mg mL−1 AM NSs or BP NSs, 0.1 mM M, 0.3 units per mL FateDH and 0.1 mM NAD+. The reaction was performed under constant pressure at 0.3 MPa. At certain time intervals, 20 μL of the sample was taken from the reaction mixture for formic acid concentration measurements after pressure release. The conversion of CO2 to formic acid using AM/M/BP NSs was also performed under the same conditions as mentioned above. The formic acid concentration was detected by a gas chromatography system (Agilent 7890A) equipped with a flame ionization detector (FID) and an Agilent HP-FFAP gas column (25 m × 0.320 mm × 0.50 μm).Conflicts of interest
There are no conflicts to declare.Acknowledgements
X. J. and Y. K. contributed equally to this study. This study was supported by the US METAvivor Early Career Investigator Award (W. T.), the National Natural Science Foundation of China (81801826, 81771966, and 21676276), the Natural Science Foundation of Guangdong Province (2016A030310023), and the Science, Technology & Innovation Commission of Shenzhen Municipality (JCYJ20180307153300735).References
- X. Chang, T. Wang and J. Gong, Energy Environ. Sci., 2016, 9, 2177 RSC.
- E. V. Kondratenko, G. Mul, J. Baltrusaitis, G. O. Larrazabal and J. Perez-Ramirez, Energy Environ. Sci., 2013, 6, 3112 RSC.
- S. Wang and X. Wang, Angew. Chem., Int. Ed., 2016, 55, 2308 CrossRef CAS.
- W.-H. Wang, Y. Himeda, J. T. Muckerman, G. F. Manbeck and E. Fujita, Chem. Rev., 2015, 115, 12936 CrossRef CAS PubMed.
- E. Romero, V. I. Novoderezhkin and R. van Grondelle, Nature, 2017, 543, 355 CrossRef CAS PubMed.
- Y. Tachibana, L. Vayssieres and J. R. Durrant, Nat. Photonics, 2012, 6, 511 CrossRef CAS.
- X. Ji, Y. Kang, Z. Su, P. Wang, G. Ma and S. Zhang, ACS Sustainable Chem. Eng., 2018, 6, 3060 CrossRef CAS.
- X. Ji, C. Liu, J. Wang, Z. Su, G. Ma and S. Zhang, J. Mater. Chem. A, 2017, 5, 5511 RSC.
- X. Ji, Z. Su, P. Wang, G. Ma and S. Zhang, Small, 2016, 12, 4753 CrossRef CAS PubMed.
- X. Ji, J. Wang, L. Mei, W. Tao, A. Barrett, Z. Su, S. Wang, G. Ma, J. Shi and S. Zhang, Adv. Funct. Mater., 2018, 28, 1705083 CrossRef.
- D. S. Choi, Y. Ni, E. Fernandez-Fueyo, M. Lee, F. Hollmann and C. B. Park, ACS Catal., 2017, 7, 1563 CrossRef CAS.
- J. Kim, S. H. Lee, F. Tieves, D. S. Choi, F. Hollmann, C. Paul and C. B. Park, Angew. Chem., Int. Ed., 2018, 57, 13825 CrossRef CAS PubMed.
- S. H. Lee, D. S. Choi, S. K. Kuk and C. B. Park, Angew. Chem., Int. Ed., 2018, 57, 7958 CrossRef CAS PubMed.
- Y. Wu, J. Ward-Bond, D. Li, S. Zhang, J. Shi and Z. Jiang, ACS Catal., 2018, 8, 5664 CrossRef CAS.
- P. Zeng, X. Ji, Z. Su and S. Zhang, RSC Adv., 2018, 8, 20557 RSC.
- J. Low, J. Yu, M. Jaroniec, S. Wageh and A. A. Al-Ghamdi, Adv. Mater., 2017, 29, 1601694 CrossRef PubMed.
- G. Yang, D. Chen, H. Ding, J. Feng, J. Z. Zhang, Y. Zhu, S. Hamid and D. W. Bahnemann, Appl. Catal., B, 2017, 219, 611 CrossRef CAS.
- Y. Tachibana, L. Vayssieres and J. R. Durrant, Nat. Photonics, 2012, 6, 511 CrossRef CAS.
- H. Li, W. Tu, Y. Zhou and Z. Zou, Adv. Sci., 2016, 3, 1500389 CrossRef PubMed.
- K. Maeda, ACS Catal., 2013, 3, 1486 CrossRef CAS.
- A. Iwase, S. Yoshino, T. Takayama, Y. H. Ng, R. Amal and A. Kudo, J. Am. Chem. Soc., 2016, 138, 10260 CrossRef CAS PubMed.
- D. Lu, H. Wang, X. Zhao, K. K. Kondamareddy, J. Ding, C. Li and P. Fang, ACS Sustainable Chem. Eng., 2017, 5, 1436 CrossRef CAS.
- L. J. Zhang, S. Li, B. K. Liu, D. J. Wang and T. F. Xie, ACS Catal., 2014, 4, 3724 CrossRef CAS.
- Z. Zhang, J. Huang, Y. Fang, M. Zhang, K. Liu and B. Dong, Adv. Mater., 2017, 29, 1500389 Search PubMed.
- Y. Liu, X. Duan, Y. Huang and X. Duan, Chem. Soc. Rev., 2018, 47, 6388 RSC.
- W. Tao, N. Kong, X. Ji, Y. Zhang, A. Sharma, J. Ouyang, B. Qi, J. Wang, N. Xie, C. Kang, H. Zhang, O. C. Farokhzad and J. S. Kim, Chem. Soc. Rev., 2019, 48, 2891 RSC.
- L. Li, Y. Yu, G. J. Ye, Q. Ge, X. Ou, H. Wu, D. Feng, X. H. Chen and Y. Zhang, Nat. Nanotechnol., 2014, 9, 372 CrossRef CAS PubMed.
- J. Kim, S. S. Baik, S. H. Ryu, Y. Sohn, S. Park, B.-G. Park, J. Denlinger, Y. Yi, H. J. Choi and K. S. Kim, Science, 2015, 349, 723 CrossRef CAS PubMed.
- B. Deng, V. Tran, Y. Xie, H. Jiang, C. Li, Q. Guo, X. Wang, H. Tian, S. J. Koester, H. Wang, J. J. Cha, Q. Xia, L. Yang and F. Xia, Nat. Commun., 2017, 8, 14474 CrossRef CAS PubMed.
- M. Pumera and Z. Sofer, Adv. Mater., 2017, 29, 1605299 CrossRef PubMed.
- J. Ji, X. Song, J. Liu, Z. Yan, C. Huo, S. Zhang, M. Su, L. Liao, W. Wang, Z. Ni, Y. Hao and H. Zeng, Nat. Commun., 2016, 7, 13352 CrossRef CAS PubMed.
- W. Tao, X. Ji, X. Xu, M. A. Islam, Z. Li, S. Chen, P. E. Saw, H. Zhang, Z. Bharwani, Z. Guo, J. Shi and O. C. Farokhzad, Angew. Chem., Int. Ed., 2017, 56, 11896 CrossRef CAS PubMed.
- X. Ji, J. Wang, Y. Kang, L. Mei, Z. Su, S. Wang, G. Ma, J. Shi and S. Zhang, ACS Catal., 2018, 8, 10732 CrossRef CAS.
- M. Zhu, S. Kim, L. Mao, M. Fujitsuka, J. Zhang, X. Wang and T. Majima, J. Am. Chem. Soc., 2017, 139, 13234 CrossRef CAS PubMed.
- W. Tao, X. Ji, X. Zhu, L. Li, J. Wang, Y. Zhang, P. E. Saw, W. Li, N. Kong, M. A. Islam, T. Gan, X. Zeng, H. Zhang, M. Mahmoudi, G. J. Tearney and O. C. Farokhzad, Adv. Mater., 2018, 33, 1802061 CrossRef PubMed.
- K. K. Sakimoto, A. B. Wong and P. Yang, Science, 2016, 351, 74 CrossRef CAS PubMed.
- C. Liu, J. J. Gallagher, K. K. Sakimoto, E. M. Nichols, C. J. Chang, M. C. Y. Chang and P. Yang, Nano Lett., 2015, 15, 3634 CrossRef CAS PubMed.
- H. Zhang, H. Liu, Z. Tian, D. Lu, Y. Yu, S. Cestellos-Blanco, K. K. Sakimoto and P. Yang, Nat. Nanotechnol., 2018, 13, 900 CrossRef CAS PubMed.
- C. Wang, Z. Sun, Y. Zheng and Y. H. Hu, J. Mater. Chem. A, 2019, 7, 865 RSC.
- S. Schlager, A. Fuchsbauer, M. Haberbauer, H. Neugebauer and N. S. Sariciftci, J. Mater. Chem. A, 2017, 5, 2429 RSC.
- F. Wang, M. Wei, D. G. Evans and X. Duan, J. Mater. Chem. A, 2016, 4, 5773 RSC.
- X. Ji, Z. Su, P. Wang, G. Ma and S. Zhang, ACS Nano, 2015, 9, 4600 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta11167k |
| This journal is © The Royal Society of Chemistry 2020 |