Non-elastic glassy coating with fouling release and resistance abilities†
Runze
Chen
,
Qingyi
Xie
,
Haohang
Zeng
,
Chunfeng
Ma
 * and
Guangzhao
Zhang
* and
Guangzhao
Zhang
 *
*
Faculty of Materials Science and Engineering, South China University of Technology, Guangzhou 510640, P. R. China. E-mail: msmcf@scut.edu.cn
First published on 25th November 2019
Abstract
Fouling release coatings are receiving growing attention because they are eco-friendly, where their elasticity plays a critical role in detaching biofouling. In this study, a non-elastic organic–inorganic hybrid coating is prepared by a facile sol–gel process by mixing the silane-terminated telomer of dodecafluoroheptyl methacrylate (DFMA) and poly(ethylene glycol) methyl ether methacrylate (PEGMA), triethoxyoctylsilane (KH832) with tetraethyl orthosilicate (TEOS). Such a coating with a tethered amphiphilic telomer is transparent and glassy. It also exhibits excellent adhesion to the substrate and high hardness, which can effectively prevent mechanical damage and also inhibit the rooting of fouling organisms. In particular, the coating has remarkable fouling release performance, even though it has a high modulus. This study clarifies that the low surface energy (∼20 mJ m−2) and low surface roughness (<3 nm) are responsible for the fouling release ability of the non-elastic coating. Moreover, the coating surface covered with non-leaching amphiphilic side chains can effectively inhibit the adhesion of marine bacteria, biofilms and diatoms. This robust hybrid coating with fouling release and fouling resistance ability is promising for use in anti-biofouling.
Introduction
Biofouling often has a negative impact on maritime industries.1 In the past few years, a number of physical and chemical approaches have been used to prevent the accumulation of fouling organisms. Using antifouling coatings containing toxic metals and organic compounds is the most effective strategy.2,3 Yet, as more and more attention is paid to marine ecological problems, there is an urgent need to develop environmentally friendly antifouling systems.As eco-friendly antifouling systems, polydimethylsiloxane (PDMS) elastomers have received increasing interest owing to their fouling release and drag-reducing abilities.4,5 Without antifoulant released, they are capable of detaching fouling organisms from surfaces with the help of an external shear force, which is attributed to their low surface energy and low modulus.6,7 However, they have weak mechanical properties, such as poor adhesion and low hardness, which make them prone to peeling from the substrate and damage.8 Besides, PDMS elastomers have poor antifouling efficacy under static conditions without strong water flow.9–11 So far, much effort has been made to improve the mechanical and antifouling properties of PDMS. For instance, introducing urea/urethane segments into PDMS elastomers can significantly increase the adhesion strength,12,13 and chemically immobilizing biocidal moieties, such as antifoulants and amphiphiles, on the elastomer surfaces can effectively enhance the static antifouling ability.14–16 However, these modifications cannot simultaneously optimize the mechanical, fouling release and fouling resistance properties.
A sol–gel procedure has been used to prepare glassy organic–inorganic hybrid materials for anti-corrosion, biomedical and light-emitting applications.17–20 The sol–gel hybrid coating can cure at room temperature yet exhibits high chemical stability and good mechanical properties.21,22 Furthermore, the hybrid coating has low surface energy upon introducing a silica-based structure. Therefore, sol–gel hybrid coatings are expected to develop into eco-friendly antifouling materials. Actually, attempts have been made by Detty and his group to develop sol–gel hybrid coatings into fouling release coatings via mixing various silanes.23–26 By studying the effects of chemical composition, surface energy and surface topography on the fouling release performance, they developed a hard coating consisting of n-octyltriethoxysilane (C8) and tetraethoxysilane (TEOS), which has higher removal rates for algae and juvenile barnacles compared with those of glass or PDMS elastomer. However, this coating is brittle when it forms a thick film and is prone to damage. Meanwhile, such a coating has limited fouling resistance owing to the lack of antifouling moieties, particularly under static conditions. PEG is a well-known fouling-resistant material that has reached the marine antifouling industry in recent years.27–29 The stability of PEG in seawater is important for its application in marine coatings and many previous reports have focused on it.30–33 A long-term exposure test in real ocean conditions indicated that PEG remains stable in a hydrophobic PDMS matrix after 2.5 years, which makes PEG promising for improving the fouling resistance of hydrophobic silica-based matrices.
In the present study, we have synthesized an amphiphilic telomer by telomerization of dodecafluoroheptyl methacrylate (DFMA), poly(ethylene glycol) methyl ether methacrylate (PEGMA) and 3-mercaptopropyl triethoxysilane (KH580). By mixing the telomer with triethoxyoctylsilane (KH832) and tetraethyl orthosilicate (TEOS), we prepared an organic–inorganic hybrid coating by tethering with an amphiphilic telomer via sol–gel procedure. The coating is transparent and glassy, so it can be applied to various substrates, including underwater windows or cameras. Moreover, such a coating with rigid inorganic and flexible organic phases has high toughness, adhesion strength to substrate and modulus. Unlike PDMS elastomer, the non-elastic coating exhibits excellent fouling release property owing to the low surface energy and high surface smoothness. Besides, the non-leaching telomer can self-enrich on the coating surface, endowing the coating with fouling resistance ability. We have examined its adhesion and hardness to evaluate the robustness. The fouling release property and mechanism were studied via the elastic modulus, surface energy and surface roughness. Furthermore, the fouling-resistant properties were evaluated by antibacterial, anti-biofilm and anti-diatom assays. Our aim is to develop a high-performance eco-friendly antifouling coating.
Results and discussion
Scheme 1 shows the synthesis of the silane-terminated telomer of dodecafluoroheptyl methacrylate (DFMA) and poly(ethylene glycol) methyl ether methacrylate (PEGMA) and the preparation of the organic–inorganic hybrid coating by facile sol–gel procedure, where the feeding molar ratio of the telomer is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (KH580/DFMA/PEGMA). For convenience, the silane-terminated telomer of DFMA and PEGMA is designated as FP. To extract the roles of PEG and DFMA, another two telomers were prepared. Following the same protocol, one telomer without DFMA was synthesized at a molar ratio of 1
2 (KH580/DFMA/PEGMA). For convenience, the silane-terminated telomer of DFMA and PEGMA is designated as FP. To extract the roles of PEG and DFMA, another two telomers were prepared. Following the same protocol, one telomer without DFMA was synthesized at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (KH580/PEGMA) and designated as P. Another telomer without PEGMA was prepared at a molar ratio of 1
4 (KH580/PEGMA) and designated as P. Another telomer without PEGMA was prepared at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (KH580/DFMA) and designated as F. The telomer was synthesized by radical polymerization with 3-mercaptopropyl triethoxysilane as the chain transfer reagent, which can introduce a silane group at one end and control the molecular weight.34,35 The successful synthesis of each telomer was determined by proton nuclear magnetic resonance spectroscopy (1H NMR, Fig. S1–S3†). The glass transition temperature (Tg) of the telomer was also determined by differential scanning calorimetry (DSC, Fig. S4†). As shown in Table S2,† the Tg of F is ∼34.6 °C, but that of FP is ∼7.8 °C, indicating that the incorporation of PEGMA decreases the rigidity of the telomer. Note that the Tg of P is hard to observe, which is probably attributed to its considerably low Tg.36 More detailed characterization data can be found in Tables S1 and S2.† FP was subsequently grafted to a hybrid matrix. The hybrid coating was designated as HC-FP-x, where x represents the weight percentage of FP/(TEOS-KH832) in each coating (x = 0, 5, 10 and 15).
4 (KH580/DFMA) and designated as F. The telomer was synthesized by radical polymerization with 3-mercaptopropyl triethoxysilane as the chain transfer reagent, which can introduce a silane group at one end and control the molecular weight.34,35 The successful synthesis of each telomer was determined by proton nuclear magnetic resonance spectroscopy (1H NMR, Fig. S1–S3†). The glass transition temperature (Tg) of the telomer was also determined by differential scanning calorimetry (DSC, Fig. S4†). As shown in Table S2,† the Tg of F is ∼34.6 °C, but that of FP is ∼7.8 °C, indicating that the incorporation of PEGMA decreases the rigidity of the telomer. Note that the Tg of P is hard to observe, which is probably attributed to its considerably low Tg.36 More detailed characterization data can be found in Tables S1 and S2.† FP was subsequently grafted to a hybrid matrix. The hybrid coating was designated as HC-FP-x, where x represents the weight percentage of FP/(TEOS-KH832) in each coating (x = 0, 5, 10 and 15).
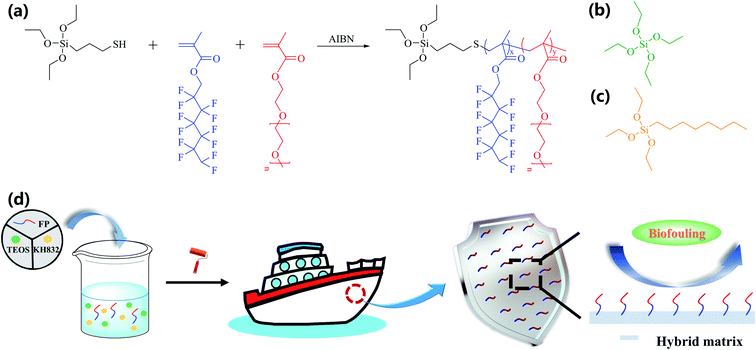 | ||
| Scheme 1 Synthetic route for telomer FP (a). TEOS (b) and KH832 (c). Schematic representation of telomer-tethered organic–inorganic hybrid coating prepared by facile sol–gel procedure (d). | ||
In the following experiment, the solution was dropped onto a glass slide (for transmittance test and adhesion test), PET (for flexibility test), steel (for adhesion test), an epoxy resin fiberglass panel (for adhesion test, nanoindentation, fouling release test, atomic force microscopy, surface wettability and mass loss test), a silicon slide (for antibacterial assay and anti-diatom assay) and a non-treated polystyrene 24-well tissue culture plate (for bacterial biofilm assay). The thickness of each film coated on the PET is ∼50 μm and on others is ∼20 μm, which is controlled by a tetrahedral wet film fabricator and determined by a coating thickness gauge (Phynix, Model Surfix).
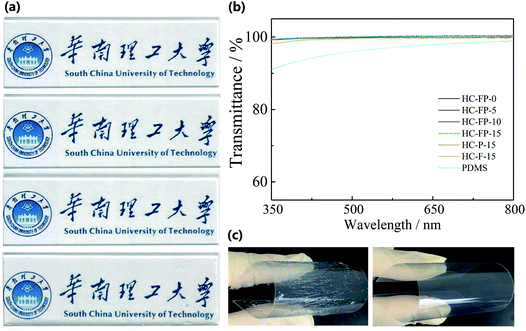
Fig. 1a and b show the transparency of the hybrid coatings. All of the coatings have good optical clarity (Fig. 1a). According to the transmittance spectra of the hybrid coatings (Fig. 1b), all of the hybrid coatings exhibit significantly high transmittance (>99%) from 400 nm to 800 nm, indicating that they have excellent optical clarity. Therefore, the transparent hybrid coatings can be applied in various substrates, such as underwater windows and cameras in the marine environment, without having an adverse effect on the transparency. For antifouling coatings, the thickness is important for their durability in harsh environments. However, previous organic–inorganic hybrid coatings generally cannot form thick coatings since they easily exhibit brittleness. Fig. 1c shows photos of HC-FP-0 and HC-FP-15 coated on PET in the flexibility test. After 100 bending cycles, numerous cracks are visible on the surface of the HC-FP-0 along the vertical direction of stress, indicating the brittleness of HC-FP-0. In contrast, no cracks are observed on the surface of HC-FP-15. This is because the soft PEG moiety acts as a plasticizer and toughens the HC-FP-15, allowing HC-FP-15 to form a thick yet tough coating to meet the requirements of harsh marine environments.
We examined the adhesion of the hybrid coatings containing telomers on an epoxy resin fiberglass panel (Fig. 2a) using an adhesion tester (Fig. 2b). All of the hybrid coatings exhibit adhesion (>0.7 MPa) higher than that of PDMS. This is because the hybrid coating covalently bonds to the substrate via the condensation of silanes with the hydroxyl.37,38 The adhesion of HC-FP-0 is ∼0.85 MPa, yet HC-FP-0 exhibits cohesive failure due to its brittleness. Upon introducing telomer FP, the adhesion is further improved to ∼1.3 MPa with adhesive failure in that the PEG segment serves as a plasticizer and toughens the hybrid coating. This is the reason why HC-P-15 also has higher adhesion than HC-FP-0. Note that the adhesion of HC-F-15 is somewhat lower than that of HC-FP-0, which also shows cohesive failure since the rigid fluorocarbon segment further increases the brittleness of the hybrid coating. Moreover, we also determined the adhesion strength of the hard coatings on steel or glass (Fig. S5†). The coatings on steel exhibit a similar trend to the coatings on the epoxy panel. Note that all HC-FP-x and HC-P-15 coatings on glass have excellent adhesion to the substrate, close to 1.8 MPa. The reason is probably that the surface of glass has much more hydroxyl groups than that of steel or epoxy resin fiberglass panel, and the silane in the hard coating tightly bonds to the surface of the glass.39,40 Anyhow, the hybrid coating containing the telomer FP has excellent adhesion to various substrates, such as metal, glass and fiberglass, which is beneficial for its long-term application in the complicated marine environment.41
To further study the mechanical properties of the coatings, the moduli and hardness of the coatings were determined by nanoindentation. Fig. 2c shows the elastic modulus of each coating. For HC-FP-0, the modulus is ∼290 MPa, owing to its rigid molecular structure. The moduli of HC-FP-5, HC-FP-10 and HC-FP-15 are ∼340 MPa, ∼380 MPa and ∼420 MPa, respectively, indicating that the surface rigidness increases with the FP content, which is attributed to the rigid fluorocarbon moiety. The moduli of HC-P-15 and HC-F-15 changed significantly. Compared to HC-FP-x, HC-P-15 has a lower modulus of ∼110 MPa owing to the presence of the soft PEG segment. For HC-F-15, the modulus is considerably high at ∼1000 MPa owing to the rigid fluorocarbon segment. Note that the modulus of the glassy hybrid coating is orders of magnitude higher than that of PDMS, clearly indicating that it is non-elastic.
Fig. 2d shows the hardness, where higher hardness indicates that the surface of the coating is more difficult to be damaged. The hardness of HC-FP-0 is ∼25 MPa. Moreover, upon introducing FP, the hardness significantly increases as the FP content increases, owing to the synergistic effect of the PEG and DFMA moieties. The hardness of HC-FP-15 is ∼57 MPa, twice as high as that of HC-FP-0, indicating that the surface of HC-FP-x is more robust than that of HC-FP-0. For HC-P-15, the hardness is ∼4 MPa, which is much lower than that of HC-FP-x owing to its low modulus. HC-F-15 has the highest modulus but it has a limited hardness of ∼36 MPa owing to its brittleness. In the marine environment, fouling organisms such as barnacles would puncture the antifouling coatings and thus damage their surfaces, leading to failure of the antifouling resistance. However, the HC-FP-x coating exhibits significantly improved hardness and robustness, which can effectively prevent mechanical damage and extend the duration of its antifouling ability.
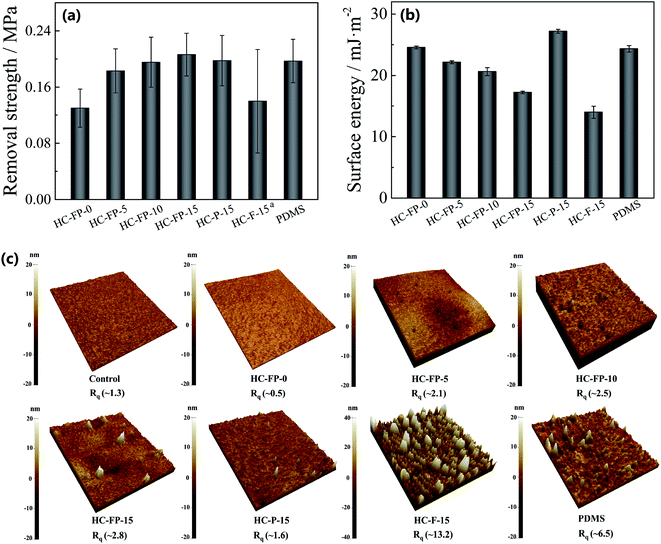
We used pseudobarnacle tests (Fig. 3a) to evaluate the fouling release ability of the coatings, where a lower removal strength represents better fouling release ability. HC-FP-0 has a removal strength of ∼0.14 MPa, lower than that of PDMS,42 indicating its excellent fouling release ability. As the FP content increases, the removal strength of HC-FP-x increases. However, HC-FP-15 still has a removal strength close to that of PDMS. For HC-P-15 and HC-F-15, the removal strengths are ∼0.20 MPa and ∼0.14 MPa, respectively, indicating that all the hybrid coatings have excellent fouling release ability. It is generally recognized that fouling release ability is mainly attributed to low surface energy and low elastic modulus.43,44 Accordingly, the hybrid coatings should have a different fouling release mechanism to elastomer.
To clarify the fouling release mechanism of the non-elastic coatings, we first examined the surface energy (SE) calculated from the water and diiodomethane contact angles (Fig. 3b). The surface energy of PDMS or HC-FP-0 is close to 24 mJ m−2 owing to their similar silica-based structure. After introducing FP, the SE of HC-FP-x gradually decreases as the FP content increases since the DFMA moiety in FP has low surface energy. Owing to the highest content of DFMA moiety, HC-F-15 has the lowest SE of ∼14 mJ m−2, much lower than that of PDMS elastomer. HC-P-15 has a higher SE of ∼27 mJ m−2 because of the presence of high SE PEG groups. As reported before,45 it is difficult for fouling organisms to adhere to a material whose SE is close to the critical surface tension γc (22–24 mJ m−2). The SE of all of the hybrid coatings ranges from 17 to 27 mJ m−2, close to the γc. Therefore, the low SE is critical for fouling release coatings.
We also studied the surface roughness of the non-elastic coatings. As shown in Fig. 3c, the Rq of PDMS is ∼6.5 nm. For HC-FP-0, it has a remarkably low Rq of less than 1 nm, close to the smoothest surface reported before,23,46 indicating its rather smooth surface. With the increase of the FP content, the Rq of HC-FP-x increases from ∼0.5 to ∼2.8 nm, indicating that FP makes the surface of HC-FP-x rougher because of the incompatibility between FP and the hybrid matrix. For HC-P-15, it also has a relatively high Rq of ∼1.6 nm compared to HC-FP-0. HC-F-15 has a considerably high Rq, probably owing to the presence of a rigid fluorocarbon segment. As discussed above, HC-FP-0 exhibits the best fouling release ability and has the smoothest surface among all the samples. Moreover, the fouling release ability of the other hybrid coatings decreases with the increase of surface roughness. Therefore, surface smoothness also plays a critical role in the fouling release of hybrid coatings. In other words, the fouling release of the hybrid coatings is mainly attributed to low surface roughness and low surface energy, which is different from that of PDMS elastomer. As we know, PDMS is an elastic material, but polytetrafluoroethylene (PTFE) is a non-elastic one with a low surface energy of ∼18.6 mJ m−2 and a high surface roughness of ∼270 nm (Rq).47 Compared with PTFE, the hybrid coatings have similar surface energy and modulus but have a remarkably smooth surface reflected by low Rq of less than 3 nm. Therefore, the hybrid coatings would exhibit better fouling release ability than PTFE. Actually, the poor antifouling property of PTFE films has been verified previously owing to its rough surface.7
Fouling resistance properties are necessary for non-elastic coatings under static conditions. Therefore, we introduced a silane-terminated amphiphilic telomer. We first examined whether the hybrid coatings release the grafted amphiphilic telomer by mass loss measurement after immersion in artificial seawater (ASW) for 14 days (Fig. S6†). The mass loss of each HC-FP-x sample is below 0.9 wt%, close to that of PDMS or HC-FP-0, indicating that the telomers are successfully grafted into the hybrid network.
As reported before,48 DFMA would migrate to the surface owing to its low SE, leading to the self-enrichment of the telomer on the coating surface. We examined the self-enriched ability of the coatings by measuring the variation of the water contact angle (WCA, Fig. 4). For HC-FP-15, after being immersed in ASW for 3 days, the WCA decreases significantly and finally reaches ∼80° since PEG comes to the surface under the migration of DFMA. However, HC-P-15 has an unchanged WCA of ∼95°, higher than that of HC-FP-15. This is because the PEG has a higher surface energy than the silica-based matrix, which is prone to be buried in the bulk. Note that the PEG content in HC-FP-15 is only 7.5 wt%, half of that in HC-P-15, yet the surface of HC-FP-15 is more hydrophilic than that of HC-P-15, indicating that PEG chains are enriched on the surface of HC-FP-15. The self-enriched ability was also confirmed by phase images (Fig. S7†) measured by atomic force microscopy (AFM). Discontinuous dark and light regions can be observed on the coating surface, where the dark region represents the hybrid matrix phase and the light region represents the telomer phase. For HC-FP-x, a large number of discontinuous regions can be observed, and the light region increases as the content of FP increases, indicating that the telomer FP migrates to the surface. However, the surface of HC-P-15 is almost even, indicating that most of the PEG segment cannot migrate to the surface of the coating. In other words, the separate PEG segment is prone to be buried in the bulk, yet FP has a trend to migrate to the surface owing to the presence of DFMA. Actually, the phase structure affects the surface roughness, as shown in Fig. 3, where the surface roughness increases with the FP content. As discussed above, the fouling release performance is related to the surface roughness, thus the fouling release ability of the hard coatings decreases with the FP content. Nonetheless, all the hard coatings still have good fouling release ability compared to PDMS.
PEG is a well-known fouling-resistant material, which prevents the adhesion of bacteria instead of killing them.49 To examine the fouling resistance ability of each coating, we selected the marine bacterium Pseudomonas sp. to evaluate the antibacterial property, where Pseudomonas sp. is widely distributed in seawater and has been largely studied as a model bacterium.50–52 As shown in Fig. 5a, the surfaces of the silicon control, PDMS, HC-FP-0 and HC-F-15 are fully covered with bacteria, indicating that they do not have antibacterial ability. However, the amount of adhered bacteria on HC-FP-x decreased with FP content and almost no bacteria can be observed on the surface of HC-FP-15, indicating that the introduction of FP significantly enhances the antibacterial ability of the hybrid coatings owing to the surface-enriched fouling-resistant PEG moiety under the migration of DFMA.53 For HC-P-15, it exhibits limited antibacterial ability. This is understandable because most of the PEG groups are buried in the bulk rather than migrating to the surface.
Fig. 5b and c show the attachment of Pseudomonas sp. biofilm on the hybrid coatings. The absorbance of the silica control is ∼2.6. PDMS and HC-FP-0 have similar absorbance to the control, indicating that they do not have anti-biofilm ability. However, the biofilm growth is significantly inhibited upon the introduction of FP. As the FP content increases, the absorbance of HC-FP-x decreases, indicating the improvement of the anti-biofilm ability. The OD of HC-P-15 is ∼1.9, much higher than that of HC-FP-x since the PEG groups cannot migrate to the surface. For HC-F-15, it has a higher absorbance than HC-P-15 because DFMA itself does not have fouling resistance ability. The inhibition of biofilm growth indicates that telomer FP is enriched on the surface upon the migration of DFMA, which gives HC-FP-x excellent fouling resistance ability.
Fig. 5d and e show the adhesion density of diatom N. incerta on the hybrid coatings. The diatom density of PDMS (∼440 cells per m2) or HC-FP-0 (∼460 cells per m2) is higher than that of the silicon control (∼360 cells per m2), indicating that the diatom favors a hydrophobic surface.54 For HC-FP-x, the diatom density is markedly lower than that of HC-FP-0 with FP content. This result indicates that the surface-enriched telomer is effective in inhibiting the adhesion of diatom. HC-P-15 and HC-F-15 have relatively higher diatom densities compared to HC-FP-x, indicating they have limited anti-diatom ability because the surface does not have fouling-resistant groups. The diatom assay results are in accordance with the anti-bacteria and anti-biofilm results, further indicating that the amphiphilic FP can be enriched on the HC-FP-x and that it can endow the hybrid coating with fouling resistance.
Conclusion
We have developed a non-elastic organic–inorganic hybrid coating tethered with an amphiphilic telomer by a facile sol–gel procedure and the optimized coating formulation in this study is HC-FP-15. The coating is transparent, glassy and robust. The robustness arises from the organic–inorganic hybrid structure. The coating has high hardness, flexibility, adhesion strength and modulus, allowing it to avoid peeling from the substrate and mechanical damage. In particular, the coating exhibits not only fouling release but also fouling resistance abilities. Unlike in the case of PDMS elastomer, the low surface energy and high surface smoothness are responsible for the fouling release ability of the non-elastic coating. The self-enrichment of the amphiphilic telomer facilitates the coating's fouling resistance. The study provides a new strategy for designing antifouling coatings, which is expected to find applications in marine anti-biofouling, medical anti-biofouling and other fields.Experimental section
Details of the experimental procedures are given in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support of the National Natural Science Foundation of China (51573061 and 51673074) and the Fundamental Research Funds for the Central Universities is acknowledged.Notes and references
- I. Banerjee, R. C. Pangule and R. S. Kane, Adv. Mater., 2011, 23, 690 CrossRef CAS PubMed.
- B. Antizar-Ladislao, Environ. Int., 2008, 34, 292 CrossRef CAS PubMed.
- C. Alzieu, Sci. Total Environ., 2000, 258, 99 CrossRef CAS PubMed.
- J. A. Callow and M. E. Callow, Nat. Commun., 2011, 2, 244 CrossRef PubMed.
- M. Lejars, A. Margaillan and C. Bressy, Chem. Rev., 2012, 112, 4347 CrossRef CAS PubMed.
- M. Berglin and P. Gatenholm, J. Adhes. Sci. Technol., 1999, 13, 713 CrossRef CAS.
- R. F. Brady and I. L. Singer, Biofouling, 2000, 15, 73 CrossRef CAS PubMed.
- Q. Y. Xie, J. S. Pan, C. F. Ma and G. Z. Zhang, Soft Matter, 2019, 15, 1087 RSC.
- R. Holland, T. M. Dugdale, R. Wetherbee, A. B. Brennan, J. A. Finlay, J. A. Callow and M. E. Callow, Biofouling, 2004, 20, 323 CrossRef CAS PubMed.
- P. J. Molino, E. Campbell and R. Wetherbee, Biofouling, 2009, 25, 685 CrossRef CAS PubMed.
- P. J. Molino, S. Childs, M. R. Eason Hubbard, J. M. Carey, M. A. Burgman and R. Wetherbee, Biofouling, 2009, 25, 149 CrossRef CAS PubMed.
- C. Liu, Q. Y. Xie, C. F. Ma and G. Z. Zhang, Ind. Eng. Chem. Res., 2016, 55, 6671 CrossRef CAS.
- A. Ekin, D. C. Webster, J. W. Daniels, S. J. Stafslien, F. Cassé, J. A. Callow and M. E. Callow, J. Coat. Technol. Res., 2007, 4, 435 CrossRef CAS.
- Q. Y. Xie, C. F. Ma and G. Z. Zhang, Adv. Mater. Interfaces, 2015, 7, 21030 CrossRef CAS PubMed.
- S. J. Stafslien, D. Christianson, J. Daniels, L. VanderWal, A. Chernykh and B. J. Chisholm, Biofouling, 2015, 31, 135 CrossRef CAS PubMed.
- P. Majumdar, E. N. Lee and B. J. Chisholm, Polymer, 2009, 50, 1124 CrossRef CAS.
- R. B. Figueira, C. J. R. Silva and E. V. Pereira, J. Coat. Technol. Res., 2015, 12, 1 CrossRef CAS.
- G. J. Owens, R. K. Singh, F. Foroutan, M. Alqaysi, C. Han, C. Mahapatra, H. Kim and J. C. Knowles, Prog. Mater. Sci., 2016, 77, 1 CrossRef CAS.
- S. Hofle, M. Bruns and A. Colsmann, Adv. Mater., 2013, 25, 4113 CrossRef PubMed.
- S. Hofacker, M. Mechtel, M. Mager and H. Kraus, Prog. Org. Coat., 2002, 45, 159 CrossRef CAS.
- B. D. Fabes and W. C. Oliver, J. Non-Cryst. Solids, 1990, 348 CrossRef CAS.
- K. K. Jena, T. K. Rout, R. Narayan and K. Raju, Polym. Int., 2012, 61, 1101 CrossRef CAS.
- Y. Tang, J. A. Finlay, G. L. Kowalke, A. E. Meyer, F. V. Bright, M. E. Callow, J. A. Callow, D. E. Wendt and M. R. Detty, Biofouling, 2005, 21, 59 CrossRef CAS PubMed.
- R. Ciriminna, F. V. Bright and M. Pagliaro, ACS Sustainable Chem. Eng., 2015, 3, 559 CrossRef CAS.
- N. Gunari, L. H. Brewer, S. M. Bennett, A. Sokolova, N. D. Kraut, J. A. Finlay, A. E. Meyer, G. C. Walker, D. E. Wendt, M. E. Callow, J. A. Callow, F. V. Bright and M. R. Detty, Biofouling, 2011, 27, 137 CrossRef CAS PubMed.
- S. M. Bennett, J. A. Finlay, N. Gunari, D. D. Wells, A. E. Meyer, G. C. Walker, M. E. Callow, J. A. Callow, F. V. Bright and M. R. Detty, Biofouling, 2010, 26, 235 CrossRef CAS PubMed.
- A. G. Nurioglu, A. C. A. Esteves and G. de With, J. Mater. Chem. B, 2015, 3, 6547–6570 RSC.
- M. Kober and B. Wesslen, J. Appl. Polym. Sci., 1994, 54, 793–803 CrossRef CAS.
- R. Murthy, C. D. Cox, M. S. Hahn and M. A. Grunlan, Biomacromolecules, 2007, 8, 3244–3252 CrossRef CAS PubMed.
- A. Camos Noguer, S. M. Olsen, S. Hvilsted and S. Kill, Prog. Org. Coat., 2017, 106, 77–86 CrossRef CAS.
- A. Camos Noguer, S. M. Olsen, S. Hvilsted and S. Kill, Prog. Org. Coat., 2017, 112, 101–108 CrossRef CAS.
- A. Camos Noguer, R. Latipovb, F. B. Madsena, A. E. Daugaarda, S. Hvilsted, S. M. Olsen and S. Kill, Prog. Org. Coat., 2018, 120, 178–189 CrossRef.
- A. Camos Noguer, S. M. Olsen, S. Hvilsted and S. Kill, J. Coat. Technol. Res., 2016, 13, 567–575 CrossRef CAS.
- C. F. Ma, H. Zhou, B. Wu and G. Z. Zhang, ACS Appl. Mater. Interfaces, 2011, 3, 455–461 CrossRef CAS PubMed.
- J. L. Ma, C. F. Ma and G. Z. Zhang, Langmuir, 2015, 31, 6471–6478 CrossRef CAS PubMed.
- A. Iborra, G. Diaz, D. Lopez, J. M. Giussi and O. Azzaroni, Eur. Polym. J., 2017, 87, 308–317 CrossRef CAS.
- L. L. Hence and J. K. West, Chem. Rev., 1990, 90, 33 CrossRef.
- J. Zhou and J. P. Lucas, Polym, 1999, 40, 5505–5512 CrossRef CAS.
- V. G. Plotnichenko, V. O. Sokolov and E. M. Dianov, Inorg. Mater., 1975, 19, 299–309 Search PubMed.
- M. R. Detty, R. Ciriminna, F. V. Bright and M. Pagliaro, Acc. Chem. Res., 2014, 47, 678–687 CrossRef CAS PubMed.
- S. K. Rath, J. G. Chavan and S. Sasane, Appl. Surf. Sci., 2010, 256, 2440 CrossRef CAS.
- H. H. Zeng, Q. Y. Xie, C. F. Ma and G. Z. Zang, ACS Appl. Polym. Mater., 2019, 1, 1689–1696 CrossRef CAS.
- R. E. Baier, E. G. Shafrin and W. A. Zisman, Science, 1968, 162, 1360 CrossRef CAS PubMed.
- R. Ciriminna, F. V. Bright and M. Pagliaro, ACS Sustainable Chem. Eng., 2015, 3, 559 CrossRef CAS.
- A. G. Nurioglu, A. C. C. Esteves and G. de With, J. Mater. Chem. B, 2015, 3, 6547 RSC.
- L. B. Capeletti, M. B. Cardoso, J. H. Z. Dos Santos and W. He, ACS Appl. Mater. Interfaces, 2016, 8, 27553 CrossRef CAS PubMed.
- C. W. Extrand and Y. Kumagai, J. Colloid Interface Sci., 1997, 191, 378–383 CrossRef CAS PubMed.
- Q. Y. Xie, H. H. Zeng, C. F. Ma and G. Z. Zhang, Adv. Mater. Interfaces, 2019, 1900535 CrossRef.
- H. Keum, J. Y. Kim, B. Yu, S. J. Yu, J. Kim, H. Jeon, D. Y. Lee, S. G. Im and S. Y. Jon, ACS Appl. Mater. Interfaces, 2017, 9, 19736–19745 CrossRef CAS PubMed.
- C. E. Zobell and E. C. Allen, J. Bacteriol., 1935, 23, 239 Search PubMed.
- K. Rasmussen and K. Øtgaard, Water Res., 2003, 37, 519 CrossRef CAS PubMed.
- L. A. Romanenko, M. Uchino, E. Falsen, G. M. Frolova, N. V. Zhukova and V. V. Mikhailov, Int. J. Syst. Evol. Microbiol., 2005, 55, 919 CrossRef CAS PubMed.
- R. Holland, T. M. Dugdale, R. Wetherbee, A. B. Brennan, J. A. Finlay, J. A. Callow and M. E. Callow, Biofouling, 2004, 20, 323 CrossRef CAS PubMed.
- J. A. Finlay, M. E. Callow, L. K. Ista, G. P. Lopez and J. A. Callow, Integr. Comp. Biol., 2002, 42, 1116 CrossRef CAS PubMed; M. S. Selim, M. A. Shenashen, S. A. El-Safty, S. A. Higazy, M. M. Selim, H. Isago and A. Elmarakbi, Prog. Mater. Sci., 2017, 87, 1 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta09794e |
| This journal is © The Royal Society of Chemistry 2020 |