Open Access Article
Open Access ArticleDesign and synthesis of chiral and regenerable [2.2]paracyclophane-based NAD(P)H models and application in biomimetic reduction of flavonoids†
Zhou-Hao
Zhu
ab,
Yi-Xuan
Ding
ab,
Bo
Wu
a and
Yong-Gui
Zhou
 *ac
*ac
aState Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China. E-mail: ygzhou@dicp.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cZhang Dayu School of Chemistry, Dalian University of Technology, Dalian 116024, China
First published on 10th September 2020
Abstract
With the rapid development of biomimetic asymmetric reduction, the demand for efficient chiral and regenerable NAD(P)H models is growing rapidly. Herein, a new class of [2.2]paracyclophane-based chiral and regenerable NAD(P)H models (CYNAMs) was designed and synthesized. The first enantioselective biomimetic reduction of tetrasubstituted alkene flavonoids has been successfully realized through enzyme-like cooperative bifunctional activation, giving chiral flavanones with up to 99% yield and 99% ee.
Introduction
Bionics is an ancient subject which is central to the development of novel agrochemicals, pharmaceuticals and materials. During metabolization in humans, reduced nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleotide phosphate (NADPH) as coenzymes play an important role as hydrogen transporters in almost 400 chemical reactions such as the citric acid cycle, glycolysis and amino acid decomposition.1 Therefore, biomimetic asymmetric reduction based on the coenzyme NAD(P)H has long attracted extensive interest of scientists. As pioneering work in this area, biomimetic asymmetric hydrogenation of heteroaromatics and imines has been achieved with regenerable and achiral dihydrophenanthridine (DHPD, Scheme 1) or its derivatives together with chiral phosphoric acid.2 Very recently, our group have reported a new type of regenerable and planarly chiral ferrocene-based NAD(P)H model (FENAM, Scheme 1).3 Consequently, a biomimetic asymmetric reduction (BMAR) of heteroaromatics, alkenes and imines has been realized using bench-stable simple Lewis acids, Brønsted acids or organic hydrogen bonding catalysts as transfer catalysts.3 In spite of remarkable contribution, an obvious drawback is that the ferrocene skeleton is not resistant to strong Brønsted acids, Lewis acids and oxidative conditions, which limits the further application in biomimetic chemistry based on the coenzyme NAD(P)H. So, the development of new and efficient regenerable NAD(P)H models still requires great attention.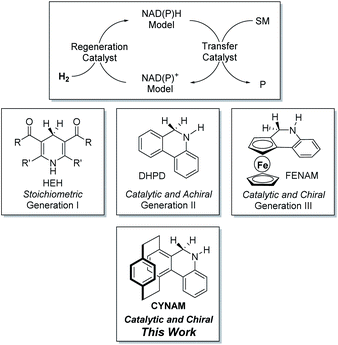 | ||
| Scheme 1 The evolution of NAD(P)H models in biomimetic reduction and our design on chiral and regenerable NAD(P)H models. | ||
As a unique type of privileged planarly chiral skeleton, [2.2]paracyclophane provides an excellent diversified platform for the development of novel and efficient chiral ligands and catalysts.4 Unlike ferrocene, [2.2]paracyclophane is surprisingly resistant to strong Brønsted acids, Lewis acids, oxidative conditions and temperature. Moreover, owing to its rigid structure and high steric bulkiness, the [2.2]paracyclophane backbone has great potential for stereocontrol.4b Furthermore, due to the ready availability and easy modification of the [2.2]paracyclophane skeleton, a series of derivatives can be easily prepared. For example, the phanephos ligand has been commercially applied in a wide range of asymmetric reactions including stereoselective hydrogenation in the synthesis of Aliskiren, which is a hypertension drug.5 Inspired by the achievements based on [2.2]paracyclophane,5,6 we envisioned that the [2.2]paracyclophane-based chiral and regenerable NAD(P)H models should be an excellent candidate. For the chiral and regenerable NAD(P)H models, some requirements must be taken into account: (1) no substituent on the active site (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and CH
N and CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH); otherwise, low reactivity would be observed due to steric hindrance; (2) the CH of active sites must be close to the planar stereogenic center, which is favourable for stereocontrol of hydride transfer. Herein, we designed and synthesized an efficient new class of [2.2]paracyclophane-based NAD(P)H models (abbreviated as CYNAMs) and successfully applied them in biomimetic asymmetric reduction of tetrasubstituted alkene flavonoids with high enantioselectivity (Scheme 1).
NH); otherwise, low reactivity would be observed due to steric hindrance; (2) the CH of active sites must be close to the planar stereogenic center, which is favourable for stereocontrol of hydride transfer. Herein, we designed and synthesized an efficient new class of [2.2]paracyclophane-based NAD(P)H models (abbreviated as CYNAMs) and successfully applied them in biomimetic asymmetric reduction of tetrasubstituted alkene flavonoids with high enantioselectivity (Scheme 1).
Results and discussion
As shown in Scheme 2, the chiral and regenerable [2.2]paracyclophane-based NAD(P)H models (CYNAMs) were conveniently prepared in a five-step sequence. The syntheses started from the known oxime ether (Sp)-1.7 Firstly, selective ortho-halogenation with Pd-catalyzed C–H activation afforded ortho-iodinated oxime (Rp)-2 in 81% yield.7 Secondly, ortho-iodinated oxime (Rp)-2 was converted into the ortho-iodinated aldehyde (Rp)-3 in 94% yield with formaldehyde and p-toluenesulfonic acid. The subsequent reduction provided the alcohol (Rp)-4. Finally, the Suzuki coupling and oxidative cyclization with MnO2 gave a series of [2.2]paracyclophane-based NAD(P)H models (CYNAMs (Sp)-6a–d) in moderate total yields.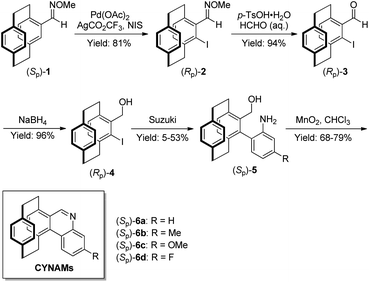 | ||
| Scheme 2 The synthesis of [2.2]paracyclophane-based NAD(P)H models (CYNAMs (Sp)-6) with planar chirality. | ||
With the new chiral NAD(P)H models (CYNAMs (Sp)-6a–d) in hand, we started to evaluate them in the biomimetic asymmetric reduction of alkenes. On one hand, for previous work on biomimetic asymmetric reduction based on NAD(P)H models, their substrate scopes are mostly confined to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds and C
N bonds and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds.2,8 However, highly efficient biomimetic asymmetric reduction of tetrasubstituted alkenes has been challenging for a long time and only a few of examples have been reported in recent years.9 On the other hand, flavanones boast an important structural framework present in many natural products, which manifests a variety of biological characteristics including anti-tumor and anti-inflammatory properties.10 Therefore, the synthesis of chiral flavanones and their derivatives has drawn great attention in the past few years.11 Herein, we attempted to activate the C
O bonds.2,8 However, highly efficient biomimetic asymmetric reduction of tetrasubstituted alkenes has been challenging for a long time and only a few of examples have been reported in recent years.9 On the other hand, flavanones boast an important structural framework present in many natural products, which manifests a variety of biological characteristics including anti-tumor and anti-inflammatory properties.10 Therefore, the synthesis of chiral flavanones and their derivatives has drawn great attention in the past few years.11 Herein, we attempted to activate the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of flavonoids in which a tetrasubstituted alkene is formed through the chelation between the oxygen atom and Lewis acids, which could be used both as a transfer catalyst and as an activator.
C bonds of flavonoids in which a tetrasubstituted alkene is formed through the chelation between the oxygen atom and Lewis acids, which could be used both as a transfer catalyst and as an activator.
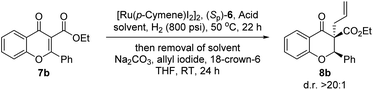
To verify the efficiency of planarly chiral NAD(P)H models, flavonoid 7b was chosen as a model substrate for biomimetic asymmetric reduction (Table 1). Firstly, in the absence of NAD(P)H models, the reduction was carried out in 1,4-dioxane with a ruthenium complex but without any Lewis acids. As expected, there was no desired product obtained (<5% conv., entry 1). When Sm(OTf)3 was added, the reduction still did not occur (<5% conv., entry 2). The above results indicated that the reaction did not undergo the process of hydrogenation. After adding NAD(P)H models, the reduction was carried out with a ruthenium complex as the regeneration catalyst but without any Lewis acids. In accordance with our expectation, there was no desired product obtained which demonstrated that Lewis acids played an important role in hydrogen transfer and activation (<5%, entry 3). To our delight, when the Lewis acid Sm(OTf)3 was added, the reductive product can be obtained. Unfortunately, the reductive product was formed as a mixture of cis and trans diastereomers which has a tendency to racemize.12 Satisfyingly, we eventually found that the in situ alkylation with allyl groups delivered highly enantioenriched stable flavanone 8b with excellent diastereoselectivity (d.r. > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and yield. A quaternary stereogenic center can be built smoothly at the same time. Next, we started to investigate the optimal reaction conditions. In the following optimization studies, when the Lewis acid Yb(OTf)3 was added, the desired product was obtained in moderate conversion (73%) and 93% enantioselectivity (entry 4), indicating the high efficiency of this novel NAD(P)H model (CYNAM). Finally, the evaluation of Lewis acids suggested that Sm(OTf)3 was optimal with regard to enantioselectivity and conversion (entries 5–7). With the transfer catalyst Sm(OTf)3, we further optimized the reaction conditions and found that the conversion could be further improved to 94% by changing the solvent from 1,4-dioxane to ethyl acetate while still maintaining the enantioselectivity (93%, entries 7–11). Conversion and enantioselectivity could be improved to >95% and 95% ee respectively using NAD(P)H models (CYNAMs (Sp)-6c) (entries 11–14). An excellent isolated yield of 8b (0.15 mmol) was achieved by prolonging the reaction time from 22 h to 24 h (99% yield, entry 15). Thus, the optimal reaction conditions were identified: flavonoids 7 (1.0 equiv.), [Ru(p-cymene)I2]2 (0.5 mol%), CYNAM (Sp)-6c (10 mol%), achiral transfer catalyst Lewis acid Sm(OTf)3 (20 mol%), H2 (800 psi), ethyl acetate and 50 °C.
1) and yield. A quaternary stereogenic center can be built smoothly at the same time. Next, we started to investigate the optimal reaction conditions. In the following optimization studies, when the Lewis acid Yb(OTf)3 was added, the desired product was obtained in moderate conversion (73%) and 93% enantioselectivity (entry 4), indicating the high efficiency of this novel NAD(P)H model (CYNAM). Finally, the evaluation of Lewis acids suggested that Sm(OTf)3 was optimal with regard to enantioselectivity and conversion (entries 5–7). With the transfer catalyst Sm(OTf)3, we further optimized the reaction conditions and found that the conversion could be further improved to 94% by changing the solvent from 1,4-dioxane to ethyl acetate while still maintaining the enantioselectivity (93%, entries 7–11). Conversion and enantioselectivity could be improved to >95% and 95% ee respectively using NAD(P)H models (CYNAMs (Sp)-6c) (entries 11–14). An excellent isolated yield of 8b (0.15 mmol) was achieved by prolonging the reaction time from 22 h to 24 h (99% yield, entry 15). Thus, the optimal reaction conditions were identified: flavonoids 7 (1.0 equiv.), [Ru(p-cymene)I2]2 (0.5 mol%), CYNAM (Sp)-6c (10 mol%), achiral transfer catalyst Lewis acid Sm(OTf)3 (20 mol%), H2 (800 psi), ethyl acetate and 50 °C.
| Entry | Acid | Solvent | Model | Conv.b (%) | eec (%) |
|---|---|---|---|---|---|
| a Reactions were carried with 7b (0.10 mmol), [Ru(p-cymene)I2]2 (0.5 mol%), (Sp)-6 (10 mol%), Lewis acid (20 mol%), solvent (2 mL), H2 (800 psi), 50 °C, and 22 h; Na2CO3 (2.0 equiv.), allyl iodide (2.0 equiv.), 18-crown-6 (15 mol%), THF (2 mL), RT, and 24 h. b Conversion and diastereoselectivity were measured by the analysis of 1H NMR spectra. c Determined by chiral HPLC. d Isolated yield for the reaction with a 0.15 mmol scale and 24 h in the step of reduction. | |||||
| 1 | — | 1,4-Dioxane | — | <5 | — |
| 2 | Sm(OTf)3 | 1,4-Dioxane | — | <5 | — |
| 3 | — | 1,4-Dioxane | (Sp)-6a | <5 | — |
| 4 | Yb(OTf)3 | 1,4-Dioxane | (Sp)-6a | 73 | 93 |
| 5 | Zn(OTf)2 | 1,4-Dioxane | (Sp)-6a | 9 | 92 |
| 6 | La(OTf)3 | 1,4-Dioxane | (Sp)-6a | 26 | 92 |
| 7 | Sm(OTf)3 | 1,4-Dioxane | (Sp)-6a | 86 | 94 |
| 8 | Sm(OTf)3 | THF | (Sp)-6a | 74 | 93 |
| 9 | Sm(OTf)3 | CHCl3 | (Sp)-6a | 89 | 65 |
| 10 | Sm(OTf)3 | Toluene | (Sp)-6a | 78 | 88 |
| 11 | Sm(OTf)3 | EA | (Sp)-6a | 94 | 93 |
| 12 | Sm(OTf)3 | EA | (Sp)-6b | >95 | 93 |
| 13 | Sm(OTf)3 | EA | (Sp)-6c | >95 | 95 |
| 14 | Sm(OTf)3 | EA | (Sp)-6d | 47 | 93 |
| 15 | Sm(OTf)3 | EA | (Sp)-6c | 99d | 95 |
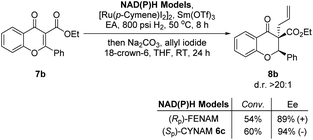
Encouraged by the above results, we next compared the catalytic properties between [2.2]paracyclophane-derived (Sp)-6c and ferrocene-derived (Rp)-FENAM, which was developed previously by us.3 With the newly synthesized (Sp)-6c as the catalyst, the model reaction proceeded smoothly under the above optimized conditions in 8 hours, giving the desired product 8b in 94% ee and 60% conversion. Nevertheless, ferrocene-derived (Rp)-FENAM only provided 89% ee and 54% conversion (Scheme 3).
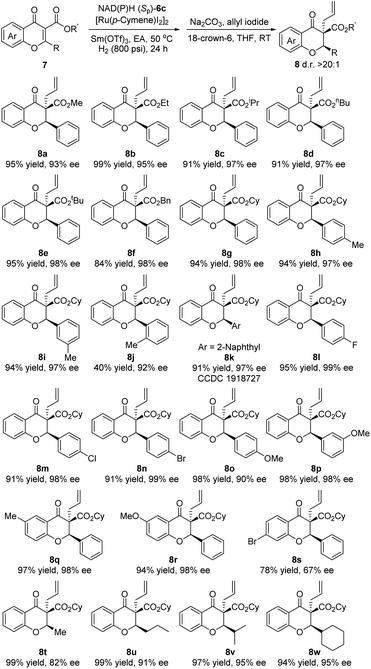
With optimal conditions in hand, the substrate scope of the biomimetic asymmetric reduction of flavonoids 7 using a catalytic amount of CYNAM (Sp)-6c was explored (Table 2). As expected, most flavonoids 7 performed well under the optimized conditions. The different esters were firstly examined in this reaction, and good yields and excellent enantioselectivities were obtained. Among them, the larger the ester is, the higher the enantioselectivity that could be reached, and the reactivity could be maintained at the same time. The best results were obtained for the ester with the cyclohexyl group (8g) and tert-butyl group (8e). Next, the steric and electronic properties of the substituent on the aryl group were explored. Either electron-donating or electron-withdrawing substituents on the aryl group could achieve excellent results (8h and 8i, 8k–8n, and 8p–8r). However, 8o which possesses 4-methoxyphenyl achieved an excellent yield but a slightly lower enantioselectivity. Notably, the substituents on the aryl group in the meta or para-position rarely influence the reaction while o-tolyl (8j) shows a more obvious steric effect, in turn decreasing the product yield and enantioselectivity. Interestingly, for 8s, only a moderate yield and enantioselectivity could be obtained. As for the alkyl-substituted substrates 8t–8w, high reactivity could be obtained. What's more, along with the enhancement of the steric hindrance of alkyl substituents, higher enantioselectivity could be achieved. The absolute configuration of 8k was assigned as (2R,3S) by X-ray diffraction analysis (for the details, see the ESI†).13
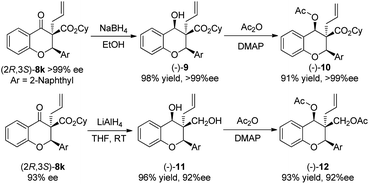
To further gain insight into the utility of the reaction, we attempted to carry out some transformations of the product 8k. First, the selective reduction of the carbonyl group of (2R,3S)-8k (>99% ee) produced the chiral alcohol 9 in 98% yield with high diastereoselectivity (d.r. > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and enantiospecificity (>99% ee). And then, the reduction of the ester and carbonyl groups of (2R,3S)-8k (93% ee) simultaneously produced the chiral diol 11 in 96% yield with high diastereoselectivity (d.r. > 20
1) and enantiospecificity (>99% ee). And then, the reduction of the ester and carbonyl groups of (2R,3S)-8k (93% ee) simultaneously produced the chiral diol 11 in 96% yield with high diastereoselectivity (d.r. > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and enantiospecificity (92% ee). In order to confirm the absolute configuration of 9 and 11, their hydroxyl groups were protected by acetyl groups so that the environment of the hydrogen atoms shown in Scheme 4 can be easily distinguished in 1H NMR. Finally, the absolute configurations were determined by using NOE spectra which are shown in Scheme 4 (for the details, see the ESI†).
1) and enantiospecificity (92% ee). In order to confirm the absolute configuration of 9 and 11, their hydroxyl groups were protected by acetyl groups so that the environment of the hydrogen atoms shown in Scheme 4 can be easily distinguished in 1H NMR. Finally, the absolute configurations were determined by using NOE spectra which are shown in Scheme 4 (for the details, see the ESI†).
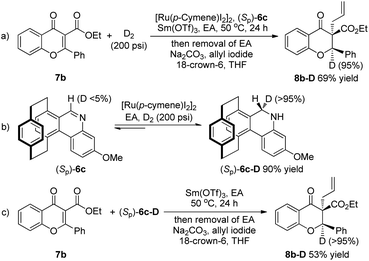
In order to investigate the reaction pathway, several isotopic labelling experiments were carried out, as summarized in Scheme 5. Substrate 7b could be reduced under a D2 atmosphere, affording the reductive deuterium product 8b-D. It was indicated that D2 was the original reductant in the biomimetic reduction reaction (Scheme 5a). Meanwhile, the chiral and regenerable NAD(P)H model (CYNAM (Sp)-6c) could be regenerated with a 90% yield in the presence of D2, and the deuterium atom was added to the less steric face. No deuterium atom incorporation was observed in the recovered NAD(P)H model (Sp)-6c (Scheme 5b). The treatment of substrate 7b with the reductive NAD(P)H model (Sp)-6c-D led to the reductive product 8b-D with 95% deuterium incorporation (Scheme 5c). The result showed that the deuterium incorporated chiral reductive NAD(P)H model (Sp)-6c-D could transfer deuterium to the flavonoid 7b in the presence of a Lewis acid, and the deuterium atom on the less steric face was selectively transferred, leading to good enantioselectivity.
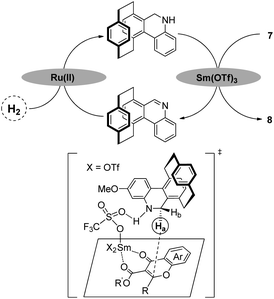
Based on the experimental results and putative mechanism of NAD(P)H model-promoted biomimetic asymmetric reduction,2a a plausible mechanism for the chiral and regenerable [2.2]paracyclophane-based NAD(P)H model-enabled biomimetic asymmetric reduction of flavonoids is illustrated (Scheme 6). At first, the chiral NAD(P)H model (Sp)-6 could be reduced to (Sp)-6-Hin situ by a ruthenium complex and hydrogen gas. Then, the reduced (Sp)-6-H could realize enantioselective reduction of flavonoids through coordination activation in the presence of a simple transfer catalyst Lewis acid (Scheme 6).
Conclusions
In conclusion, we have successfully designed and synthesized a new class of chiral and regenerable NAD(P)H models (CYNAMs) based on a planarly chiral [2.2]paracyclophane skeleton, and successfully applied them to biomimetic asymmetric reduction of flavonoids. A broad range of highly enantiomerically enriched flavanones bearing a quaternary carbon could be conveniently prepared with up to 99% yield and 99% ee. Efforts are underway to expand the applications of CYNAMs to other transformations in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (21532006 and 21690074) and the Chinese Academy of Sciences (XDB17020300) for financial support.References
- (a) B. J. Wallar and J. D. Lipscomb, Chem. Rev., 1996, 96, 2625 CrossRef; (b) J. Gebicki, A. Marcinek and J. Zielonka, Acc. Chem. Res., 2004, 37, 379 CrossRef CAS; (c) H. Lin, Org. Biomol. Chem., 2007, 5, 2541 RSC; (d) R. H. Houtkooper, C. Cantó, R. J. Wanders and J. Auwerx, Endocr. Rev., 2010, 31, 194 CrossRef CAS; (e) H. Wu, C. Tian, X. Song, C. Liu, D. Yang and Z. Jiang, Green Chem., 2013, 15, 1773 RSC; (f) C. T. Walsh, B. P. Tu and Y. Tang, Chem. Rev., 2018, 118, 1460 CrossRef.
- (a) Q.-A. Chen, K. Gao, Y. Duan, Z.-S. Ye, L. Shi, Y. Yang and Y.-G. Zhou, J. Am. Chem. Soc., 2012, 134, 2442 CrossRef; (b) L.-Q. Lu, Y. Li, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 8382 ( Angew. Chem. , 2013 , 125 , 8540 ) CrossRef CAS; (c) Z.-P. Chen, M.-W. Chen, R.-N. Guo and Y.-G. Zhou, Org. Lett., 2014, 16, 1406 CrossRef CAS; (d) L.-Q. Lu, Y. Li, K. Junge and M. Beller, J. Am. Chem. Soc., 2015, 137, 2763 CrossRef CAS; (e) M.-W. Chen, B. Wu, Z.-P. Chen, L. Shi and Y.-G. Zhou, Org. Lett., 2016, 18, 4650 CrossRef CAS.
- (a) J. Wang, Z.-H. Zhu, M.-W. Chen, Q.-A. Chen and Y.-G. Zhou, Angew. Chem., Int. Ed., 2019, 58, 1813 ( Angew. Chem. , 2019 , 131 , 1827 ) CrossRef; (b) J. Wang, Z.-B. Zhao, Y. Zhao, G. Luo, Z.-H. Zhu, Y. Luo and Y.-G. Zhou, J. Org. Chem., 2020, 85, 2355 CrossRef CAS.
- (a) S. Bräse, S. Dahmen, S. Höfener, F. Lauterwasser, M. Kreis and R. E. Ziegert, Synlett, 2004, 15, 2647 CrossRef; (b) Z. Hassan, E. Spuling, D. M. Knoll, J. Lahann and S. Bräse, Chem. Soc. Rev., 2018, 47, 6947 RSC; (c) C. Hicks, B. Duffy and G. C. Hargaden, Org. Chem. Front., 2014, 1, 716 RSC; (d) J. Paradies, Synthesis, 2011, 23, 3749 CrossRef; (e) A. A. Aly and A. B. Brown, Tetrahedron, 2009, 65, 8055 CrossRef.
- E. Cini, L. Banfi, G. Barreca, L. Carcone, L. Malpezzi, F. Manetti, G. Marras, M. Rasparini, R. Riva, S. Roseblade, A. Russo, M. Taddei, R. Vitale and A. Zanotti-Gerosa, Org. Process Res. Dev., 2016, 20, 270 CrossRef.
- E. Xie, S. Huang and X. Lin, Org. Lett., 2019, 21, 3682 CrossRef CAS.
- J. J. P. Kramer, C. Yildiz, M. Nieger and S. Bräse, Eur. J. Org. Chem., 2014, 2014, 1287 CrossRef CAS.
- (a) N. Kanomata and T. Nakata, J. Am. Chem. Soc., 2000, 122, 4563 CrossRef CAS; (b) N.-X. Wang and J. Zhao, Synlett, 2007, 18, 2785 CrossRef; (c) C.-B. Bai, N.-X. Wang, Y. Xing and X.-W. Lan, Synlett, 2017, 28, 402 CAS; (d) S. G. Ouellet, A. M. Walji and D. W. C. Macmillan, Acc. Chem. Res., 2007, 40, 1327 CrossRef CAS; (e) M. Rueping, J. Dufour and F. R. Schoepke, Green Chem., 2011, 13, 1084 RSC; (f) C. Zheng and S.-L. You, Chem. Soc. Rev., 2012, 41, 2498 RSC; (g) A. M. F. Phillips and A. J. L. Pombeiro, Org. Biomol. Chem., 2017, 15, 2307 RSC; (h) C. Zhu, K. Saito, M. Yamanaka and T. Akiyama, Acc. Chem. Res., 2015, 48, 388 CrossRef CAS; (i) C. Zhu and T. Akiyama, Org. Lett., 2009, 11, 4180 CrossRef CAS; (j) C. Zhu and T. Akiyama, Adv. Synth. Catal., 2010, 352, 1846 CrossRef CAS; (k) A. Henseler, M. Kato, K. Mori and T. Akiyama, Angew. Chem., Int. Ed., 2011, 50, 8180 ( Angew. Chem. , 2011 , 123 , 8330 ) CrossRef CAS; (l) T. Sakamoto, K. Mori and T. Akiyama, Org. Lett., 2012, 14, 3312 CrossRef CAS.
- For the selected reviews and papers on asymmetric hydrogenation of alkenes, see: (a) S. Kraft, K. Ryan and R. B. Kargbo, J. Am. Chem. Soc., 2017, 139, 11630 CrossRef; (b) X. Cui and K. Burgess, Chem. Rev., 2005, 105, 3272 CrossRef; (c) M. V. Troutman, D. H. Appella and S. L. Buchwald, J. Am. Chem. Soc., 1999, 121, 4916 CrossRef; (d) M. G. Schrems, E. Neumann and A. Pfaltz, Angew. Chem., Int. Ed., 2007, 46, 8274 ( Angew. Chem. , 2007 , 119 , 8422 ) CrossRef; (e) Y.-T. Liu, J.-Q. Chen, L.-P. Li, X.-Y. Shao, J.-H. Xie and Q.-L. Zhou, Org. Lett., 2017, 19, 3231 CrossRef; (f) Y.-T. Liu, L.-P. Li, J.-H. Xie and Q.-L. Zhou, Angew. Chem., Int. Ed., 2017, 56, 12708 ( Angew. Chem. , 2017 , 129 , 12882 ) CrossRef; (g) Q. Wang, W. Huang, H. Yuan, Q. Cai, L. Chen, H. Lv and X. Zhang, J. Am. Chem. Soc., 2014, 136, 16120 CrossRef CAS.
- (a) The Flavonoids: Advances in Research Since 1980, ed. J. B. Harborne, Chapman and Hall, New York, 1988 Search PubMed; (b) J. B. Harborne and C. A. Williams, Nat. Prod. Rep., 1995, 12, 639 RSC; (c) L. C. Chang and A. D. Kinghorn, in Bioactive Compounds from Natural Sources: Isolation Characterisation and Biological Properties, ed. C. Tringali, Taylor & Francis Ltd., London, 2001, ch. 5 Search PubMed; (d) Flavonoids: Chemistry, Biochemistry and Applications, ed. M. Andersen and K. R. Markham, Taylor & Francis Ltd., London, 2006 Search PubMed; (e) H. Y. Chen, K. D. Dykstra, E. T. Birzin, K. Frisch, W. Chan, Y. T. Yang, R. T. Mosley, F. DiNinno, S. P. Rohrer, J. M. Schaeffer and M. L. Hammond, Bioorg. Med. Chem. Lett., 2004, 14, 1417 CrossRef.
- (a) H.-J. Kabbe and A. Widdig, Angew. Chem., Int. Ed., 1982, 21, 247 ( Angew. Chem. , 1982 , 94 , 254 ) CrossRef; (b) S. Ramadas and G. L. D. Krupadanam, Tetrahedron: Asymmetry, 2004, 15, 3381 CrossRef; (c) M. K. Brown, S. J. Degrado and A. H. Hoveyda, Angew. Chem., Int. Ed., 2005, 44, 5306 ( Angew. Chem. , 2005 , 117 , 5440 ) CrossRef; (d) K. J. Hodgetts, Tetrahedron, 2005, 61, 6860 CrossRef.
- M. M. Biddle, M. Lin and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 3830 CrossRef.
- CCDC 1918727 contain supplementary crystallographic data of (−)-8k.†.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data of compounds and crystallographic data. CCDC 1918727 (−)-8k. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc04188b |
| This journal is © The Royal Society of Chemistry 2020 |