Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Light-intensity switch enabled nonsynchronous growth of fluorinated raspberry-like nanoparticles†
Shantao
Han
,
Yu
Gu
,
Mingyu
Ma
and
Mao
Chen
 *
*
State Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science, Fudan University, Shanghai 200433, China. E-mail: chenmao@fudan.edu.cn; Web: http://polymaolab.com/
First published on 10th September 2020
Abstract
Raspberry-like (RB) nanoparticles hold potential for diverse applications due to their hierarchical morphology. Here we developed a novel tandem synthetic approach of nonsynchronous growth based on photo-mediated reversible-deactivation radical polymerization, enabling simple, efficient and bottom-up synthesis of RB nanoparticles of uniform sizes at quantitative conversions of fluorinated monomers. Chain transfer agents of different chain lengths, concentrations and chemical compositions were varied to tune the diameter of RB particles. Importantly, fluorinated RB nanoparticles obtained with this method allow facile post modifications via both covalent bond formation and intermolecular physical interactions without disrupting the RB morphology. The facile nature of this method and versatility of the obtained fluorinated RB materials open new opportunities for the development of functional materials using nanoparticles.
Introduction
Nonspherical colloidal particles with anisotropic structures have been widely investigated due to their importance in fabricating functional materials.1 Among them, raspberry-like (RB) particles have gained considerable attention due to their surface roughness, large surface areas and high level of scattering ratios,2,3 leading to applications such as drug delivery,4 catalyst supports,5 photonic materials6 and superhydrophobic coatings.7 While reversible-deactivation radical polymerization (RDRP) has enabled the generation of polymeric particles with various morphologies8–10 ranging from spheres, worms, and vesicles to ordered inverse mesophases through the operationally simple polymerization induced self-assembly (PISA) pathway,11–15 it still remains challenging to generate RB particles based on PISA. Conventionally, RB particles have been obtained via the combination of small corona and large core particles via physical or chemical interactions,16,17 as well as emulsion polymerization.18,19 However, these strategies often suffer from complex operating processes or driving force of multiphase separation. Moreover, while post-synthetic modification represents an attractive approach to functionalize polymeric colloids, its utility for RB particles was confined due to lack of modifiable substituents and requirement of multistep processes.20,21 Therefore, the development of a facile PISA approach to prepare versatile RB particles would provide improved opportunities for materials engineering.The employment of light as a low-cost and environmentally sustainable stimulus in RDRP22–32 (i.e., photoinduced electron transfer–reversible addition-fragmentation chain-transfer (PET–RAFT) polymerization)33–35 has aroused a lot of interest, promoting the preparation of polymeric colloids without intermediate purification.36–39 Motivated by the outstanding physicochemical properties of fluoropolymers,40,41 we have developed photo-RDRP of fluorinated alkenes.42,43 While the fluorine–fluorine (F–F) interaction has been adopted to generate various morphologies,44–47 the preparation of fluorinated RB particles remains unexploited. Recently, kinetic mediation by light intensity in photo-RDRP has been demonstrated by Boyer24 and Miyake.25 We envisioned that by regulating the polymerization rate through visible light, self-assembly of fluorous substances would lead to nonsynchronous growth of successively-generated particles, resulting in polymeric colloids of different sizes, which would fuse to provide the RB morphology.
Herein, we report a one-pot, bottom-up synthesis of fluorinated RB particles through tandem photo-mediated nonsynchronous growth for the first time (Scheme 1), which represents a convenient and practical pathway to control the morphology of nanoparticles via the photo-RDRP of pentafluorostyrene (PFS). Moreover, the poly(PFS) (PPFS) backbone enables post modification of RB nanoparticles via both chemical and physical interactions, as demonstrated by cross-linking, substitution and aggregation-induced emission (AIE) experiments, providing an attractive platform for materials engineering.
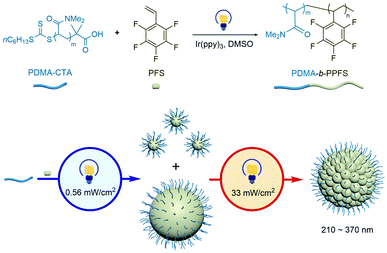 | ||
| Scheme 1 Synthesis of PPFS RB nanoparticles via a tandem photo-mediated approach based on photo-RDRP. | ||
Results and discussion
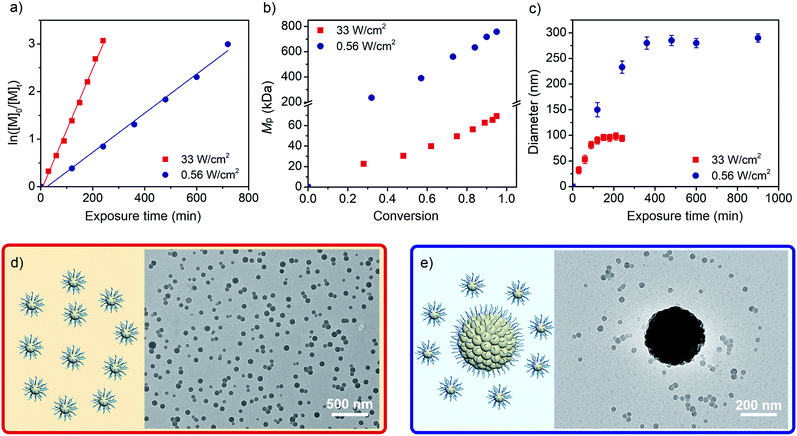
At the beginning of our study, poly(dimethylacrylamide) (PDMA54, Mn = 5.64 × 103 Da, Đ = 1.06) terminated with a trithiocarbonate group was prepared as a chain-transfer agent (CTA), and employed in the photo-RDRP of PFS using tris(2-phenylpyridine)iridium as a photocatalyst (PC) ([PFS]/[PDMA54-CTA]/[PC] = 300/1/0.1) under visible-light irradiation (400–750 nm, Fig. S1†) at room temperature. Dimethyl sulfoxide (DMSO) was adopted as solvent. In the formed copolymers, the PDMA block is solvophilic and the PPFS block is solvophobic. After optimization, light intensities of 33 and 0.56 mW cm−2 have been employed for the strong- and weak-light irradiations, respectively (Table S1†).As shown in Fig. 1a, while the strong-light irradiation enables faster polymerization compared to weak-light (kp = 0.77 vs. 0.25 h−1), both processes exhibit first-order kinetics during propagations. Although a two-stage regime of kinetics has been detected in PISA,48,49 our observations are consistent with RAFT polymerization of PFS under thermal conditions,44 where the absence of the first stage for homogeneous polymerization is attributed to the fast nucleation which occurred at very low degrees of polymerization (DPs) of the fluorinated core-forming block as driven by the F–F interaction.
The reaction mixtures were analyzed by size-exclusion chromatography (SEC). The peak molecular weight (Mp) was adopted to indicate the changing trend of the molar mass of PPFS. The Mp values increase with PFS conversions under both strong- and weak-light irradiations (Mp = 22.7–69.1 vs. 235–746 kDa, Fig. 1b, S2, and S3, Tables S2 and S3†). The much higher Mp values observed for reaction mixtures under weak-light irradiation could be due to the low initiation efficiency of the CTA during the synthesis of the fluoropolymer. Under strong-light irradiation, more CTAs could be initiated, affording lower molecular weights at similar monomer conversions. As analyzed by dynamic light scattering (DLS, Fig. 1c and S4†), the diameters of particles first increase with the exposure time, and remain almost constant subsequently under both conditions. Meanwhile, decreasing the light intensity increases the final size of particles from about 92 to 318 nm. The nonlinear relationship between the colloidal volume and exposure time (Fig. S5†)37 suggests that a secondary nucleation could take place during the photo-RDRP process.
Next, the particles generated under both conditions were characterized by transmission electron microscopy (TEM). As shown in Fig. 1d, spheres of hydration diameter = 90 ± 10 nm were obtained using strong-light irradiation. Similarly, when other ratios of [PFS]/[PDMA54-CTA] (from 100/1 to 500/1) were employed to provide copolymers of different lengths of the fluorinated segment, only a spherical morphology was observed (diameter = 83 to 119 nm, Mp = 24.3–123 kDa, Fig. S6, S7 and Table S4†), which is different from the morphology evolution trend detected in other photo-PISA processes.8,36 In comparison, when weak-light intensity was used under otherwise identical conditions, large particles (341 nm) and a few small spheres (∼30 nm) have been simultaneously observed by TEM at 82% conversion of PFS (Fig. 1e).
Under weak-light irradiation, photoredox catalysis would generate relatively fewer propagating chains and lead to slower monomer consumption rates compared to strong-light irradiation. As a result, a portion of PDMA-CTA would first grow into fluorinated polymers and lead to the generation of fluorous particles via self-assembly. These particles would absorb PFS in solvent, subsequently provide fluoropolymers with high molar masses due to the increased molar ratio of the monomer to growing chain,42 and evolve into large particles during the relatively long-time polymerization. Meanwhile, the reaction between remaining PDMA-CTA and PFS dissolved in solvent would generate new fluorous particles of small sizes, which would have time to further fuse with large ones (Fig. S9†). In contrast, strong-light irradiation at the beginning of the reaction would promote the generation of more propagating chains, and enable high initiation efficiencies of PDMA-CTA (Fig. S2 and Table S2†). The formation of lots of fluorinated chains at the early stage would accelerate the formation of fluorous particles, which don't have enough time or remaining PFS to allow their growth into large ones.
Although the fusion of small and large particles could take place under weak-light irradiation, the remaining PFS absorbed in fused particles would act as plasticizers to increase the mobility of polymers.45 Before the complete consumption of PFS, the slow polymerization rate under weak-light irradiation could provide enough time for polymers to undergo chain movement, which would reduce the surface roughness of fused particles (Fig. S11†). We envisioned that accelerating the monomer consumption at the late-stage of polymerization could benefit maintaining the surface roughness.
Next, we attempted to apply weak- and strong-light irradiation successively to photo-RDRP in a one-pot fashion (Fig. 2a–d). After optimization of the switching timing (Fig. S10†), we found that when the light intensity was changed at high conversions (84–95%) of PFS, RB particles of uniform sizes were successfully obtained (327–341 nm, particle size distribution (PSD) = 1.05–1.14, Table S5†). During the reaction, formation and entanglement of fluoropolymers between different particles could stabilize the fused particles.50
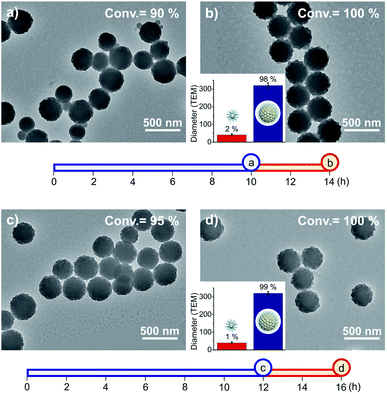 | ||
Fig. 2 TEM images of fluorinated particles prepared by successive photo-RDRP using two light intensities. [PFS]/[PDMA54-CTA]/[PC] = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1. (a and c) Reaction mixtures were exposed to weak-light irradiation for 10 and 12 h, respectively. (b and d) Strong-light irradiation (4 h) was subsequently used following (a) and (c). The size and distribution of particles depicted in Fig. 2b and d were obtained by analysing approximately 400 random particles. The two axes exhibit the exposure times of two light intensities: blue colour for 0.56 mW cm−2, red colour for 33 mW cm−2. 0.1. (a and c) Reaction mixtures were exposed to weak-light irradiation for 10 and 12 h, respectively. (b and d) Strong-light irradiation (4 h) was subsequently used following (a) and (c). The size and distribution of particles depicted in Fig. 2b and d were obtained by analysing approximately 400 random particles. The two axes exhibit the exposure times of two light intensities: blue colour for 0.56 mW cm−2, red colour for 33 mW cm−2. | ||
According to the hypothesis, the fluorous cores should have higher PFS concentrations than that in solvent. Therefore, this method should lead to different populations of molar masses for polymers grown in fluorous cores and generated from CTA and PFS in solvents. When the reaction mixtures were analyzed by SEC, bimodal distributions were observed (Mp = 455–956 and 33.7–51.6 kDa, Fig. S8†), and the proportions of higher Mp values increased with the exposure time.
With the optimized method, RB nanoparticles were synthesized at a variety of ratios of [PFS]/[PDMA54-CTA] (100/1–500/1), and characterized by scanning electron microscopy (SEM). RB particles exhibited a narrow particle size distribution without purification (PSD = 1.05–1.28, Dh = 317 to 368 nm, Table 1, entries 1 to 4). The diameter of RB particles increased with the dosage of PFS relative to the CTA (Fig. 3), attributing to fluoropolymers of higher molar masses.51,52 When the DP of PDMA increased from 28 to 196 ([PFS]/[PDMAn-CTA] = 200/1), the diameter of RB particles decreased from 329 to 211 nm (PSD = 1.10–1.04, entries 2 and 5–7, Fig. S16†).52 When another macro-initiator (i.e., polyethylene glycol (PEG) substituted CTA, entries 8) was employed, RB particles (Dh = 352 nm, PSD = 1.13, Fig. S12c†) were successfully generated, manifesting the versatility of this method. All particles in Table 1 exhibited bimodal molecular weight distributions indicated by SEC (Tables S6, and S7 and Fig. S13–S15†).
| Entry | CTA | [PFS]/[CTA] | D h (nm) | PSDa |
|---|---|---|---|---|
| a Hydration diameter (Dh) and particle size distribution (PSD) of RB particles determined by DLS. b CTA was substituted by PEG113. | ||||
| 1 | PDMA54 | 100/1 | 317 | 1.28 |
| 2 | PDMA54 | 200/1 | 332 | 1.07 |
| 3 | PDMA54 | 300/1 | 347 | 1.07 |
| 4 | PDMA54 | 500/1 | 368 | 1.05 |
| 5 | PDMA28 | 200/1 | 329 | 1.10 |
| 6 | PDMA100 | 200/1 | 276 | 1.08 |
| 7 | PDMA196 | 200/1 | 211 | 1.04 |
| 8 | PEG113b | 200/1 | 352 | 1.13 |
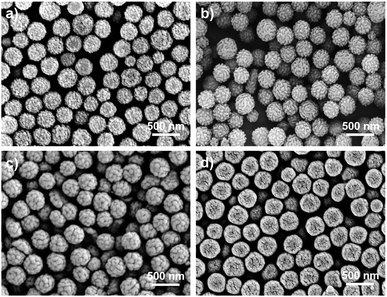 | ||
| Fig. 3 SEM images of RB particles synthesized at [PFS]/[PDMA54-CTA] = (a) 100/1, (b) 200/1, (c) 300/1, and (d) 500/1. | ||
The obtained particles were characterized using an energy dispersive spectrometer (EDS, Fig. 4a). The EDS spectra exhibited a uniform distribution of C and F elements on the RB particles (Fig. 4b, c, S17 and Table S8†). The glass transition temperature (Tg) of the RB particles (PDMA54-b-PPFS4588, DP of PFS was calculated based on Mp, entry 2, Table S6†) was analyzed by differential scanning calorimetry (DSC, Fig. 4d), affording a Tg of 17 °C higher than that of PDMA54-b-PPFS50, owing to the increased molecular weight.
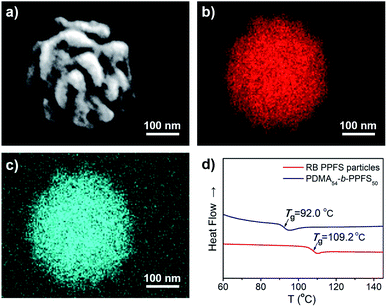 | ||
| Fig. 4 (a) SEM image of the RB particle. (b and c) Mapping images of elemental C and F. (d) DSC profiles of RB particles and PDMA54-b-PPFS50. | ||
An advantage of PPFS is the ease in post-synthetic modification by nucleophilic aromatic substitution on the –C6F5 group, facilitating the preparation of functional polymers.53,54 In our studies, when RB particles were treated with 1,2-ethanedithiol, cross-linked colloids were generated without a clear morphological change (Fig. 5a and b). Compared to original RB particles, thermal stability of the rough surfaces of the cross-linked counterparts has dramatically increased for over 30 °C (80 vs. 115 °C, Fig. S20†).
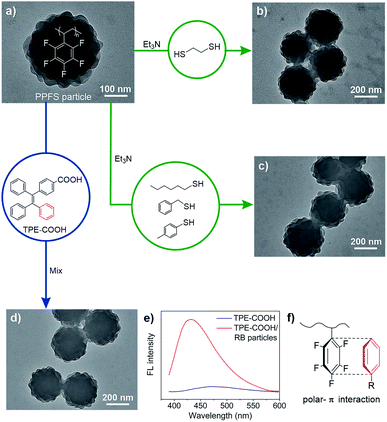 | ||
| Fig. 5 TEM images of RB particles before and after modification. (a) Original particles. (b) Treated with 1,2-ethanedithiol. (c) Treated with p-toluenethiol (hexanethiol and benzylthiol were used in Fig. S20†). (d) Treated with TPE-COOH. (e) Fluorescence profiles of TPE-COOH, and the mixture of TPE-COOH/RB particles. (f) Schematic illustration of polar-π interactions between –C6F5 of the particles and phenyl of TPE. | ||
Moreover, three types of nucleophiles including alkyl, benzyl and aryl thiols have been used to react with RB particles at room temperature. As determined by 19F nuclear magnetic resonance, the para-fluorine atom of the –C6F5 group could be efficiently converted in the nucleophilic aromatic substitution, while the RB morphology was maintained (Fig. 5c and Table S10†), furnishing opportunities for loading various molecules onto RB particles under mild and metal-free conditions via C–S bond formation.
Compounds with tetraphenylethylene (TPE) units show AIE behavior, and have stimulated biomedical and optoelectronic applications.55 Upon mixing a carboxyl substituted TPE (TPE-COOH) with RB particles, TPE-COOH was encapsulated into RB particles as indicated by the promoted AIE expression (Fig. 5d, e and S22†), exhibiting an enhanced fluorescence at 430 nm and a blue-shift of 40 nm compared to TPE-COOH due to restricted intramolecular rotation.56,57 The successful incorporation of TPE-COOH is probably due to the polar-π interaction58,59 between –C6F5 from the host particle and phenyl from the TPE guest (Fig. 5f).
Conclusion
In conclusion, we have developed a tandem approach based on nonsynchronous growth via photo-RDRP that enables the one-pot and in situ synthesis of RB nanoparticles with uniform sizes at quantitative monomer conversion and under mild conditions. The successive visible-light irradiation from weak-to strong-light intensity has allowed the generation and fusion of large and small colloids, facilitating the synthesis of RB particles of a variety of sizes. Moreover, the fluorinated RB particles could be modified by both chemical and physical interactions, broadening opportunities to access fluorinated nanomaterials with various physicochemical properties. Given the broad interest in nonspherical colloids, fluorinated materials and photochemistry, we expect this method to be useful for providing versatile PPFS RB particles for advanced materials engineering.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the NSFC (no. 21704016 and 21971044), and start-up funding from Fudan University.Notes and references
- W. Li, H. Palis, R. Mérindol, J. Majimel, S. Ravaine and E. Duguet, Chem. Soc. Rev., 2020, 49, 1955–1976 RSC.
- C. Lu and M. Urban, ACS Nano, 2015, 9, 3119–3124 CrossRef CAS.
- S. Liu, S. Yin, J. Duvigneau and G. J. Vancso, ACS Nano, 2020, 14, 1623–1634 CrossRef CAS.
- H. Song, M. Yu, Y. Lu, Z. Gu, Y. Yang, M. Zhang, J. Fu and C. Yu, J. Am. Chem. Soc., 2017, 139, 18247–18254 CrossRef CAS.
- A. Kumar, N. Kumari, S. Dubbu, S. Kumar, T. Kwon, J. H. Koo, J. Lim, I. Kim, Y. K. Cho, J. Rho and I. S. Lee, Angew. Chem., Int. Ed., 2020, 59, 9460–9469 CrossRef CAS.
- Y. Lan, A. Caciagli, G. Guidetti, Z. Yu, J. Liu, V. E. Johansen, M. Kamp, C. Abell, S. Vignolini, O. A. Scherman and E. Eiser, Nat. Commun., 2018, 9, 3614 CrossRef.
- S. Samanta, S. L. Banerjee, S. K. Ghosh and N. K. Singha, ACS Appl. Mater. Interfaces, 2019, 11, 44722–44734 CrossRef CAS.
- X. Wang and Z. An, Macromol. Rapid Commun., 2019, 40, e1800325 CrossRef.
- F. D'Agosto, J. Rieger and M. Lansalot, Angew. Chem., Int. Ed., 2020, 59, 8368–8392 CrossRef.
- N. J. W. Penfold, J. Yeow, C. Boyer and S. P. Armes, ACS Macro Lett., 2019, 8, 1029–1054 CrossRef CAS.
- Y. Ding, M. Cai, Z. Cui, L. Huang, L. Wang, X. Lu and Y. Cai, Angew. Chem., Int. Ed., 2018, 57, 1053–1056 CrossRef CAS.
- C. Bergerbit, F. Baffie, A. Wolpers, P. Y. Dugas, O. Boyron, M. Taam, M. Lansalot, V. Monteil and F. D'Agosto, Angew. Chem., Int. Ed., 2020, 59, 10385–10390 CrossRef CAS.
- P. Yang, Y. Ning, T. J. Neal, E. R. Jones, B. R. Parker and S. P. Armes, Chem. Sci., 2019, 10, 4200–4208 RSC.
- W. Zhao, H. T. Ta, C. Zhang and A. K. Whittaker, Biomacromolecules, 2017, 18, 1145–1156 CrossRef CAS.
- R. Zeng, Y. Chen, L. Zhang and J. Tan, Polym. Chem., 2020, 11, 4591–4603 RSC.
- W. Hou, H. Wang, Y. Cui, Y. Liu, X. Ma and H. Zhao, Macromolecules, 2019, 52, 8404–8414 CrossRef CAS.
- R. P. M. Höller, M. Dulle, S. Thomä, M. Mayer, A. M. Steiner, S. Förster, A. Fery, C. Kuttner and M. Chanana, ACS Nano, 2016, 10, 5740–5750 CrossRef.
- X. Qiao, P. Y. Dugas, B. Charleux, M. Lansalot and E. Bourgeat-Lami, Macromolecules, 2015, 48, 545–556 CrossRef CAS.
- Y. Sun, Y. Yin, M. Chen, S. Zhou and L. Wu, Polym. Chem., 2013, 4, 3020–3027 RSC.
- W. Jiang, C. M. Grozea, Z. Shi and G. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 2629–2638 CrossRef CAS.
- D. Lee, J. W. Hong, C. Park, H. Lee, J. E. Lee, T. Hyeon and S. R. Paik, ACS Nano, 2014, 8, 8887–8895 CrossRef CAS.
- S. Dadashi-Silab, S. Doran and Y. Yagci, Chem. Rev., 2016, 116, 10212–10275 CrossRef CAS.
- M. Chen, M. Zhong and J. A. Johnson, Chem. Rev., 2016, 116, 10167–10211 CrossRef CAS.
- J. Xu, K. Jung, A. Atme, S. Shanmugam and C. Boyer, J. Am. Chem. Soc., 2014, 136, 5508–5519 CrossRef CAS.
- M. D. Ryan, R. M. Pearson, T. A. French and G. M. Miyake, Macromolecules, 2017, 50, 4616–4622 CrossRef CAS.
- C. Tian, P. Wang, Y. Ni, L. Zhang, Z. Cheng and X. Zhu, Angew. Chem., Int. Ed., 2020, 59, 3910–3916 CrossRef CAS.
- A. Anastasaki, B. Oschmann, J. Willenbacher, A. Melker, M. H. C. Van Son, N. P. Truong, M. W. Schulze, E. H. Discekici, A. J. McGrath, T. P. Davis, C. M. Bates and C. J. Hawker, Angew. Chem., Int. Ed., 2017, 56, 14483–14487 CrossRef CAS.
- R. Whitfield, K. Parkatzidis, M. Rolland, N. P. Truong and A. Anastasaki, Angew. Chem., Int. Ed., 2019, 58, 13323–13328 CrossRef CAS.
- V. Kottisch, M. J. Supej and B. P. Fors, Angew. Chem., Int. Ed., 2018, 57, 8260–8264 CrossRef CAS.
- E. E. Stache, V. Kottisch and B. P. Fors, J. Am. Chem. Soc., 2020, 142, 4581–4585 CrossRef CAS.
- J. Jiang, G. Ye, F. Lorandi, Z. Liu, Y. Liu, T. Hu, J. Chen, Y. Lu and K. Matyjaszewski, Angew. Chem., Int. Ed., 2019, 58, 12096–12101 CrossRef CAS.
- G. Szczepaniak, M. Łagodzińska, S. Dadashi-Silab, A. Gorczyński and K. Matyjaszewski, Chem. Sci., 2020, 11, 8809–8816 RSC.
- N. Corrigan, J. Yeow, P. Judzewitsch, J. Xu and C. Boyer, Angew. Chem., Int. Ed., 2019, 58, 5170–5189 CrossRef CAS.
- N. Corrigan and C. Boyer, ACS Macro Lett., 2019, 8, 812–818 CrossRef CAS.
- S. Xu, J. Yeow and C. Boyer, ACS Macro Lett., 2018, 7, 1376–1382 CrossRef CAS.
- J. Yeow and C. Boyer, Adv. Sci., 2017, 4, 1700137 CrossRef.
- L. Yu, X. Dai, Y. Zhang, Z. Zeng, L. Zhang and J. Tan, Macromolecules, 2019, 52, 7267–7277 CrossRef CAS.
- L. Wang, Y. Ding, Q. Liu, Q. Zhao, X. Dai, X. Lu and Y. Cai, ACS Macro Lett., 2019, 8, 623–628 CrossRef CAS.
- Q. Xu, C. Tian, L. Zhang, Z. Cheng and X. Zhu, Macromol. Rapid Commun., 2019, 40, e1800327 CrossRef.
- A. Vitale, R. Bongiovanni and B. Ameduri, Chem. Rev., 2015, 115, 8835–8866 CrossRef CAS.
- E. H. Discekici, A. Anastasaki, R. Kaminker, J. Willenbacher, N. P. Truong, C. Fleischmann, B. Oschmann, D. J. Lunn, J. Read de Alaniz, T. P. Davis, C. M. Bates and C. J. Hawker, J. Am. Chem. Soc., 2017, 139, 5939–5945 CrossRef CAS.
- H. Gong, Y. Gu, Y. Zhao, Q. Quan, S. Han and M. Chen, Angew. Chem., Int. Ed., 2020, 59, 919–927 CrossRef CAS.
- K. Jiang, S. Han, M. Ma, L. Zhang, Y. Zhao and M. Chen, J. Am. Chem. Soc., 2020, 142, 7108–7115 CrossRef CAS.
- F. Lv, Z. An and P. Wu, Macromolecules, 2019, 53, 367–373 CrossRef.
- F. Lv, Z. An and P. Wu, Nat. Commun., 2019, 10, 1397 CrossRef.
- B. Couturaud, P. G. Georgiou, S. Varlas, J. R. Jones, M. C. Arno, J. C. Foster and R. K. O'Reilly, Macromol. Rapid Commun., 2019, 40, e1800460 CrossRef.
- M. Huo, Y. Zhang, M. Zeng, L. Liu, Y. Wei and J. Yuan, Macromolecules, 2017, 50, 8192–8201 CrossRef CAS.
- J. Tan, Y. Bai, X. Zhang and L. Zhang, Polym. Chem., 2016, 7, 2372–2380 RSC.
- Y. Zhang, G. Han, M. Cao, T. Guo and W. Zhang, Macromolecules, 2018, 51, 4397–4406 CrossRef CAS.
- Q. Quan, H. Wen, S. Han, Z. Wang, Z. Shao and M. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 24319–24327 CrossRef CAS.
- J. Tan, Q. Xu, Y. Zhang, C. Huang, X. Li, J. He and L. Zhang, Macromolecules, 2018, 51, 7396–7406 CrossRef CAS.
- Y. Zhang, M. Cao, G. Han, T. Guo, T. Ying and W. Zhang, Macromolecules, 2018, 51, 5440–5449 CrossRef CAS.
- G. Delaittre and L. Barner, Polym. Chem., 2018, 9, 2679–2684 RSC.
- J.-M. Noy, Y. Li, W. Smolan and P. J. Roth, Macromolecules, 2019, 52, 3083–3091 CrossRef CAS.
- J. Mei, Y. Hong, J. W. Lam, A. Qin, Y. Tang and B. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS.
- G. Liang, J. W. Y. Lam, W. Qin, J. Li, N. Xie and B. Tang, Chem. Commun., 2014, 50, 1725–1727 RSC.
- W. Bai, Z. Wang, J. Tong, J. Mei, A. Qin, J. Sun and B. Tang, Chem. Commun., 2015, 51, 1089–1091 RSC.
- N. ten Brummelhuis and M. T. Heilmann, Macromolecules, 2016, 49, 6879–6887 CrossRef CAS.
- Z. Huang, X. Chen, G. Wu, P. Metrangolo, D. Whitaker, J. A. McCune and O. A. Scherman, J. Am. Chem. Soc., 2020, 142, 7356–7361 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc04141f |
| This journal is © The Royal Society of Chemistry 2020 |