Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of photo- and chemo-stable near-infrared-emitting dyes: linear-shape benzo-rosol and its derivatives as unique ratiometric bioimaging platforms†
Mingchong
Dai
 ,
Ye Jin
Reo
,
Ye Jin
Reo
 ,
Chang Wook
Song
,
Yun Jae
Yang
and
Kyo Han
Ahn
,
Chang Wook
Song
,
Yun Jae
Yang
and
Kyo Han
Ahn
 *
*
Department of Chemistry, Pohang University of Science and Technology (POSTECH), 77 Cheongam-Ro, Nam-Gu, Pohang, Gyungbuk 37673, Republic of Korea. E-mail: ahn@postech.ac.kr
First published on 6th August 2020
Abstract
Microscopic imaging aided with fluorescent probes has revolutionized our understanding of biological systems. Organic fluorophores and probes thus continue to evolve for bioimaging applications. Fluorophores such as cyanines and hemicyanines emit in the near-infrared (NIR) region and thus allow deeper imaging with minimal autofluorescence; however, they show limited photo- and chemo-stability, demanding new robust NIR fluorophores. Such photo- and chemo-stable NIR fluorophores, linear-shape π-extended rosol and rosamine analogues, are disclosed here which provide bright fluorescence images in cells as well as in tissues by confocal laser-scanning microscopy. Furthermore, they offer unique ratiometric imaging platforms for activatable probes with dual excitation and dual emission capability, as demonstrated with a 2,4-dinitrophenyl ether derivative of benzo-rosol.
Introduction
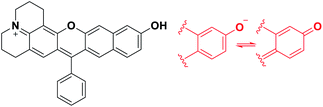
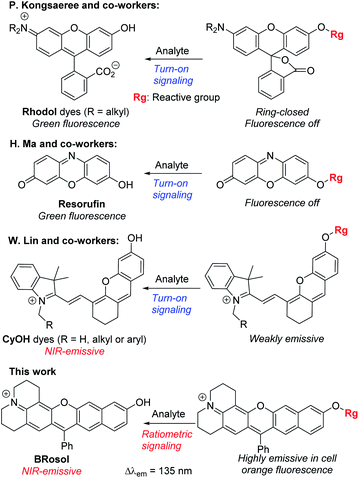
Microscopic imaging aided with a fluorescent probe is essential for studying biological processes in living systems. Accordingly, fluorescent organic molecules (fluorophores) and probes have been evolved for bioimaging applications. Fluorophores that emit in the deep-red/near-infrared (NIR) wavelength region have received particular attention for deep-tissue or whole body imaging applications,1,2 because with such dyes we can minimize the autofluorescence interference from innate biomolecules and thus increase the imaging depth. Cyanines (Cy) and analogues constitute a representative class of deep-red/NIR dyes. For example, ICG, an FDA-approved Cy7 dye, has been importantly used for tumour imaging and photodynamic therapy. A key structural feature of Cy dyes is that two identical nitrogen-containing heterocycles, one with a positive charge, are resonance-stabilized through “flexible” polymethine units. Cy dyes have limited photo-stability,3 however, thwarting their use for long-term bioimaging applications. In addition, Cy dyes and their asymmetric analogues hemicyanines seem to have limited chemical stability (chemo-stability) toward some reactive biological species, such as hypochlorous acid, hydrogen sulfide or bisulfite, as inferred from the various cyanine- and hemicyanine-based activatable probes for these species (Table S1, ESI†). Therefore, new NIR-emitting dyes that are photo- and chemo-stable are in great demand.Besides, for application in biomolecular tagging or detection of biological analytes, fluorophores with a functional arm are required. In particular, fluorophores with a functional arm through which we can modulate the emission properties are highly valuable.4 With such “armed” fluorophores we can develop the so-called reaction-based or activatable probes for biological analytes.5,6 For example, the rhodol system, a structural hybrid of rhodamine and fluorescein dyes, and resorufin offer an opportunity for the development of activatable probes; When the reactive group (Rg) attached onto the hydroxyl group of such a dye is selectively cleaved by a target analyte, it generates the parent dye with accompanying fluorescence changes and thus enables fluorescence detection (Scheme 1). This sensing scheme has been widely explored with other aryl alcohol (ArOH)-type fluorophores as well.7–9 Typical rhodols and also resorufin, however, absorb and emit in a rather shorter wavelength region, which, during microscopic imaging of tissues, can cause significant autofluorescence from innate biomolecules such as flavoproteins. To minimize the autofluorescence interference, which mostly ranges from the blue to orange wavelength region, fluorophores that emit in longer wavelengths (λem ≥ 630 nm) are in great demand.2 Recently, Lin and co-workers developed a new class of ArOH-type fluorophores, so-called CyOH dyes,9 which is a hybrid system of cyanine and fluorescein dyes. The CyOH dyes emit in the NIR region, alleviating autofluorescence and also the shallow imaging depth in tissue imaging. Accordingly, they are increasingly used to develop fluorescent probes for various analytes, such as tyrosinase,10 esterases,11 β-glucuronidase,12 keloid,13 phosphatase,14 peptidases,15 and cytochrome P450,16 by introducing the corresponding reactive group to the hydroxyl arm.
Although CyOH dyes have promising features such that they emit in the NIR wavelength region and have an hydroxyl functional arm through which their emission properties can be modulated, we have found that they have limited photostability (see below) as well as limited chemostability.17
Besides, the CyOH dyes in the O-functionalized form (ArORg) are weakly emissive, as in the case of rhodol and resorufin dyes: accordingly, the analyte-triggered conversion from ArORg to ArOH results in a turn-on type fluorescence response (Scheme 1). Such “single intensity-based” detection is widely used for bioimaging studies; however, it raises a serious reliability issue in quantitative analysis because the fluorescence intensity is inherently sensitive to environmental factors, such as polarity and viscosity changes. The reliability issue can be solved with ratiometric probes, which allow monitoring of fluorescence intensity ratio changes that are not so sensitive to the environmental fluctuations. Therefore, there is a strong need for new ArOH-type dyes whose O-functionalized forms also fluoresce but in different emission windows. Such dyes will find widespread use for the development of activatable probes with ratiometric imaging capability.
In this contribution, we wish to report the NIR-emitting dyes that show high photo- and chemo-stability, along with large Stokes shifts. The new dyes have π-extended features of rosol and rosamine dyes, which have hydroxyl and amino functional arms, respectively. Notably, their O- and N-functionalized derivatives also emit strong fluorescence with large peak separations from the parent dyes at the physiological pH, offering highly promising dye platforms for the development of ratiometric imaging probes. Surely, they provide bright fluorescence images in cells as well as in tissues by confocal laser-scanning microscopy (CLSM).
Results and discussion
Design and synthesis
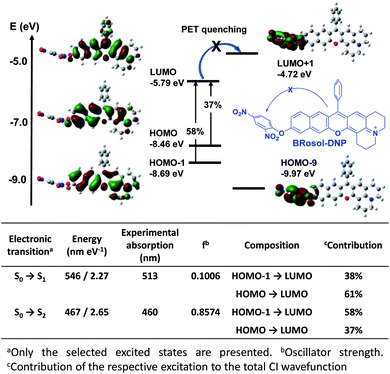
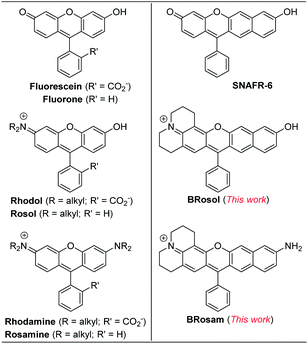
To develop NIR-emitting dyes that have high photo- and chemo-stability, we focused on linear-shape benzene-fused rosol (benzorosol: BRosol) analogues, from which the corresponding benzene-fused rosamine (benzorosamine: BRosam) derivatives can be also derived (Fig. 1). The new dyes belong to the push–pull type dipolar dyes having a hydroxyl or amino donor, which also acts as the functional arm. A linear-shape, benzene-fused fluorone such as SNAFR-6 is reported by Strongin and co-workers,18 but linear-shape benzene-fused rosol or rosamine dyes have not been reported yet. Inspired by their studies, we have investigated the linear-shape benzene-fused rosol and rosamine systems. From our previous experience on benzene-fused coumarin19 and rhodamine dyes20 we expected that the linear-shape benzorosol and its analogues would emit in longer wavelengths, preferably in the NIR region, along with stronger fluorescence and larger Stokes shifts in comparison with the corresponding bent-shape isomers. Furthermore, we expected that the benzene-fused dye core which has no flexible vinyl units would bestow the desired photo- and chemo-stability in bioimaging applications; We suspect that the flexible vinyl unit(s) conjugated with the cationic heterocycle present in Cy and hemicyanine dyes is responsible for their limited photo- and chemo-stability.The synthetic route to BRosol was established by adopting the synthetic procedure of SNAFRs (Scheme 2).18 Starting from 2,7-dimethoxynaphthalene (3) to 7-benzoyl-8-methoxyjulolidine (2), which, in turn, was prepared from 8-methoxyjulolidine (1) through the Friedel–Crafts acylation with benzoyl chloride, afforded the tertiary alcohol 4. Treatment of compound 4 with boron tribromide at room temperature afforded BRosol through ring-closing followed by demethylation. Starting from commercially available chemicals, through three steps we were able to synthesize BRosol. At the tunable functional arm, the hydroxyl group of BRosol, we can readily introduce functional groups, such as propargyl (BRosol-P), acetyl (BRosol-E), and 2,4-dinitrophenyl (BRosol-DNP) through standard chemical conversions. BRosol-P will be useful for bioconjugation by click chemistry.21BRosol-E and BRosol-DNP could potentially be ratiometric probes for esterase and biothiols, respectively. In the last section, we demonstrate that BRosol-DNP indeed can be used to sense glutathione (GSH), a major biothiol, with a ratiometric fluorescence response under the dual excitation and emission conditions.22,23BRosol can be also functionalized to provide o-bromo derivative, Br-BRosol, which would allow further derivatization through transition metal-catalyzed coupling reactions.
BRosol can further provide the corresponding amine analogues, BRosam dyes; Through the Buchwald–Hartwig reaction of the BRosol-triflate with dimethylamine or benzophenone imine, the corresponding benzorosamine BRosam 2 and the imine intermediate 5 were readily prepared, respectively. Hydrolysis of the diphenylimino group of compound 5 afforded the corresponding benzorosamine with a simple amino substituent, BRosam 1. The free amino group of BRosam 1 provides a tunable arm for further functionalization. For example, we can introduce a p-nitrobenzyloxycarbonyl (p-NBC) group to it, affording the p-nitrobenzylcarbamate derivative BRosam-pNBC. All the new compounds were fully characterized by 1H NMR, 13C NMR, and HRMS analyses (ESI†).
Photophysical properties of BRosol and its derivatives
The absorption and emission spectra of BRosol and its derivatives (BRosol-E and BRosol-P) were compared in organic and also in aqueous media. The compounds have higher absorbance and fluorescence intensity in organic solvent such as dichloromethane, acetonitrile and ethanol (Fig. S1 in the ESI,†Table 1), but lower absorbance and fluorescence in PBS (10 mM, pH 7.4). Notably, inspite of the low fluorescence in the buffer only, BRosol and its derivatives provide bright cellular fluorescence images under normal imaging conditions (at 10 μM fluorophore with 5% laser power; see below). The unique cellular environment, which is more viscous and hydrophobic compared with that of aqueous, and the dipolar nature of the fluorophores are likely to be responsible for the contrasting emission behaviour. BRosol and its derivatives including those of BRosam belong to dipolar dyes, whose emission behaviour is highly dependent on media. In general, dipolar dyes are weakly or poorly emissive in aqueous media but become highly fluorescent in a less polar and more viscous environment.24 Accordingly, dipolar dyes display pronounced changes in their emission intensity compared to that of the “resonance-symmetric” dyes such as rhodamine, fluorescein, and cyanine dyes when going from aqueous media to the cellular medium.| Medium | BRosol | ||||
|---|---|---|---|---|---|
| λ abs (nm) | λ em (nm) | Stoke shift (nm) | ε | Φ f | |
| a The fluorescence quantum yields determined using Nile blue (ΦF = 0.27 in EtOH) as a reference fluorophore. The concentration of BRosol was 10 μM in the organic solvent (containing 1% DMSO) or 1.0 μM in PBS (containing 0.1% DMSO), the concentration at which the dye shows no aggregation-caused quenching. | |||||
| PBS pH 7.4 | 516 | 586 | 70 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
0.021 |
| CH2Cl2 | 525 | 611 | 86 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.276 |
| CH3CN | 515 | 589 | 74 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.192 |
| Ethanol | 520 | 595 | 75 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.103 |
| 0.3 EtOH/PBS (pH 7.4) | 515 | 590 | 75 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.037 |
| 615 | 725 | 110 | 4800 | 0.020 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| BRosol-E | |||||
| PBS pH 7.4 | 453 | 601 | 148 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.088 |
| CH2Cl2 | 478 | 591 | 113 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.316 |
| CH3CN | 512 | 595 | 83 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.202 |
| Ethanol | 471 | 595 | 124 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.088 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| BRosol-P | |||||
| PBS pH 7.4 | 476 | 591 | 115 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.064 |
| CH2Cl2 | 517 | 585 | 68 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.273 |
| CH3CN | 466 | 590 | 124 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.183 |
| Ethanol | 514 | 589 | 75 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.108 |
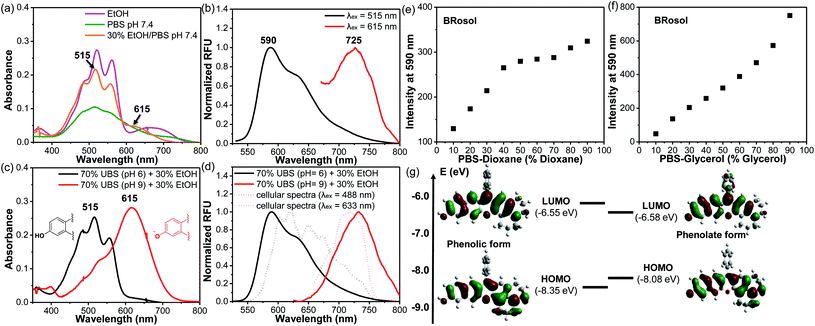
The photophysical properties of BRosol were measured in a mixed solvent system of 30% ethanol in PBS (pH 7.4). In the mixed medium, in addition to the absorption band of the phenolic form of BRosol (λabs = 515 nm), that of the phenolate form gave substantial fluorescence (λabs = 615 nm) (Fig. 2a). When being excited at each of the two absorption maxima, emissions from the orange (λem = 590 nm) and NIR (λem = 725 nm) regions were observed, respectively (Fig. 2b).
The hydroxyl group of BRosol has pKa = 7.4, which is less acidic than that of the CyOH fluorophore (pKa ∼5.6). Thus, BRosol exists both in the phenolic and phenolate forms in an approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in the cytosol (Fig. S2 in the ESI†). Thus, BRosol would enable cellular fluorescence imaging in the NIR region. Based on the pKa value, we obtained the absorption and emission spectra of the phenolic and phenolate forms of BRosol in the mixed solvent system of 30% EtOH + 70% UBS (Universal Buffer System, at pH 6.0 and pH 9.0 respectively) (Fig. 2c and d). At pH 6.0, BRosol mostly exists in its phenolic form, having λabs = 515 nm and λem = 590 nm. However, at pH 9, BRosol mainly exists in its phenolate form, displaying the absorption and emission maxima at λabs = 615 nm and λem = 725 nm, respectively. The normalized emission spectra of the phenolic and phenolate forms of BRosol (Fig. 2d) are matched well with those obtained at pH 7.4 (Fig. 2b). The polarity- and viscosity-dependent emission behaviour of BRosol shown in Fig. 2e and f, respectively, indicates that it becomes strongly fluorescent in a less polar and more viscous environment. The environment-sensitive emission behaviour supports that BRosol and its derivatives are dipolar dyes. The quantum yield of BRosol in organic solvent is quite high (Φf = 28% in CH2Cl2) but reduces in PBS (Φf = 2.1%). In PBS, BRosol and its derivatives seem to exhibit aggregation-induced quenching (ACQ) even in the linear absorbance–concentration range (Fig. S3†), hence we measured the quantum yields of the dyes in PBS at a low concentration (1.0 μM) to avoid the ACQ effect. ACQ is a common issue for hydrophobic dyes. To inhibit ACQ in the case of Brosol, it would be necessary to introduce a steric group in the benzene ring in BRosol. The photophysical data of BRosol and its derivatives are listed in Table 1.
1 ratio in the cytosol (Fig. S2 in the ESI†). Thus, BRosol would enable cellular fluorescence imaging in the NIR region. Based on the pKa value, we obtained the absorption and emission spectra of the phenolic and phenolate forms of BRosol in the mixed solvent system of 30% EtOH + 70% UBS (Universal Buffer System, at pH 6.0 and pH 9.0 respectively) (Fig. 2c and d). At pH 6.0, BRosol mostly exists in its phenolic form, having λabs = 515 nm and λem = 590 nm. However, at pH 9, BRosol mainly exists in its phenolate form, displaying the absorption and emission maxima at λabs = 615 nm and λem = 725 nm, respectively. The normalized emission spectra of the phenolic and phenolate forms of BRosol (Fig. 2d) are matched well with those obtained at pH 7.4 (Fig. 2b). The polarity- and viscosity-dependent emission behaviour of BRosol shown in Fig. 2e and f, respectively, indicates that it becomes strongly fluorescent in a less polar and more viscous environment. The environment-sensitive emission behaviour supports that BRosol and its derivatives are dipolar dyes. The quantum yield of BRosol in organic solvent is quite high (Φf = 28% in CH2Cl2) but reduces in PBS (Φf = 2.1%). In PBS, BRosol and its derivatives seem to exhibit aggregation-induced quenching (ACQ) even in the linear absorbance–concentration range (Fig. S3†), hence we measured the quantum yields of the dyes in PBS at a low concentration (1.0 μM) to avoid the ACQ effect. ACQ is a common issue for hydrophobic dyes. To inhibit ACQ in the case of Brosol, it would be necessary to introduce a steric group in the benzene ring in BRosol. The photophysical data of BRosol and its derivatives are listed in Table 1.
It is notable that the absorbance from the phenolic form of BRosol becomes insignificant above 600 nm, whereas that of the phenolate form displays the maximum at 615 nm (Fig. 2c). Therefore, we can selectively excite the phenolate form over the phenolic form by irradiation at 615 nm or higher. This is an important feature for the ratiometric imaging under dual excitation conditions. Suppose a ratiometric probe generated from BRosol, labeled ArORg, is converted to ArOH through activation by an analyte, we can monitor the formation of the enzymatic product separately from the probe by following the phenolate form.
The calculated HOMO–LUMO energy gap of the phenolate form (ketone) was 0.3 eV smaller than that of the phenolic form of BRosol (Fig. 2g), which corroborates the red-shifted absorption and emission bands of the phenolate form. Both forms have highly conjugated electronic distributions.
At this stage, we have briefly assessed the absorption and emission behaviour of Br-BRosol; its absorption spectra in different solvents indicated that the phenolate form becomes major in ethanol, which is likely due to the more acidic hydroxyl group (pKa = 5.8) (Fig. S4 and S5 in the ESI†). Accordingly, the NIR emission from the phenolate form is much stronger than the red emission from the phenolic form (Table S2†). A further study to derivatize Br-BRosol and apply it to develop ratiometric probes is the next concern.
Photophysical properties of BRosam and its derivatives
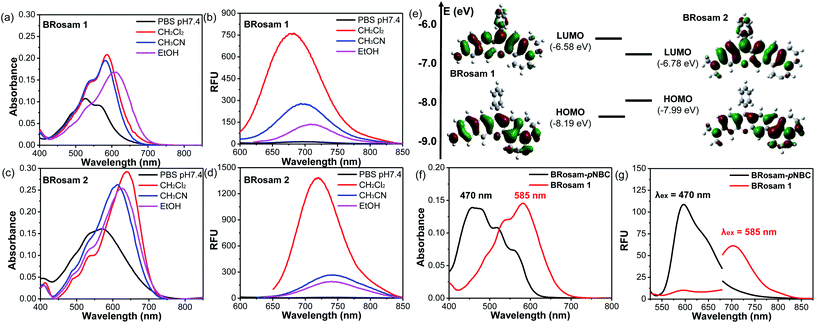
Starting from BRosol, we can readily synthesize the corresponding benzo-rosamine analogues, BRosam. The BRosam compounds lack the carboxy group that forms the spirolactone equilibrium species and thus behave differently from the corresponding rhodamine fluorophores. The BRosam dyes 1 and 2 emit in the NIR region in ethanol, at 708 nm and 740 nm respectively. Thus, they are also of interest as a new class of NIR-emitting fluorophores. We briefly evaluated their photophysical properties. The BRosam dyes emitted fluorescence insensitive to pH changes in the range from pH 4–12, because the aryl amines are poorly basic (Fig. S6 in the ESI†). This is a distinctive feature from the ArOH-type fluorophores that have a more acidic hydroxyl group. The BRosam dyes are also strongly emissive in dichloromethane but weakly emissive in PBS (pH 7.4) (Fig. 3a–d), as in the case of BRosol. However, they also provided bright cellular images under excitation at low laser powers (see below). Furthermore, both BRosam 1 and BRosam 2 have large Stokes shifts of more than 170 nm (Table 2) in PBS (pH 7.4).| Medium | BRosam 1 | ||||
|---|---|---|---|---|---|
| λ abs (nm) | λ em (nm) | Stoke shift (nm) | ε | Φ f | |
| a Fluorescence quantum yields determined using Nile blue (ΦF = 0.27 in EtOH) as a reference dye. The concentration of each dye was 10 μM in the organic solvent (containing 1% DMSO) or 1.0 μM in PBS (containing 0.1% DMSO). | |||||
| PBS pH 7.4 | 526 | 700 | 174 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
0.008 |
| CH2Cl2 | 586 | 681 | 95 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.184 |
| CH3CN | 581 | 693 | 112 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.068 |
| Ethanol | 610 | 708 | 92 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.027 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| BRosam 2 | |||||
| PBS pH 7.4 | 572 | 742 | 170 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.006 |
| CH2Cl2 | 637 | 720 | 83 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.172 |
| CH3CN | 611 | 743 | 132 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.059 |
| Ethanol | 626 | 740 | 114 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.041 |
Theoretical calculations (Fig. 3e) at the ground state indicated that, compared to BRosam 1, BRosam 2 has the smaller HOMO–LUMO gap, and thus absorbs and emits the longer wavelengths in solution. However, their cellular spectra displayed emission peaks at similar wavelengths. Interestingly, their maximum emission wavelengths, λem = 707 nm and 710 nm respectively in the cellular environment, are not so shifted from that of BRosol. We can readily functionalize BRosam at the free amine site, for example, providing a carbamate derivative, BRosam-pNBC (Scheme 2). Such a p-nitrophenyl carbamate could be explored to develop a nitroreductase probe:25 a possible conversion of the carbamate to the corresponding amine by the enzyme would cause a dramatic color change from blue to red (Fig. 3f). The large absorption change is likely due to a change in the intramolecular charge-transfer (ICT) upon the conversion. Thus, BRosam also could offer a novel dye platform for the development of ratiometric fluorescent probes (Fig. 3g and S7, ESI†).
Photo- and chemo-stability of the new fluorophores and their derivatives
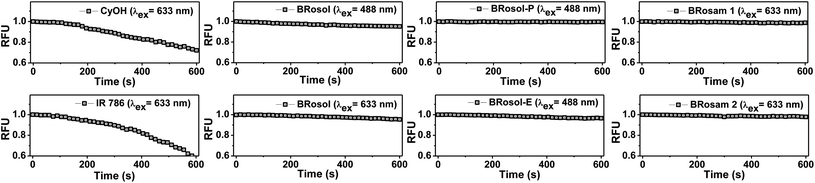
BRosol is stable in solution (30% EtOH/PBS at pH 7.4): It maintained its emission intensity, more than 90%, at 37 °C when monitored at every hour for 24 h (Fig. S8 in the ESI†). It should be noted that cyanine dyes, such as Cy7 and Qcy7, in PBS lose their emission intensity progressively.26,27As NIR-emitting dyes, currently cyanine and hemicyanine compounds or their analogues are widely used for bioimaging applications. However, owing to the flexible polymethine structure conjugated with the cationic heterocycle moiety, they have low photostability, thwarting their use in long-term monitoring of biological processes. When we monitored the fluorescence intensity of CyOH and IR 786, a hemicyanine and a Cy7 dye respectively, in 30% EtOH/PBS (pH 7.4) by continuous laser (633 nm) irradiation for 10 min, they showed a substantial decrease in the emission intensity (Fig. 4). In contrast, BRosol, BRosam 1, and BRosam 2 under the same laser irradiation conditions show slight changes in the emission intensity. BRosol and its derivatives BRosol-P and BRosol-E also maintain their emission intensity even under irradiation at 488 nm. Thus, the new fluorophores and their derivatives are highly photostable, showing minor changes in the emission intensity under the imaging conditions by confocal laser scanning microscopy.
As mentioned above, the cyanine and hemicyanine moieties are used to develop various activatable probes to detect hypochlorous acid,28 hydrogen sulfide29 or bisulfite,30,31 which, in turn, suggests that these fluorophores respond to the “reactive” biological species.17 Therefore, we have checked the stability of BRosol in the presence of the reactive chemical species each at 100 μM in 30% EtOH-PBS (100 mM; pH 7.4) at room temperature (∼25 °C) for 1 h. We used a rather high concentration of the buffer to eliminate any possible effect by pH change upon addition of the analytes. Under the given conditions BRosol is found to be stable toward the reactive species (Fig. S9 in the ESI†).
The high photo- and chemo-stability of the new fluorophores are likely due to their robust structural feature that consists of highly fused benzene rings instead of the flexible vinyl unit conjugated with the cationic heterocycle as in the case of cyanine and hemicyanine fluorophores. The cationic polymethine moiety seems to be responsible for the photo-degradation and also the sensitivity toward nucleophilic sulfur species.
Comparison between BRosol and CyOH
To point out the advantageous features of BRosol as the ratiometric sensing platform, we have compared its basic photophysical properties with those of a CyOH fluorophore (Table 3 and S3†). The large Stokes shifts, the large emission peak separation, and the bright cellular fluorescence of BRosol as well as its O-functionalized derivatives (see below), in addition to their high photo- and chemo-stability (chemical stability), are highly desirable features for bioimaging applications.| CyOH (R = Me)a turn-on sensing platform | BRosol ratiometric sensing platform | |||
|---|---|---|---|---|
| Phenolic (also, O-functionalized) form | Phenolate form | Phenolic (also, O-functionalized) form | Phenolate form | |
a Measured in PBS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 1 MeOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
b Measured in PBS 1.
b Measured in PBS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 7 EtOH = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
c See details in Fig. S11 (ESI).
d See details in Fig. 4. 3.
c See details in Fig. S11 (ESI).
d See details in Fig. 4.
|
||||
| λ abs/λem | 654/677 nm | 690/716 nm | 515/590 nm | 615/725 nm |
| Stokes shift | 23 nm | 26 nm | 75 nm | 110 nm |
| Δλem; pKa | 39 nm; pKa = 5.6 | 135 nm; pKa = 7.4 | ||
| Φ f in CH2Cl2 | 0.033 | 0.276 | ||
| Cellular brightness | Dark | Bright | Bright | Bright |
| Chemo-stabilityc | Response to bisulfite | Stable to bioanalytes | ||
| Photo-stabilityd | Limited photo-stability | High photo-stability | ||
At this point, it is also worthwhile to compare BRosol with SNARF-1, which has the o-carboxy substituent and thus can be classified as a π-extended rhodol analogue, benzorhodol (Fig. S10 in the ESI†). SNARF-1 and its analogues are widely used for measuring of intracellular pHs.32 The common features of SNARF-1, in comparison to BRosol, are that it has a similar pKa value of 7.4 and both the phenolic and phenolate forms are emissive. On the other hand, for SNARF-1, both the phenolic and phenolate forms have rather small Stokes shifts (31 nm and 48 nm, respectively), and the emission peak separation between the phenolic and phenolate forms is much smaller (Δλem = 50 nm: λem = 586 nm at pH 6; λem = 636 nm at pH 9) than that of BRosol. Such bent-shape dipolar dyes generally emit in the shorter wavelengths and have smaller Stokes shifts compared with the corresponding linear-shape analogues.19,20
Cellular imaging evaluation of BRosol and BRosam derivatives
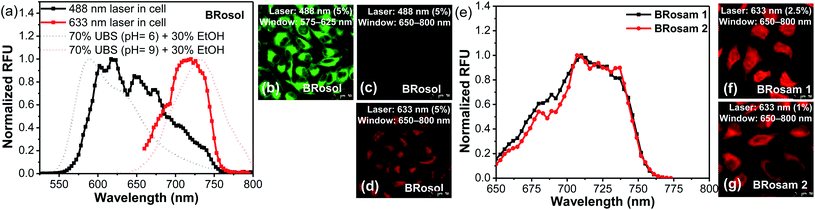
Prior to cellular imaging, we have evaluated the biocompatibility of the new dyes. BRosol and its derivatives are biocompatible, as indicated by the cell viability assay conducted for each fluorophore at 5.0, 10, and 20 μM for 12 h. The BRosam dyes exhibited a little cellular toxicity at a higher concentration of 20 μM, but, at the 10 μM level, the cell viability was more than 90% even after 12 h (Fig. S11 in the ESI†).For the cellular imaging, first we obtained the cellular emission spectra of BRosol under excitation at different wavelengths (laser sources: 488 nm, 514 nm, 561 nm, 594 nm and 633 nm) at 1–5% laser power (Fig. 5 and S12 in the ESI†). Under excitation at 488 nm, at which the phenolic form of BRosol has a significantly higher absorbance than its phenolate form, the cellular emission spectrum overlaps well with that of the phenolic form observed in solution at pH 6.0. Under excitation at both 514 nm and 561 nm, the cellular emission spectra cover both the phenolic and phenolate forms. Under excitation at 594 nm and further at 663 nm where the phenolic form has insignificant absorbance, the cellular spectra overlap well with the emission spectrum of the phenolate form obtained in the pH 9.0 solution. According to the cellular emission spectra obtained under excitation at 488 nm and 633 nm, respectively, during cellular imaging by confocal laser-scanning microscopy (CLSM), we can resolve the emissions from the phenolic and phenolate forms of BRosol through separate windows, 575–625 nm and 650–800 nm respectively (Fig. 5b–d). Thus, fluorescent probes based on BRosol are expected to enable ratiometric cellular imaging with a minimal spectral overlap under the dual excitation/emission conditions. Indeed, this is the case, as demonstrated with a BRosol-derived biothiol probe (see below). In the case of BRosam dyes, their cellular emission spectra are very similar: They are broad and cover a wide spectral range from 650–750 nm (Fig. 5e). BRosam dyes also provide very bright fluorescence images in cells at a lower laser power (1.0–2.5% at 633 nm, Fig. 5f and g), offering novel NIR-emitting dyes for bioimaging applications.
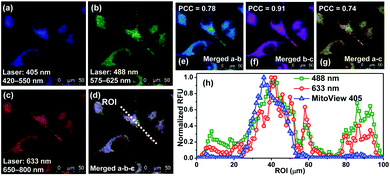
At this point, we conducted dye colocalization experiments to figure out the cellular localization of BRosol (Fig. 6), with respect to its phenolic and phenolate forms. Because BRosol contains a hydrophobic and cationic moiety, we suspected whether it, either in the phenolic or phenolate form, could localize in the mitochondria. To this end, we chose a commercial blue dye MitoView 405 that selectively stains the mitochondria. HeLa cells coincubated with BRosol and MitoView 405 for 15 min at 37 °C were fluorescently imaged by confocal laser scanning microscopy (CLSM). By choosing appropriate excitation wavelengths (405 nm, 488 nm, and 633 nm) and emission windows (blue, green, and red), it was possible to separate fluorescence images from MitoView 405, the phenolic BRosol, and the BRosol phenolate. The resulting colocalization data indicate that BRosol, either in the phenolic or phenolate form, mostly localizes in the mitochondria (Fig. 6): The intensity profiles along the region-of-interest (ROI) show a small discrepancy region and the Pearson's colocalization coefficient (PCC) values are quite high (PCC = 0.78 between the images of MitoView40 and phenolic BRosol; PCC = 0.74 between the images of MitoView40 and BRosol phenolate). Both the phenolic and phenolate forms of BRosol have a higher PCC value of 0.91.
Imaging of tissues with BRosol and BRosam derivatives
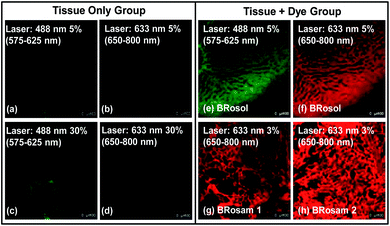
A big advantage of deep-red/NIR emitting dyes is that they enable florescence imaging with minimal autofluorescence, fluorescence interference from innate biomolecules. All the dyes provide bright fluorescence images from the green-orange and deep-red emission channels, respectively (Fig. 7). Accordingly, at the low laser power (≤5%), by using the new dyes we can obtain bright fluorescence images of tissues with little autofluorescence interference.Application of BRosol as a ratiometric imaging platform
To demonstrate the potential of BRosol as a ratiometric imaging platform, BRosol-DNP (DNP represents the 2,4-dinitrophenyl group) was studied as a new biothiol probe, as other aryl DNP ethers are known to sense biothiols such as glutathione (GSH).33–35 For example, GSH displaces the DNP moiety in related aryl DNP ethers (ArO-DNP) through nucleophilic aromatic substitution, generating the corresponding aryl alcohols (ArOH). This conversion was mostly accompanied by a fluorescence turn-on response, owing to the fluorescence quenching effect by the nitro group through the photo-induced electron transfer (PET) mechanism. However, unlike the common cases, BRosol-DNP emitted strong fluorescence in a wide emission window from the green to red regions when excited at its absorption maximum, 460 nm (Fig. S13 in the ESI†). As the absorption and emission spectra of BRosol itself appear at different wavelengths from the probe, it can be used to detect GSH with ratiometric signal responses (see below).The “non-quenched” emission behaviour from the DNP-substituted BRosol is likely due to the lower HOMO energy level of the BRosol moiety compared to the LUMO level of the DNP, which makes the possible PET quenching difficult. Thanks to the highly conjugated nature of BRosol and thus the lower LUMO energy level (Fig. 8), the commonly observed PET-quenching from the nitroaryl group is suppressed in both the dinitrophenyl- and mononitrophenyl-substituted BRosol derivatives (BRosol-NBE: Fig. S14 and S15 in the ESI†). Significant orbital overlaps between the LUMO and HOMO (also HOMO−1) also support the highly fluorescent nature of the dinitrophenyl derivatives. In other words, the highly conjugated feature of BRosol and thus its lower LUMO energy level is an added merit, from which we can generate the fluorescent nitroaryl derivatives.
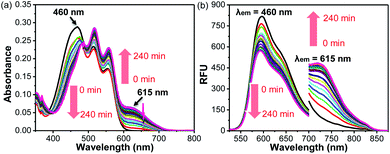
Given that BRosol and BRosol-DNP emit fluorescence in different channels, we moved to evaluate the ratiometric imaging capability of BRosol-DNP toward intracellular GSH under the dual excitation/emission conditions. First, we evaluated the ratiometric sensing behavior of BRosol-DNP toward GSH in the mixed medium of 30% EtOH/PBS (pH 7.4) at 37 °C. In the presence of GSH at a biologically relevant concentration (10 mM), the emission peak from BRosol-DNP (10 μM) at 590 nm decreased gradually while a new peak at 725 nm increased, which was ascribed to the BRosol molecules produced from the supposed nucleophilic aromatic substitution by GSH. Concurrently, the absorption peak at 460 nm from the probe decreased while that at 615 nm (from the BRosol produced) increased (Fig. 9a and b). There were no further signal changes after 180 min under the conditions (Fig. S16 in the ESI†).
BRosol-DNP also showed high selectivity toward GSH over other potentially competing analytes; It exhibited prominent absorption and emission spectral changes only toward GSH over the other analytes, when examined for the biothiols at their biologically relevant concentrations and for the other analytes at 1.0 mM level. Only hydrogen sulfide and cysteine may cause slight interference in detecting intracellular GSH levels, as shown in the selectivity data presented by the ratio values in the absorbance (A615/A460) or in the fluorescence intensity (I725/I590) estimated by the peak height (Fig. S17 in the ESI†). The basal ratio (I725/I590) values are due to the residual fluorescence of the probe in the NIR region. The time-course fluorescence response of BRosol-DNP toward GSH at different concentrations exhibits concentration-dependent changes (Fig. S18 in the ESI†).
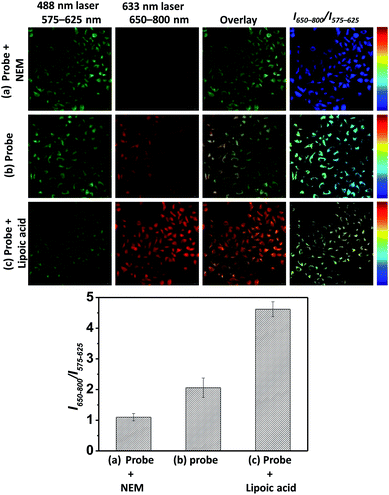
In light of the ratiometric sensing capability of BRosol-DNP toward GSH in solution, next we applied it to observe intracellular GSH levels through ratiometric fluorescence imaging under the dual excitation/emission conditions. HeLa cells incubated with BRosol-DNP (10 μM) at 37 °C for 2 h were subjected to fluorescence imaging using confocal laser scanning microscopy. As a negative control experiment, prior to incubation with the probe, HeLa cells were pre-incubated for 30 min with N-ethylmaleimide (NEM, 100 μM), which consumes GSH to form the corresponding conjugate addition product (Fig. 10a). As a positive control, the time HeLa cells were pre-incubated for one day with lipoic acid (500 μM), which increases the intracellular GSH level (Fig. 10c).36 The cellular fluorescence images indicate a lower level of intracellular GSH in the negative control, whereas an opposite result in the case of the positive control experiment. A comparison of the signal intensity ratios between the red and green images (I650–800/I575–625) provides the relative GSH levels quantitatively. At this point, it should be noted that fluorescence of such phenolic probes would be dependent on pH changes in general. Therefore, we should take care of such interference in real applications. In this regard, the use of Br-BRosol that has a lower pKa value would be better than the use of BRosol for the probe development.
In short, BRosol offers a unique ratiometric sensing platform capable of fluorescence microscopic imaging under the dual excitation/emission conditions, as demonstrated here with BRosol-DNP. Thus, a door is open to develop various fluorescent probes with ratiometric imaging capability under the dual excitation/emission conditions, by introducing other analyte-specific reactive groups to BRosol and its analogues at the hydroxyl arm.
Conclusions
NIR-emitting compounds such as cyanine and hemicyanine dyes are widely used for bioimaging. Their structures are characterized by a polymethine moiety conjugated to a cationic heterocycle, which seems to be the feature responsible for their limited photostability. The key structural component is also known to react with some reactive oxygen species and biothiols, as indicated by a number of so-called reaction-based probes. Therefore, there is a strong demand to develop new NIR fluorophores that have high photostability as well as high chemo-stability toward those reactive species. Such NIR fluorophores are disclosed here, which have a functional arm and, furthermore, provide novel ratiometric imaging platforms. The new fluorophore system, benzorosol, belongs to aryl alcohol-type NIR fluorophores, of which O-functionalized derivatives also provide bright cellular fluorescence in contrast to indolinum-containing hemicyanine dyes. Both the free hydroxyl and O-functionalized forms have large Stokes shifts as well as a large emission peak separation, enabling ratiometric bioimaging under the dual excitation and dual emission conditions. From benzorosol, the corresponding benzorosamine compounds can be readily prepared, which possess similar photophysical properties to the parent benzorosol. The ratiometric imaging capability of benzorosol is demonstrated by its 2,4-dinitrophenyl ether derivative, which selectively detects cellular glutathione. The new fluorophore systems have several notable features, such as ease of synthesis, NIR emission, high photo- and chemo-stability, large Stokes shifts, biocompatibility, and high cellular brightness. As robust NIR fluorophores, they hold great promise for bioconjugation as well as for the development of ratiometric imaging probes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the following programs: Global Research Laboratory Program (2014K1A1A2064569) through the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning; Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1A2C2085438).Notes and references
- C. Shi, J. B. Wu and D. Pan, J. Biomed. Opt., 2016, 21, 050901 CrossRef PubMed.
- Y. W. Jun, H. R. Kim, Y. J. Reo, M. Dai and K. H. Ahn, Chem. Sci., 2017, 8, 7696–7704 RSC.
- A. Bera, D. Bagchi and S. K. Pal, J. Phys. Chem. A, 2019, 123, 7550–7557 CrossRef CAS PubMed.
- H. Chen, B. Dong, Y. Tang and W. Lin, Acc. Chem. Res., 2017, 50, 1410–1422 CrossRef CAS PubMed.
- S. Singha, Y. W. Jun, S. Sakar and K. H. Ahn, Acc. Chem. Res., 2019, 52, 2571–2581 CrossRef CAS PubMed.
- E. Lacivita, M. Leopoldo, F. Berardi, N. A. Colabufo and R. Perrone, Curr. Med. Chem., 2012, 19, 4731–4741 CrossRef CAS PubMed.
- K. Tiensomjitr, R. Noorat, K. Wechakorn, S. Prabpai, K. Suksen, P. Kanjanasirirat, Y. Pewkliang, S. Borwornpinyo and P. Kongsaeree, Spectrochim. Acta, Part A, 2017, 185, 228–233 CrossRef CAS PubMed.
- Z. Li, X. Li, X. Gao, Y. Zhang, W. Shi and H. Ma, Anal. Chem., 2013, 85, 3926–3932 CrossRef CAS PubMed.
- L. Yuan, W. Lin, S. Zhao, W. Gao, B. Chen, L. He and S. Zhu, J. Am. Chem. Soc., 2012, 134, 13510–13523 CrossRef CAS PubMed.
- X. Wu, L. Li, W. Shi, Q. Gong and H. Ma, Angew. Chem., Int. Ed., 2016, 55, 14728–14732 CrossRef CAS PubMed.
- S. H. Yang, Q. Sun, H. Xiong, S. Y. Liu, B. Moosavi, W. C. Yang and G. F. Yang, Chem. Commun., 2017, 53, 3952–3955 RSC.
- Y. Jin, X. Tian, L. Jin, Y. Cui, T. Liu, Z. Yu, X. Huo, J. Cui, C. Sun, C. Wang, J. Ning, B. Zhang, L. Feng and X. Ma, Anal. Chem., 2018, 90, 3276–3283 CrossRef CAS PubMed.
- Q. Miao, D. C. Yeo, C. Wiraja, J. Zhang, X. Ning, C. Xu and K. Pu, Angew. Chem., Int. Ed., 2018, 57, 1256–1260 CrossRef CAS PubMed.
- X. Gao, G. Ma, C. Jiang, L. Zeng, S. Jiang, P. Huang and J. Lin, Anal. Chem., 2019, 91, 7112–7117 CrossRef CAS PubMed.
- Z. Luo, L. Feng, R. An, G. Duan, R. Yan, H. Shi, J. He, Z. Zhou, C. Ji, H. Y. Chen and D. Ye, Chem. – Eur. J., 2017, 23, 14778–14785 CrossRef CAS PubMed.
- J. Ning, T. Liu, P. Dong, W. Wang, G. Ge, B. Wang, Z. Yu, L. Shi, X. Tian, X. Huo, L. Feng, C. Wang, C. Sun, J. Cui, T. D. James and X. Ma, J. Am. Chem. Soc., 2019, 141, 1126–1134 CrossRef CAS PubMed.
- H. J. Park, C. W. Song, S. Sakar, Y. W. Jun, Y. J. Reo, M. Dai and K. H. Ahn, Chem. Commun., 2020, 56, 7025–7028 RSC.
- (a) Y. Yang, M. Lowry, X. Xu, J. O. Escobedo, M. Sibrian-Vazquez, L. Wong, C. M. Schowalter, T. J. Jensen, F. R. Fronczek, I. M. Warner and R. M. Strongin, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 8829–8834 CrossRef CAS PubMed; (b) R. M. Strongin, I. M. Warner, Y. Yang, M. Lowry, S. O. Fakayode, J. O. Escobedo-Cordova and X. Xu, WO 2008/011508 A1, 2008 Search PubMed; (c) R. M. Strongin, M. Sibrian-Vazquez, Z. O. Escobedo-Cordova and M. A. Lowry, WO 2013/003815, 2013 Search PubMed.
- D. Kim, P. X. Qui, H. Moon, Y. W. Jun and K. H. Ahn, Asian J. Org. Chem., 2014, 3, 1089–1096 CrossRef CAS.
- M. Dai, H. Lee, Y. J. Yang, M. Santra, C. W. Song, Y. W. Jun, Y. J. Reo, W. J. Kim and K. H. Ahn, Chem. – Eur. J., 2020 DOI:10.1002/chem.202001163.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS PubMed.
- D. Maity, A. Raj, P. K. Samanta, D. Karthigeyan, T. K. Kundu, S. K. Pati and T. Govindaraju, RSC Adv., 2014, 4, 11147–11151 RSC.
- X. Han, X. Song, F. Yu and L. Chen, Chem. Sci., 2017, 8, 6991–7002 RSC.
- R. Misra and S. P. Bhattacharyya, Intramolecular Charge Transfer, Theory and Applications, Wiley-VCH, 2018 Search PubMed.
- X. Tian, Z. Li, Y. Sun, P. Wang and H. Ma, Anal. Chem., 2018, 90, 13759–13766 CrossRef CAS PubMed.
- N. Karton-Lifshin, E. Segal, L. Omer, P. M. ortnoy, R. Satchi-Fainaro and D. Shabat, J. Am. Chem. Soc., 2011, 133, 10960–10965 CrossRef CAS PubMed.
- N. Karton-Lifshin, L. Albertazzi, M. Bendikov, P. S. Baran and D. Shabat, J. Am. Chem. Soc., 2012, 134, 20412–20420 CrossRef CAS PubMed.
- H. Yu, Y. Wu, Y. Hu, X. Gao, Q. Liang, J. Xu and S. Shao, Talanta, 2017, 165, 625–631 CrossRef CAS PubMed.
- Y. Chen, C. Zhu, Z. Yang, J. Chen, Y. He, Y. Jiao, W. He, L. Qiu, J. Cen and Z. Guo, Angew. Chem., Int. Ed., 2013, 52, 1688–1691 CrossRef CAS PubMed.
- Y. Liu, K. Li, M. Wu, Y. Liu, Y. Xie and X. Yu, Chem. Commun., 2015, 51, 10236–10239 RSC.
- Y. Zhang, L. Guan, H. Yu, Y. Yan, L. Du, Y. Liu, M. Sun, D. Huang and S. Wang, Anal. Chem., 2016, 88, 4426–4431 CrossRef CAS PubMed.
- T. M. Zurawik, A. Pomorski, A. Belczyk-Ciesielska, G. Goch, K. Niedzwiedzka, R. Kucharczyk, A. Krezel and W. Bal, PLoS One, 2016, 11(8), e0161353 CrossRef PubMed.
- H. Maeda, H. Matsuno, M. Ushida, K. Katayama, K. Saeki and N. Itoh, Angew. Chem., Int. Ed., 2005, 44, 2922–2925 CrossRef CAS PubMed.
- J. Bouffard, Y. Kim, T. M. Swager, R. Weissleder and S. A. Hilderbrand, Org. Lett., 2008, 10, 37–40 CrossRef CAS PubMed.
- Q. Liu, A. Li, X. Li, B. Li, Y. Zhang, J. Li and Y. Guo, Sens. Actuators, B, 2019, 10, 820–830 CrossRef.
- B. Hultberg, A. Andersson and A. Isaksson, Toxicol, 2002, 175, 103–110 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc03314f |
| This journal is © The Royal Society of Chemistry 2020 |

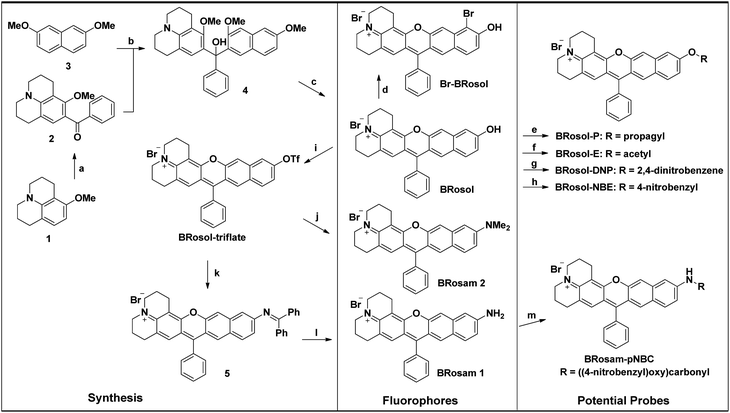
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH, Pd2(dba)3, Xantphos, Cs2CO3, toluene, 100 °C, overnight; (l) NaOAc, HONH2·HCl, MeOH, room temp., 1 h, 51%(2 steps); (m) 4-nitrobenzyl chloroformate, DMAP, pyridine, CH2Cl2, room temp., overnight, 86%.
NH, Pd2(dba)3, Xantphos, Cs2CO3, toluene, 100 °C, overnight; (l) NaOAc, HONH2·HCl, MeOH, room temp., 1 h, 51%(2 steps); (m) 4-nitrobenzyl chloroformate, DMAP, pyridine, CH2Cl2, room temp., overnight, 86%.