Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible light driven deuteration of formyl C–H and hydridic C(sp3)–H bonds in feedstock chemicals and pharmaceutical molecules†
Yulong
Kuang
 ,
Hui
Cao
,
Haidi
Tang
,
Junhong
Chew
,
Wei
Chen
,
Xiangcheng
Shi
and
Jie
Wu
,
Hui
Cao
,
Haidi
Tang
,
Junhong
Chew
,
Wei
Chen
,
Xiangcheng
Shi
and
Jie
Wu
 *
*
Department of Chemistry, National University of Singapore, 3 Science Drive 3, 117543, Republic of Singapore. E-mail: chmjie@nus.edu.sg
First published on 5th August 2020
Abstract
Deuterium labelled compounds are of significant importance in chemical mechanism investigations, mass spectrometric studies, diagnoses of drug metabolisms, and pharmaceutical discovery. Herein, we report an efficient hydrogen deuterium exchange reaction using deuterium oxide (D2O) as the deuterium source, enabled by merging a tetra-n-butylammonium decatungstate (TBADT) hydrogen atom transfer photocatalyst and a thiol catalyst under light irradiation at 390 nm. This deuteration protocol is effective with formyl C–H bonds and a wide range of hydridic C(sp3)–H bonds (e.g. α-oxy, α-thioxy, α-amino, benzylic, and unactivated tertiary C(sp3)–H bonds). It has been successfully applied to the high incorporation of deuterium in 38 feedstock chemicals, 15 pharmaceutical compounds, and 6 drug precursors. Sequential deuteration between formyl C–H bonds of aldehydes and other activated hydridic C(sp3)–H bonds can be achieved in a selective manner.
Introduction
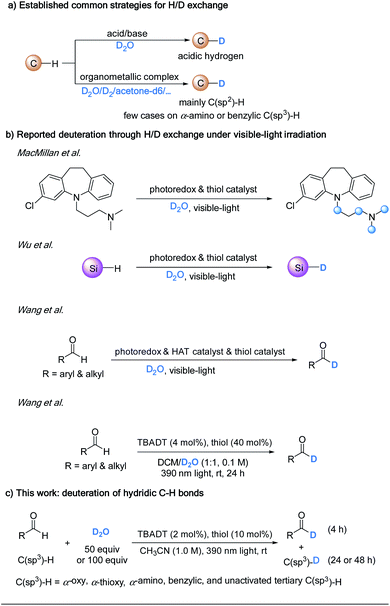
Deuterium labelling of organic molecules is of vital importance in many aspects.1 Deuterated compounds have been widely applied for the investigation of reaction mechanisms2 and diagnosis of drug metabolisms.3 The introduction of deuterium into pharmaceutical molecules can alter the absorption, distribution, metabolism, excretion, and toxicity (ADME-Tox) properties.1 In 2017, the U.S. Food and Drug Administration (FDA) approved SD-809 (Austedo) for the treatment of chorea associated with Huntington's disease.4 Austedo has OCD3 groups in place of the two OCH3 groups on the phenyl ring of its parent FDA approved drug, Xenazine, but shows similar efficacy at lower doses and has a longer duration of action. Currently, there are many other deuterium-containing drugs under clinical testing, such as d6-dextromethorphan (AVP-786).5 The importance of introducing deuterium into drug molecules originates from its significant kinetic isotope effects (KIEs) in enzyme-catalyzed metabolizing reactions (e.g., cytochrome P450 enzyme,6 aldehyde oxidase,7 and monoamine oxidase8), resulting in a slower oxidative metabolism of the drug compounds. Therefore, it is important to incorporate deuterium atoms at readily metabolized sites in pharmaceutical compounds, especially the activated hydridic C(sp3)–H bonds.Deuterium can be incorporated into a molecule by halogen/deuterium exchange9 or by reductive deuteration,10 which require the preparation of suitable precursors. A more atom- and step-economic pathway for the introduction of deuterium is hydrogen deuterium (H/D) exchange.11 Two well-known methods for H/D exchange are pH-dependent12 and transition-metal catalyzed H/D exchange.13 While the acid/base system is used to achieve the deuteration of acidic hydrogens, transition-metal catalysis is mainly applied to C(sp2)–H deuteration. Transition-metal catalysis has also been occasionally utilized in H/D exchange of C(sp3)–H bonds, including α-amino14 and benzylic C–H bonds15 (Scheme 1a).
In comparison, the emergence of photocatalysis has provided enormous opportunities for regioselective C(sp3)–H functionalization.16 MacMillan et al. have reported a hydrogen isotope exchange at α-amino C(sp3)–H bonds by the combination of a photoredox catalyst to generate the carbon radical, and a thiol catalyst to transfer the isotopic atom from isotopically labelled water (Scheme 1b).17 Our lab has reported a related visible-light-mediated deuteration of silanes using D2O as the deuterium source through the synergistic effect of a photoredox catalyst and a thiol catalyst.18 However, the substrate scope of photoredox catalyzed C(sp3)–H functionalization is limited, because it is successful only when the redox potential of the C–H substrate is matched with that of the photocatalyst.19 Wang et al. have recently reported a H/D exchange at formyl groups of both aromatic and aliphatic aldehydes, by merging a photoredox catalyst, a hydrogen atom transfer (HAT) catalyst, and a thiol catalyst.20
Direct HAT photocatalysis offers a broad substrate scope in C(sp3)–H functionalization without the need to match redox potentials.21,22 Among the direct HAT photocatalysts, tetra-n-butylammonium decatungstate (TBADT) represents a versatile one which can activate formyl C–H and a wide range of C(sp3)–H bonds.23 We envision that by taking advantage of a direct HAT photocatalyst,21 a variety of formyl C–H and hydridic C(sp3)–H bonds can be deuterated. In line with our continuing interest in direct HAT photocatalyzed C–H functionalization,22 we report herein that by implementing the cooperative effect of TBADT with a thiol catalyst, various types of formyl C–H and electron-rich C(sp3)–H bonds can be deuterated using deuterium oxide as the source of deuterium under irradiation by near-ultraviolet light (390 nm). Notably, during the process of our study, a similar catalytic system was reported by Wang et al. for the deuteration of only formyl C–H bonds.24 In stark contrast, with the addition of tetra-n-butylammonium bromide (TBAB) in our hand, the HAT efficiency was significantly improved, and the deuteration scope can go far beyond formyl C–H bonds, enabling the selective deuteration of a wide range of hydridic C(sp3)–H bonds, including α-oxy, α-thioxy, α-amino, benzylic and unactivated tertiary C(sp3)–H bonds (Scheme 1c). This provides a convenient and effective protocol for the regioselective incorporation of deuterium into drug molecules and their precursors and promises to find wide application in biological and pharmaceutical studies.
Results and discussion
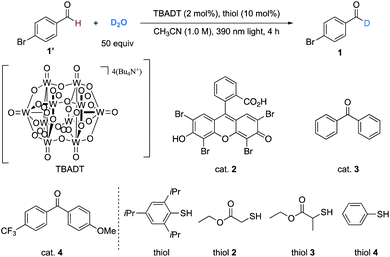
Our investigation began with the deuteration of 4-bromobenzaldehyde as a model reaction using D2O as the deuterium source. Direct HAT photocatalysts in combination of thiol catalysts (BDE of 2,4,6-triisopropylbenzenethiol = 80 kcal mol−1)10b were evaluated because the formyl radical could not directly abstract deuterium atom from D2O (BDE of H2O = 119 kcal mol−1vs. BDE of formyl C(sp2)–H in benzaldehyde = 86.9 kcal mol−1).25 After extensive evaluation (Table 1), we established that the combination of TBADT (2 mol%), 2,4,6-triisopropylbenzenethiol (10 mol%), and D2O (50 equiv.) in acetonitrile (1.0 M) irradiated by 390 nm Kessil light (80 W) was the optimal condition, affording product 1 in 85% isolated yield with 94% deuterium incorporation within 4 hours (Table 1, entry 1). Light, TBADT, the thiol catalyst, and solvent were of significance in achieving a high deuteration efficiency (entries 2–5). Changing TBADT to other types of HAT photocatalysts (entries 6–8) or changing the thiol catalyst (entries 9–11) resulted in lower levels of deuterium incorporation. A less concentrated reaction or switching CH3CN to other solvents would cause decreasing of the deuteration ratio (entries 12–16).| Entry | Deviation | Yieldb (%) | D-inc.c (%) |
|---|---|---|---|
| a Reactions were conducted following the optimal conditions with indicated deviation. b Yield was determined by the analysis of 1H NMR spectra of the crude product mixture using CH2Br2 as an internal standard. c Deuterium incorporation was determined by the analysis of 1H NMR spectra of the products. d Yield in the parentheses was the isolated yield. e Reaction was irradiated under a blue light emitting diode (LED, 24 W) for 24 h. | |||
| 1 | No change | 99% (85%)d | 94% |
| 2 | No light | 99% | 0% |
| 3 | No TBADT | 99% | 19% |
| 4 | No thiol | 99% | 30% |
| 5 | Neat | 99% | 14% |
| 6e | Cat. 2 (5 mol%) instead of TBADT | 99% | 0% |
| 7 | Cat. 3 (10 mol%) instead of TBADT | 94% | 38% |
| 8 | Cat. 4 (10 mol%) instead of TBADT | 99% | 28% |
| 9 | Thiol 2 instead of thiol | 99% | 40% |
| 10 | Thiol 3 instead of thiol | 99% | 27% |
| 11 | Thiol 4 instead of thiol | 99% | 83% |
| 12 | CH3CN (0.5 M) | 99% | 89% |
| 13 | CH3CN (0.2 M) | 99% | 88% |
| 14 | Acetone (1.0 M) | 99% | 87% |
| 15 | CH2Cl2 (1.0 M) | 99% | 90% |
| 16 | tBuOH (1.0 M) | 99% | 19% |
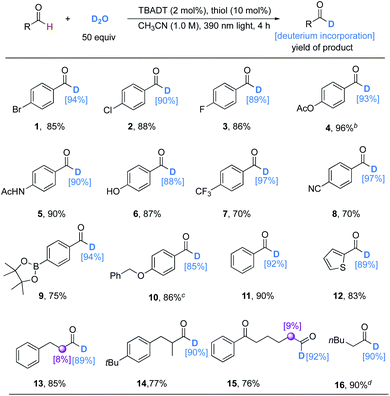
With the optimal conditions in hand, we evaluated the reaction scope with respect to aldehydes (Table 2). The results were similar to those of Wang's, but lower amounts of catalysts and D2O and a shorter reaction time were required. The transformations performed well with aromatic aldehydes to give products in generally good to excellent (70% to 96%) isolated yields and deuterium incorporation (88% to 97%). Functionalities ranging from electron-withdrawing substituents such as halides (1–3), trifluoromethyl (7), cyanides (8), and boronate esters (9), to electron-donating substituents, including amides (5), phenols (6), and ethers (10), were well tolerated. However, 4-acetoxybenzaldehyde gave very low deuterium incorporation (14%) under these conditions. Changing the light source to a 365 nm LED (24 W) with an extended reaction time (24 h) could deliver product 4 with 93% deuterium incorporation. Heteroaromatic aldehydes like 2-thenaldehyde proceeded well to afford product 12 with high deuterium incorporation. This protocol could also be applied to both linear and branched alkyl aldehydes with good regioselectivity and excellent deuterium incorporation (13–16). Even the volatile hexanal (bp = 129 °C) underwent 90% selective deuteration of its formyl group (16).
| a Unless otherwise noted, the reactions were conducted under the optimized conditions for 4 h. Isolated yields are given. The deuterium incorporation was determined by the analysis of the 1H NMR spectra of the crude products. b 365 nm LED light (24 W) was used instead of 390 nm Kessil light (80 W) for 24 h. c Reaction time is 8 h. d Due to the volatility of product 16, the results were determined by the analysis of the crude 1H NMR spectra with the reaction conducted in CD3CN (1.0 M) using CH2Br2 as an internal standard. |
|---|
|
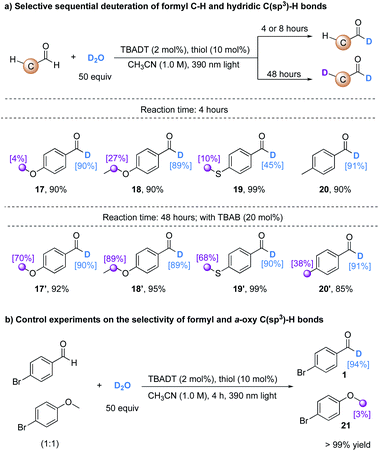
TBADT is a well-known powerful photocatalyst which can activate not only formyl C–H bonds, but also activated and unactivated hydridic C(sp3)–H bonds.23 During our study of deuteration of the formyl C–H bond in 4-methoxybenzaldehyde, we observed that this formyl C–H bond underwent H/D exchange in a selective fashion within the first 4 h of light irradiation (17), and the methoxy group was subsequently deuterated with 70% deuterium incorporation, giving 17′ by prolonging the reaction time to 48 h, assisted by the addition of a phase transfer catalyst TBAB (20 mol%) to accelerate the reaction rate by transferring more decatungstate anions ([W10O32]4−) to the organic phase. This selective sequential deuteration can be extended to aldehydes bearing α-oxy, α-thioxy, or benzylic C(sp3)–H bonds (17–20, 17′–20′, Table 3a). The examination of the site selectivity established a trend that more hydridic C(sp3)–H bonds undergo C–H isotopic exchange to a higher extent. High levels of deuterium incorporation are beneficial in the application of deuterated compounds as internal standards in pharmaceutical studies.1 To further demonstrate the selectivity of our H/D exchange protocol, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 4-bromobenzaldehyde and 4-bromoanisole was treated under the optimal reaction conditions with 4 h light irradiation, achieving a deuteration selectivity >10
1 mixture of 4-bromobenzaldehyde and 4-bromoanisole was treated under the optimal reaction conditions with 4 h light irradiation, achieving a deuteration selectivity >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, highlighting the excellent reactivity of formyl C–H bonds in the direct HAT photocatalysis (Table 3b).
1, highlighting the excellent reactivity of formyl C–H bonds in the direct HAT photocatalysis (Table 3b).
| a Reactions were conducted under the optimized conditions. TBAB (20 mol%) was added when the reaction time was 48 h. Isolated yields are given. The deuterium incorporation was determined by the analysis of the 1H NMR spectra of products. |
|---|
|
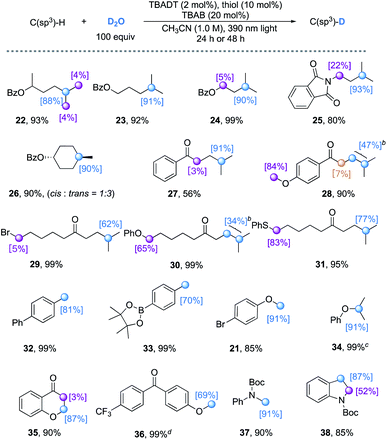
With an extended reaction time (24–48 h), the TBADT/thiol catalyzed H/D exchange also proceeds with various hydridic C(sp3)–H bonds. Both the polarity effect and steric effect play an important role in determining the regioselectivity in TBADT-catalyzed C–H functionalizations.23c As illustrated in Table 4, the tertiary C(sp3)–H bonds in alkanes bearing remote electron-withdrawing functional groups such as esters, amides, and ketones can be selectively deuterated (22–31). Both cyclic and acyclic alkanes afforded the corresponding products with moderate to good deuterium incorporation. When a substrate bearing both activated C(sp3)–H bonds and a tertiary C–H bond was used (25, 28, 30, and 31), both C–H bonds underwent H/D exchange to some extent. Addition of 20 mol% TBAB was crucial for an efficient H/D exchange, as significantly lower deuterium incorporation was observed in the absence of TBAB (e.g. 36% deuterium incorporation for 22 without TBAB). The benzylic (32 and 33), α-ether (21 and 34–36) and α-amino (37 and 38) C(sp3)–H bonds were all feasible substrates for this H/D exchange protocol. Trifluoromethylbenzene can be used as a cosolvent to improve the deuterium incorporation (e.g.36, from 37% to 69%) when the substrate is poorly soluble in acetonitrile.
| a Unless otherwise noted, reactions were conducted under optimized conditions with the addition of 20 mol% TBAB, irradiated by 390 nm light for 24 or 48 h. Isolated yields are given. The deuterium incorporation was determined by the analysis of the 1H NMR spectra of products. b The overall deuterium incorporation ratio of three C(sp3)–H bonds is given. c Due to the volatility of the substrate, the yield was determined by the analysis of the crude 1H NMR spectra using CH2Br2 as an internal standard. d C6H5CF3 (1.0 M) was added to solubilize the starting substrate. |
|---|
|
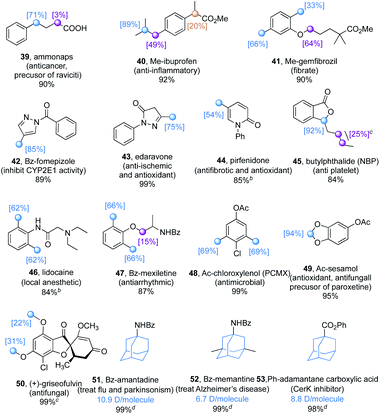
The promising functional group compatibility and the wide scope of hydridic C(sp3)–H bonds suitable for this dual catalytic method promoted an evaluation of the deuteration of a library of commercially available pharmaceuticals (Table 5), especially those with benzylic C–H bonds and methoxy moieties that are prone to metabolic oxidation.26 While isotopic exchange was preferred at the benzylic C–H bond of the acid form of ammonaps (39), decarboxylation was observed with ibuprofen and gemfibrozil, which possess a secondary and a tertiary acid moiety respectively. This is probably due to the TBADT promoted photoredox side reactions,27 and secondary and tertiary acids are more labile than the primary acid due to the generation of more stable carbon radicals. After methylation to avoid the undesired decarboxylation, the H/D exchange occurred at the tertiary and benzylic C–H bonds of ibuprofen methylester 40, as well as at the benzylic and α-oxy C–H bonds of gemfibrozil methylester 41. Heterocyclic drugs, such as benzoyl protected fomepizole (42), edaravone (43) and pirfenidone (44), were deuterated selectively at the benzylic C–H bonds. Notably, the dual catalytic system is not efficient with basic substrates probably due to the acidic nature of TBADT. To overcome this limitation, the free N–H was protected (42) or trifluoroacetic acid (TFA) was added (44) to achieve a smooth H/D exchange. Butylphthalide (45), TFA acidified lidocaine (46), benzoyl protected mexiletine (47), and acetyl protected chloroxylenol (48) could be converted to isotopically enriched products by deuterium incorporation which occurred mainly at the benzylic C–H bonds. Moreover, acetyl protected sesamol, a natural product and a precursor of paroxetine, could be deuterated selectively at the acetal moiety (49). (+)-Griseofulvin, an antifungal drug, was converted to the deuterated product 50 with moderate deuterium incorporation at the phenyl methoxy groups. Adamantane derivatives are an important family of drug molecules.28 For instance, amantadine is an antiviral drug effectively against various strains of influenza and is also used to treat Parkinson's disease, memantine is used to treat Alzheimer's disease, and 1-adamantanecarboxylic acid is a CerK inhibitor. The N-benzoyl protected amantadine (51) and memantine (52), and phenyl adamantane carboxylate (53) could be deuterated with more than 5 deuterium atoms per molecule and with less than 0.1% of unlabelled compound remaining, meeting the requirement for their usage as internal standards in ADME-Tox biostudies.
| a Unless otherwise noted, reactions were conducted under optimized conditions with the addition of 20 mol% TBAB, irradiated by 390 nm light for 24 or 48 h. Isolated yields are given. The deuterium incorporation was determined by the analysis of the 1H NMR spectra of products. b 3.0 equiv TFA was added. c The average deuterium incorporation was given. d C6H5CF3 (1.0 M) was added. |
|---|
|
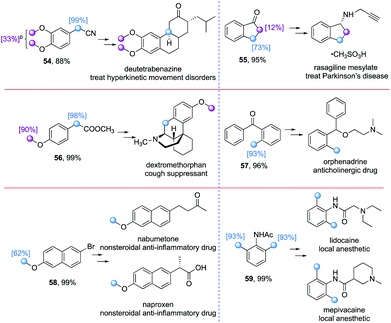
In addition to the late stage deuteration of pharmaceutical compounds, deuterium labelling of drug precursors for de novo synthesis also represents an important pathway to introduce deuterium atoms selectively into complex drug molecules. We first attempted to synthesize the precursor for the deuterated drug Austedo (deutetrabenazine, Table 6). However, the level of deuteration of the phenyl methoxy groups in compound 54 was low (33%), while there was full deuteration at the benzylic C–H bonds even though they are relatively electron deficient. Indanone (55), the precursor of rasagiline mesylate, which is used to treat Parkinson's disease, could be deuterated at the benzylic position (73%), with the α-carbonyl C–H bonds being deuterated to some extent (12%). Both the phenyl methoxy and benzylic C(sp3)–H bonds in the precursor of dextromethorphan underwent H/D exchange effectively (56). 2-Methylbenzophenone (57), 2-bromo-6-methoxynaphthalene (58), and N-(2,6-dimethylphenyl)acetamide (59) proceeded the H/D exchange smoothly with moderate to excellent deuterium incorporation (62–93%) at the activated hydridic C(sp3)–H bonds.
| a Unless otherwise noted, reactions were conducted under optimized conditions with the addition of 20 mol% TBAB, irradiated by 390 nm light for 48 h. Isolated yields are given. The deuteration incorporation was determined by the analysis of 1H NMR spectra of products. b The average deuterium incorporation was given. |
|---|
|
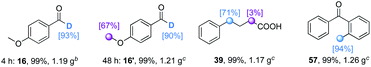
The method is scalable (Table 7). After the treatment of 4-methoxybenzaldehyde under the optimized conditions for 4 h using a lower catalyst loading (1 mol% TBADT and 5 mol% thiol), a gram-scale synthesis of deuterium labeled 16 could be achieved with an even higher (93%) deuterium level. The selective sequential deuteration of 4-anisaldehyde was also successful, producing both the formyl C–H and α-oxy C(sp3)–H labeled product 16′. Further gram-scale synthesis of the deuterated acid form of ammonaps 39 and an orphenadrine precursor 57 afforded results comparable to those obtained in the synthesis on a 0.2 mmol scale.
| a Unless otherwise noted, the reaction was conducted on a gram-scale under the optimized conditions. Isolated yields are given. The deuterium incorporation was determined by the analysis of 1H NMR spectra of products. b Reaction was conducted for 4 h by using TBADT (1 mol%) and thiol (5 mol%) in CH3CN (2.0 M). c 20 mol% TBAB was added. |
|---|
|
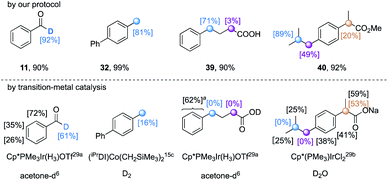
Compared to photoredox catalysis,19 the use of TBADT as a direct HAT photocatalyst exhibits a wider substrate scope without the limitation of required redox matching. Aldehydic and a variety of hydridic C(sp3)–H bonds (e.g. α-oxy, α-thioxy, α-amino, benzylic, and unactivated tertiary C(sp3)–H bonds) underwent efficient H/D exchange through the synergistic merging of TBADT and a thiol catalyst. The regioselectivity thus is orthogonal and complementary to existing pH-dependent H/D exchange (acidic protons) and transition-metal catalysis (mainly C(sp2)–H bonds). We highlighted several H/D exchange products obtained under our conditions that exhibited orthogonal regioselectivity to those obtained by reported transition-metal catalysis (Table 8).15c,29
| a Average deuterium incorporation was given. |
|---|
|
Mechanism studies
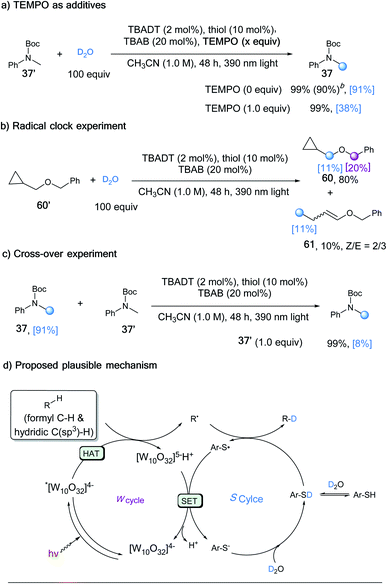
A series of control experiments were conducted to further elucidate the reaction mechanism. The deuterium incorporation was significantly lower in the presence of TEMPO (38% vs. 91%, Scheme 2a), which supported the radical process. When radical clock substrate 60′ was applied to our standard reaction conditions, the ring opening product 61 was generated, even though with a low deuterium ratio (Scheme 2b). To further probe the reaction process, deuterated 37 and un-deuterated 37′ were subjected to the reaction conditions in the absence of D2O, which resulted in a product with only 8% deuterium incorporation (Scheme 2c). This control experiment indicated that the deuterated product would also undergo H/D exchange, with the generated carbon radical intermediate capturing hydrogen atoms more favourably than deuterium atoms.A plausible mechanism was proposed in light of all the experimental data and precedent related literature (Scheme 2d).23 The transient carbon radical intermediate was generated through a HAT process between the aldehyde/alkane substrate and photo-excited *TBADT. In the presence of excess amounts of D2O, the H/D exchange of the thiol catalyst leads to dominant formation of deuterated thiol.18 The carbon radical abstracts a deuterium atom from the deuterated thiol to deliver the desired deuterated product and a thiyl radical. A single electron transfer between the thiyl radical and [W10O32]5−H+ would regenerate the TBADT catalyst and deuterated thiol after deuteration with D2O.23a,30
Conclusions
A synergistic catalytic system comprised of the direct HAT photocatalyst TBADT and a thiol catalyst was developed for H/D exchange with formyl C–H and hydridic C(sp3)–H bonds, which is orthogonal and complementary to existing pH-dependent H/D exchange and transition-metal catalyzed H/D exchange. A wide range of C–H bonds underwent the H/D exchange effectively, including the formyl C–H, α-oxy, α-thioxy, α-amino, benzylic, and unactivated tertiary C(sp3)–H bonds, as a result of the absence of required redox matching between the direct HAT photocatalyst and the substrate. The developed protocol is easily scalable. The ability to introduce deuterium atoms into unique positions of pharmaceutical molecules and drug precursors provides a new diagnostic tool for ADME-Tox studies attempting to develop new molecular therapies. Current efforts are devoted to applying this method to more pharmaceutical candidates and their bioactivity evaluation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support provided by the Ministry of Education (MOE) of Singapore (MOE2017-T2-2-081) and National University of Singapore (R-143-000-B60-114).Notes and references
- J. Atzrodt, V. Derdau, W. J. Kerr and M. Reid, Angew. Chem., Int. Ed., 2018, 57, 1758 CrossRef CAS PubMed.
- (a) M. Gómez-Gallego and M. A. Sierra, Chem. Rev., 2011, 111, 4857 CrossRef PubMed; (b) E. M. Simmons and J. F. Hartwig, Angew. Chem., Int. Ed., 2012, 51, 3066 CrossRef CAS PubMed.
- (a) E. M. Isin, C. S. Elmore, G. N. Nilsson, R. A. Thompson and L. Weidolf, Chem. Res. Toxicol., 2012, 25, 532 Search PubMed; (b) C. S. Elmore and R. A. Bragg, Bioorg. Med. Chem. Lett., 2015, 25, 167 CrossRef CAS PubMed.
- (a) Y.-A. Heo and L. J. Scott, Drugs, 2017, 77, 1857 CrossRef CAS PubMed; (b) A. Mullard, Nat. Rev. Drug Discovery, 2017, 16, 305 Search PubMed.
- L. Nguyen, A. L. Scandinaro and R. R. Matsumoto, Pharmacol., Biochem. Behav., 2017, 161, 30 CrossRef CAS PubMed.
- (a) F. P. Guengerich, Chem. Res. Toxicol., 2001, 14, 611 Search PubMed; (b) S. D. Nelson and W. F. Trager, Drug Metab. Dispos., 2003, 31, 1481 CrossRef CAS PubMed; (c) G. Chowdhury, M. W. Calcutt, L. D. Nagy and F. P. Guengerich, Biochemistry, 2012, 51, 9995 CrossRef CAS PubMed.
- R. Sharma, T. J. Strelevitz, H. Gao, A. J. Clark, K. Schildknegt, R. S. Obach, S. L. Ripp, D. K. Spracklin, L. M. Tremaine and A. D. N. Vaz, Drug Metab. Dispos., 2012, 40, 625 CrossRef CAS.
- R. V. Dunn, K. R. Marshall, A. W. Munro and N. S. Scrutton, FEBS J., 2008, 275, 3850 CrossRef CAS PubMed.
- (a) F. Alonso, I. P. Beletskaya and M. Yus, Chem. Rev., 2002, 102, 4009 CrossRef CAS PubMed; (b) C. Liu, Z. Chen, C. Su, X. Zhao, Q. Gao, G.-H. Ning, H. Zhu, W. Tang, K. Leng, W. Fu, B. Tian, X. Peng, J. Li, Q.-H. Xu, W. Zhou and K. P. Loh, Nat. Commun., 2018, 9, 80 CrossRef PubMed; (c) X. Wang, M.-H. Zhu, D. P. Schuman, D. Zhong, W.-Y. Wang, L.-Y. Wu, W. Liu, B. M. Stoltz and W.-B. Liu, J. Am. Chem. Soc., 2018, 140, 10970 CrossRef CAS PubMed.
- (a) T. Shao, Y. Li, N. Ma, C. Li, G. Chai, X. Zhao, B. Qiao and Z. Jiang, iScience, 2019, 16, 410 CrossRef CAS PubMed; (b) M. Zhang, X.-A. Yuan, C. Zhu and J. Xie, Angew. Chem., Int. Ed., 2019, 58, 312 CrossRef CAS PubMed.
- J. Atzrodt, V. Derdau, T. Fey and J. Zimmermann, Angew. Chem., Int. Ed., 2007, 46, 7744 CrossRef CAS PubMed.
- (a) T. R. Puleo, A. J. Strong and J. S. Bandar, J. Am. Chem. Soc., 2019, 141, 1467 CrossRef CAS PubMed; (b) H. V. Thulasiram, R. M. Phan, S. B. Rivera and C. D. Poulter, J. Org. Chem., 2006, 71, 1739 CrossRef CAS PubMed.
- (a) R. P. Yu, D. Hesk, N. Rivera, I. Pelczer and P. J. Chirik, Nature, 2016, 529, 195 CrossRef PubMed; (b) J. Atzrodt, V. Derdau, W. J. Kerr and M. Reid, Angew. Chem., Int. Ed., 2018, 57, 3022 CrossRef CAS PubMed.
- (a) L. V. A. Hale and N. K. Szymczak, J. Am. Chem. Soc., 2016, 138, 13489 CrossRef CAS PubMed; (b) M. Valero, R. Weck, S. Güssregen, J. Atzrodt and V. Derdau, Angew. Chem., Int. Ed., 2018, 57, 8159 CrossRef CAS PubMed.
- (a) H. Sajiki, F. Aoki, H. Esaki, T. Maegawa and K. Hirota, Org. Lett., 2004, 6, 1485 CrossRef CAS PubMed; (b) H. Sajiki, N. Ito, H. Esaki, T. Maesawa, T. Maegawa and K. Hirota, Tetrahedron Lett., 2005, 46, 6995 CrossRef CAS; (c) W. N. Palmer and P. J. Chirik, ACS Catal., 2017, 7, 5674 CrossRef CAS PubMed.
- S. Protti, M. Fagnoni and D. Ravelli, ChemCatChem, 2015, 7, 1516 CrossRef CAS.
- Y. Y. Loh, K. Nagao, A. J. Hoover, D. Hesk, N. R. Rivera, S. L. Colletti, I. W. Davies and D. W. C. MacMillan, Science, 2017, 358, 1182 CrossRef CAS PubMed.
- R. Zhou, J. Li, H. W. Cheo, R. Chua, G. Zhan, Z. Hou and J. Wu, Chem. Sci., 2019, 10, 7340 RSC.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed.
- Y. Zhang, P. Ji, Y. Dong, Y. Wei and W. Wang, ACS Catal., 2020, 10, 2226 CrossRef CAS.
- L. Capaldo and D. Ravelli, Eur. J. Org. Chem., 2017, 2056 CrossRef CAS PubMed.
- (a) X.-Z. Fan, J.-W. Rong, H.-L. Wu, Q. Zhou, H.-P. Deng, J. D. Tan, C.-W. Xue, L.-Z. Wu, H.-R. Tao and J. Wu, Angew. Chem., Int. Ed., 2018, 57, 8514 CrossRef CAS PubMed; (b) X. Fan, P. Xiao, Z. Jiao, T. Yang, X. Dai, W. Xu, J. D. Tan, G. Cui, H. Su, W. Fang and J. Wu, Angew. Chem., Int. Ed., 2019, 58, 12580 CrossRef CAS PubMed; (c) Y. Kuang, K. Wang, X. Shi, X. Huang, E. Meggers and J. Wu, Angew. Chem., Int. Ed., 2019, 58, 16859 CrossRef CAS PubMed; (d) H. Cao, Y. Kuang, X. Shi, K. L. Wong, B. B. Tan, J. M. C. Kwan, X. Liu and J. Wu, Nat. Commun., 2020, 11, 1956 CrossRef CAS PubMed.
- (a) M. D. Tzirakis, I. N. Lykakis and M. Orfanopoulos, Chem. Soc. Rev., 2009, 38, 2609 RSC; (b) V. D. Waele, O. Poizat, M. Fagnoni, A. Bagno and D. Ravelli, ACS Catal., 2016, 6, 7174 CrossRef CAS; (c) D. Ravelli, M. Fagnoni, T. Fukuyama, T. Nishikawa and I. Ryu, ACS Catal., 2018, 8, 701 CrossRef CAS; (d) G. Laudadio, Y. Deng, K. Wal, D. Ravelli, M. Nuño, M. Fagnoni, D. Guthrie, Y. Sun and T. Noël, Science, 2020, 369, 92 CrossRef CAS PubMed.
- J. Dong, X. Wang, Z. Wang, H. Song, Y. Liu and Q. Wang, Chem. Sci., 2020, 11, 1026 RSC.
- R. K. Solly and S. W. Benson, J. Am. Chem. Soc., 1971, 93, 1592 CrossRef CAS.
- M. Trunzer, B. Faller and A. Zimmerlin, J. Med. Chem., 2009, 52, 329 CrossRef CAS PubMed.
- L. Capaldo, L. Buzzetti, D. Merli, M. Fagnoni and D. Ravelli, J. Org. Chem., 2016, 81, 7102 CrossRef CAS PubMed.
- K. Spilovska, F. Zemek, J. Korabecuny, E. Nepovimova, O. Soukup, M. Windisch and K. Kuca, Curr. Med. Chem., 2016, 23, 3245 CrossRef CAS PubMed.
- (a) C. M. Yung, M. B. Skaddan and R. G. Bergman, J. Am. Chem. Soc., 2004, 126, 13033 CrossRef CAS PubMed; (b) S. R. Klei, J. T. Golden, T. D. Tilley and R. G. Bergman, J. Am. Chem. Soc., 2002, 124, 2092 CrossRef CAS PubMed.
- I. Ryu, A. Tani, T. Fukuyama, D. Ravelli, M. Fagnoni and A. Albini, Angew. Chem., Int. Ed., 2011, 50, 1869 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and NMR spectra of all new compounds. See DOI: 10.1039/d0sc02661a |
| This journal is © The Royal Society of Chemistry 2020 |