Open Access Article
Open Access ArticlePyrazolone ligation-mediated versatile sequential bioconjugations†
Melrose
Mailig
 and
Fa
Liu
and
Fa
Liu
 *
*
Novo Nordisk Research Center, 530 Fairview Avenue North, Seattle, WA 98109, USA. E-mail: falx@novonordisk.com
First published on 24th June 2020
Abstract
Bioconjugation chemistries are critical tools in biotherapeutics discovery. The past efforts have been exclusively focused on two-segment conjugations. However, emerging research directions, such as polypharmacy biotherapeutics, desire multiple-component bioconjugations where more than two pharmacologically related biomolecules can be assembled into a single construct in high efficiency. We present here a set of sequential bioconjugation chemistries centered on a pyrazolone structural motif. It starts with a clickable “pyrazolone ligation” between a hydrazine group and a β-ketoester moiety followed by the conjugation between the newly formed pyrazolone core and an aldehyde-bearing biomolecule through a Knoevenagel reaction forming a Michael addition acceptor that can effectively capture a thiol-bearing biomolecule. When utilized intermolecularly, it quickly assembles four segments together forming a quadruple functional construct. When applied intramolecularly, it offers a set of highly diverse biomolecule scaffolds including stapled peptides and poly-macrocyclic peptides. We envision broad utilities of such sequential ligation chemistries.
Introduction
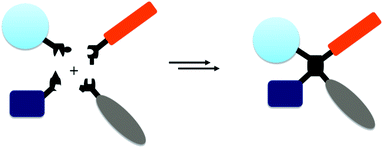
Bioconjugation chemistries are being routinely employed in the discovery of biotherapeutics. They play crucial roles in constructing dual functional single molecule constructs by joining two bioactive entities through a covalent linkage.1 One notable success is the regulatory approval of 8 antibody–drug conjugate (ADC) drugs by the end of 2019, such as Adcetris and Kadcyla.2 In addition, these methods offer powerful tools enabling fundamental research at the interface of chemistry and biology.A significant number of bioconjugation reactions have been established,1,3 a frequently-utilized short list consists of alkyne–azide cycloaddition,4 oxime/hydrazone ligation,5 Staudinger ligation,6 tetrazine ligation,7 alkylation of cysteine thiol,1a,3 and acylation of the ε-amine of lysine.1a,3 The past efforts have been exclusively focused on improving the conversion efficiency, reaction kinetics, selectivity, and the drug-likeness of the newly-formed junctions. Currently these criteria can often be satisfactorily met.8 However, new directions constantly emerge in pharmaceutical and biotechnology field that raise the bar for our capabilities in generating new chemical classes. For instance, the integration of multiple pharmacological elements such as multiple agonists and/or antagonists, tissue targeting motif, and pharmacokinetic modulators into a single therapeutic construct represents a highly promising direction.9 In addition, multiple modalities, such as small molecule,10 antibody,11 protein,12 peptide,13 oligonucleotide,14 gene14 and cell therapies,15 presently have all been well-embraced by the community. The desire of efficiently integrating multiple pharmacological components, possibly in heterogeneous molecular formats, requires the development of new bioconjugation chemistries as the existing methods are designed for connecting two molecules. Driven by such needs, we have been embarking on a new field that focuses on developing chemistries which can covalently joint multiple components together with high efficiency (Fig. 1).
Here we describe a set of sequential bioconjugation chemistries centered on a pyrazolone structural motif. It begins with a clickable “pyrazolone ligation” between a hydrazine group and a β-ketoester moiety generating a 5-membered cyclic junction site. Subsequently the resulting pyrazolone can be readily conjugated to an aldehyde through a Knoevenagel reaction forming a Michael addition acceptor that can effectively capture a free thiol group. When such chemistry is utilized intermolecularly, it can quickly assemble four conjugation partners together forming a quadruple functional construct. When engaging intramolecular reactions, it offers a set of highly diverse biomolecule scaffolds including further derivatized stapled peptides and bicyclic peptides, and potentially tricyclic peptides.
Results and discussion
Pyrazolone ligation
We envisioned the 5-membered cyclic functional group pyrazolone could serve as a core structural motif that may enable an efficient covalent assembly of multiple biomolecules into a single construct. Such vision was based on our hypothesis that the pyrazolone core could be formed through the conjugation between a β-ketoester-bearing biomolecule and a hydrazine-bearing biomolecule, and the fact that it has been reported as reactive toward aldehyde-bearing biomolecules providing the possibility of subsequent conjugations.16The synthesis of pyrazolone compounds through a tandem reaction between β-ketoester and hydrazine has been practiced in classical medicinal chemistry. Although it was typically conducted under conditions that are too harsh to biomolecules.17 To the best of our knowledge, there have been no reported attempt of employing such chemistry to bioconjugation. On the other hand, despite that hydrazone ligation is an attractive procedure attributed to the specificity and simplicity; its usefulness is significantly limited by the reversibility.5,18 Our proposed “pyrazolone ligation”, involving a tandem process of hydrazone ligation-lactam cyclization which locks the meta-stable hydrazone bond into an irreversible structure moiety, should offer an improved alternative to the hydrazone ligation.
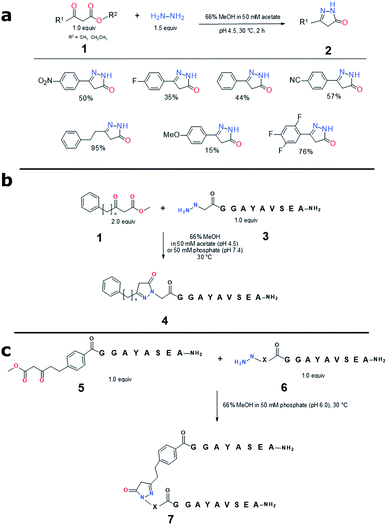
We started our investigation by examining the reactivity of different β-ketoesters 1 with hydrazine (Fig. 2a). Among the initially assessed compounds, it was found that β-oxo-benzenepentanoic methyl ester possessed significantly faster reaction kinetics than β-oxo-benzenepropanoic methyl ester and its derivatives. In addition, the electron-withdrawing substituents on the latter were favorable for the ligation rate while the electron-donating groups were detrimental. In all cases, there was no non-cyclized hydrazone intermediate observed in the reaction mixture, suggesting this thermodynamically favorable 5-membered lactam cyclization is fast while the hydrazone formation represents the rate-limiting step. In addition, it was confirmed that such tandem reaction between alkyl hydrazine and β-oxo-benzenepentanoic methyl ester was highly regioselective, 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for methylhydrazine and single product for bulkier α-methylbenzylhydrazine as determined by LC-MS and NMR (Fig. S1†). Such data suggests that the conjugation between β-oxo-benzenepentanoic methyl ester- and hydrazine-containing peptides should be highly regioselective.
4 for methylhydrazine and single product for bulkier α-methylbenzylhydrazine as determined by LC-MS and NMR (Fig. S1†). Such data suggests that the conjugation between β-oxo-benzenepentanoic methyl ester- and hydrazine-containing peptides should be highly regioselective.
In the next step, we studied the structure-reaction kinetics relationship of the pyrazolone ligation by derivatizing both β-ketoester and hydrazine segments. It was found that the contraction by one methylene or expansion up to two methylene groups between the phenyl group and β-ketoester unit had negligible impact on the ligation rate with the hydrazine peptide 3 (Fig. 2b, S2 and Table S1†). In contrast, among the hydrazine groups examined by ligating peptides 6 to the β-ketoester-bearing peptide 5, 2-hydrazinyl acetyl group outperformed others (Fig. 2c, S3 and Table S2†). In addition, the 2-hydrazinyl acetyl-peptides can be readily prepared and possess a favorable chemical stability profile. Furthermore, for all these ligations involving short peptides, only a single product was observed on UPLC-MS (Fig. S2 and S3†). The collective data led us to choose β-oxo-benzenepentanoic methyl ester and 2-hydrazinyl acetyl as our preferred pyrazolone ligation precursors, and they were used in the following studies where the scope of the ligation was evaluated.
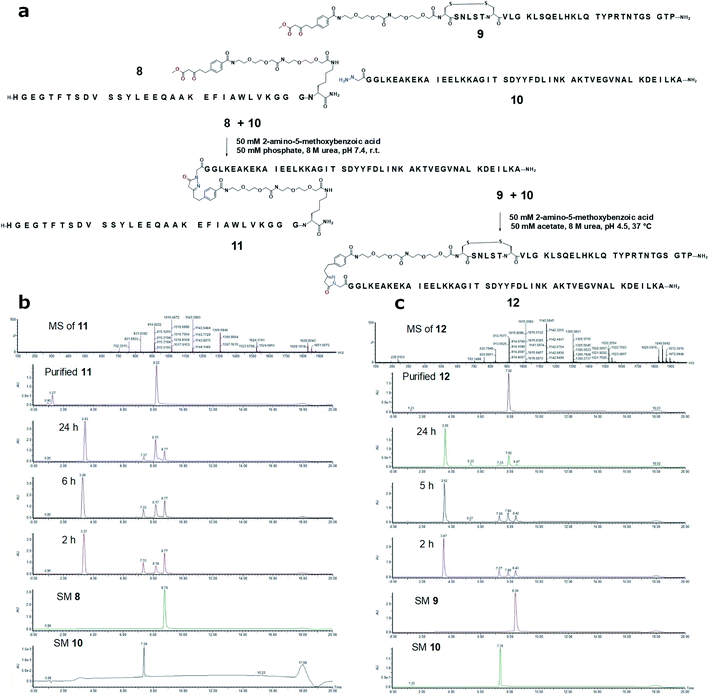
In the first application case study, we aimed to conjugate a known albumin binding domain (ABDcon)19 to the C-terminus of a glucagon-like peptide 1 (GLP-1) analog (Fig. 3a). The resulting construct was expected to be fully active based on the well-established GLP-1 structure–activity relationship (SAR)20 while possessing prolonged in vivo half-life attributed to the albumin binding. The synthesis of both GLP-1 analog 8 and the ABDcon peptide 10 were completed smoothly by applying the standard Fmoc/tBu-based solid phase peptide synthesis (SPPS). The pre-made β-ketoester building block 4-(5-methoxy-3,5-dioxopentyl)benzoic acid S-8 (Fig. S12 and S13†) was incorporated to the GLP-1 sequence as the last on-resin coupling step following the Mtt removal from C-terminal Lys side chain and the subsequent couplings of two mini-PEG linker (8-amino-3,6-dioxaoctanoic acid, or “OEG”). Similarly, the hydrazine was also introduced as the final step during the synthesis of ABDcon 10 by treating the resin-bound N-terminal bromoacetylated peptide with hydrazine in DMSO. The standard resin cleavage condition (TFA/H2O for 8 and TFA/TIS/H2O for 10) and reverse-phase (RP)-HPLC purification afforded both peptides in good yield and excellent purity (Fig. S17 and S22†).
The pyrazolone ligation condition between peptides 8 and 10 was screened against various parameters including pH (4.5, 7.4),5 temperature (r.t., 30, 40 °C),5 catalyst (2-amino-5-methoxybenzoic acid, aniline, 3,5-diaminobenzoic acid, at 10 eq. and 50 mM),21 urea (0–8 M), NaCl (0, 0.15, 3 M),22 and buffer salts (acetate, phosphate, C-(1H-imidazol-2-yl)-methylamine, N,N-dimethylethylenediamine, p-phenylenediamine) (Table S3†).23 It was found that both 50 mM catalyst 2-amino-5-methoxybenzoic acid and 8 M urea were critical for high conversion efficiency. Under such conditions, the impact of pH on reaction rate was not significant, and the ligation achieved around 80% completion after 6 h at 40 °C, or 1 day at 30 °C, or 2 days at r.t (Table S4†). In a modest scale-up ligation reaction, peptides 8 and 10 were mixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with a final concentration of 800 μM of each in the pH 7.4 buffer with 8 M urea and 50 mM catalyst 2-amino-5-methoxybenzoic acid. 20 mg of the ligated product GLP-1-ABDcon 11 was obtained with an isolation yield of 55% after 48 h agitation at r.t. and subsequent RP-HPLC purification (Fig. 3a and b).
1 molar ratio with a final concentration of 800 μM of each in the pH 7.4 buffer with 8 M urea and 50 mM catalyst 2-amino-5-methoxybenzoic acid. 20 mg of the ligated product GLP-1-ABDcon 11 was obtained with an isolation yield of 55% after 48 h agitation at r.t. and subsequent RP-HPLC purification (Fig. 3a and b).
The pyrazolone ligation was next assessed on constructing an ABDcon–salmon calcitonin (sCT) conjugate (Fig. 3a and c). Differently from GLP-1, sCT bears one disulfide and the conjugation site needs to be at its N-terminus where the modification doesn't compromise its biological activities.24 The ligation of peptides 9 and 10 was conducted by mixing both peptides at 500 μM final concentration in pH 4.5 buffer (50 mM acetate) in the presence of 8 M urea and 50 mM 2-amino-5-methoxybenzoic acid overnight at 37 °C. 11.8 mg of ABDcon–sCT conjugate 12 was obtained after RP-HPLC purification with an isolation yield of 64%.
With two successful case studies, we chemically stressed the ligation products 11 and 12 to evaluate the stability of the newly-formed pyrazolone junction. We monitored the UPLC traces of both GLP-1-ABDcon and ABDcon–sCT conjugates in pH 4.5 and 7.5 buffers at 4, 25 and 37 °C over 7 days (Fig. S4 and S5†). ABDcon–sCT 12 demonstrated highly favorable stability profiles at all conditions with a negligible amount of degraded products detected. In contrast, despite being stable at 4 °C, a significant amount of degradation was observed for GLP-1-ABDcon 11, in particular at pH 4.5, 37 °C. The strikingly different stability suggested the observed degradation of GLP-1-ABDcon took place primarily on the peptide sequence rather than at the pyrazolone junction. These case studies collectively established the pyrazolone ligation as an attractive new bioconjugation tool.
Pyrazolone-mediated sequential conjugations
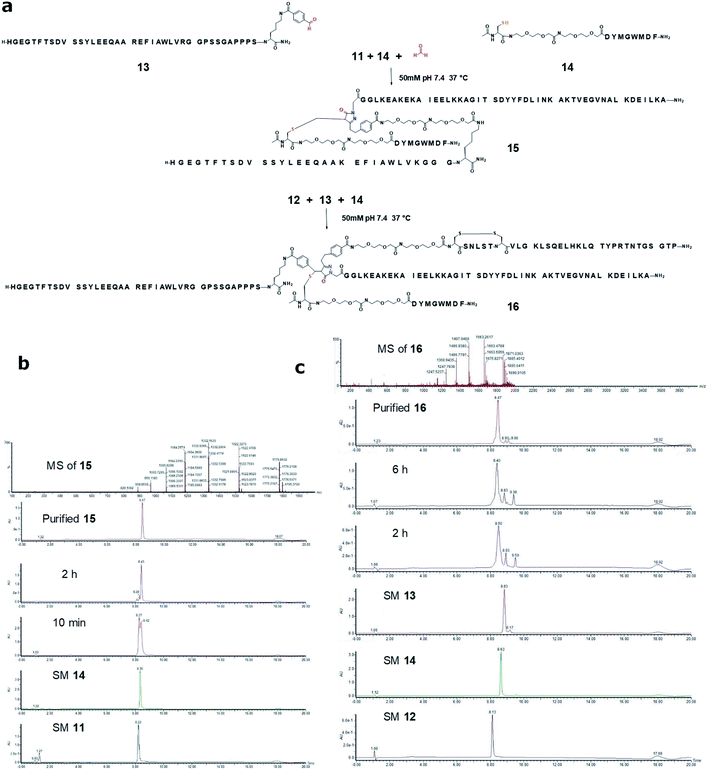
It was reported decades ago that pyrazolone can react with the aldehyde group through Knoevenagel condensation forming a Michael addition acceptor that however can react with another pyrazolone at a significantly faster rate than the first step, forming a single product consisting of one aldehyde and two pyrazolone units (Fig. S6†).25 By treating the carbohydrates with phenylpyrazolone, this chemistry has provided a useful method for determining the carbohydrate composition of natural polysaccharides or glycoproteins.25 More recently, the application of such reaction has been extended to the preparation of antibody drug conjugates.16 In one case, a pre-made pyrazolone-tagged cytotoxic agent was loaded to a Her2 antibody, bearing two aldehyde groups providing a drug to antibody ratio of 4 to 1.16b To realize our goal of assembling multiple biomolecules into a single construct, the reactivity between pyrazolone and aldehyde offers promise. However, the resulting construct bearing two pyrazolone segments is often undesired for constructing multiple functional therapeutic biomolecules. To address this, we proposed to trap the highly reactive Knoevenagel condensation product by an external thiol-containing biomolecule through an extremely fast thiol 1,4-addition. Such strategy not only prevents further reaction with a second pyrazolone segment post initial Knoevenagel condensation, but also allows us to incorporate a fourth biomolecule segment into the product, realizing a true sequential bioconjugation process.We first chose formaldehyde, and a short peptide Cholecystokinin 26-33 (CCK-8)26 which was extended by two OEG and one acetylated Cys residue at the N-terminus (peptide 14), to test the sequential conjugation. A slightly molar excess amount of formaldehyde (final concentration: 414 μM) and CCK-8 thiol 14 (290 μM) were mixed with GLP-1-ABDcon 11 (276 μM) in a phosphate buffer at neutral pH and r.t. It was found the Knoevenagel condensation between GLP-1-ABDcon 11 and formaldehyde and the subsequent Michael addition with CCK8 thiol 14 proceeded cleanly with fast kinetics. The tandem process was completed within 60 min and pyrazolone peptide 11 was quantitatively converted to a trifunctional construct 15 consisting of albumin binding domain, sCT and CCK8 with an isolation yield of 65% (9.5 mg) (Fig. 4a and b).
We next substituted formaldehyde with an aldehyde-containing GLP-1 derivative 13 and re-assessed the sequential conjugation by using ABDcon–sCT conjugate 12 and CCK8 thiol 14. The final concentration of peptides 12, 13, and 14 was 130, 136 and 136 μM respectively. It was found that the transformation of peptides 12, 13 and 14 took place equally efficiently to the one of peptides 11, 14 and formaldehyde. The reaction was completed within 60 min providing a quadri-functional construct 16 consisting of albumin binding domain, GLP-1, sCT and CCK8 with an isolation yield of 60% (5.8 mg) (Fig. 4a and c).
These two applications established an unprecedent sequential conjugation procedure involving pyrazolone ligation, Knoevenagel condensation and Michael addition. It demonstrated high efficiency in assembling multiple building segments, each possessing a unique pharmacological property, into a trifunctional or quadri-functional construct.
Pyrazolone-mediated versatile macrocyclization
The high efficiency of pyrazolone-centered intermolecular sequential conjugation inspired us to further explore its application in intramolecular reactions. Such intramolecular processes could offer versatile methods to quickly access novel macrocyclic peptide scaffolds, a molecular format that is often highlighted by its antibody-mimicking potency and significantly improved chemical, biophysical and enzymatic stabilities.We started testing the intramolecular pyrazolone ligation by placing the β-ketoester and hydrazine groups at the side chain of residues Lys17 and Lys24 of a GLP-1 analog. They were incorporated to the resin-bound peptide through Lys(ivDde) and Lys(Mtt) chemistries respectively. Immediately after resin-cleavage by TFA/H2O, it was found that the major crude product was the desired GLP-1 analog with Lys17–Lys24 side chain crosslinked by a pyrazolone motif, peptide 19 (Fig. 5a and S25†). Such result suggested the intramolecular pyrazolone ligation took place concurrently during the resin-cleavage step. The kinetics were extremely fast, which was in stark contrast to the intermolecular ligation where the reaction rate was significantly slower. This GLP-1 case study establishes the intramolecular pyrazolone ligation as an effective alternative method for peptide crosslinking, including α-helix stabilization through side-chain stapling.
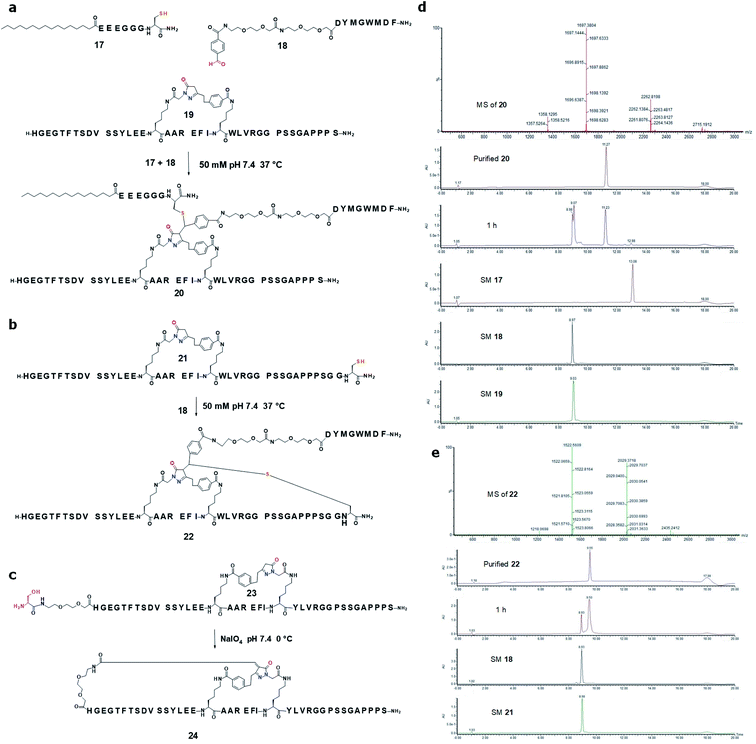
Uniquely different from the well-established alpha-helix stapling protocols, the pyrazolone ligation-mediated stapling offers the opportunity of post-stapling modification of the linker, a feature that has not been reported previously. We first treated the pyrazolone stapled GLP-1 analog 19 with formaldehyde and an albumin binder palmitic acid motif 17 in phosphate buffer at neutral pH and 37 °C. Palmitic acid peptide 17 was decorated by a free thiol via a solubility-enhancing spacer. The final concentration of 17, 19 and formaldehyde were 316 μM, 300 μM and 450 μM respectively. The reaction proceeded smoothly and completed within 30 min, offering a palmitic acid protracted stapled GLP-analog S-3 with an isolation yield of 70% (5.6 mg) (Fig. S7†). We secondly attempted to introduce a second therapeutic segment in addition to the time-action modulating palmitoyl segment. The conjugation was conducted by mixing the pyrazolone stapled GLP-1 19 (final concentration: 500 μM), a CCK-8 peptide 18 (550 μM) bearing an aldehyde group and the palmitoyl segment 17 (550 μM) under the same condition. Similarly, the reaction proceeded cleanly and was completed within 60 min providing a palmitoylated dual agonist 20 consisting of CCK-8 and stapled GLP-1 with an isolation yield of 62% (6.3 mg) (Fig. 5a and d). The bis-pyrazolone product S-4 was observed as a minor side product in this reaction (Fig. S8†).
We then explored the pyrazolone-mediated poly-macrocyclization. We first introduced a Cys residue to the C-terminus of pyrazolone-stapled GLP-1 19. It was confirmed that the free thiol was fully compatible with the intramolecular pyrazolone ligation process affording the desired product 21 (Fig. S26†). Upon treatment with a formaldehyde (final concentration: 44 μM) in neutral phosphate buffer at 37 °C, the C-terminal region of 21 (29 μM) was cyclized to the pyrazolone core through a tandem process involving intermolecular Knoevenagel condensation–intramolecular Michael addition producing a bicyclic peptide S-5 with an isolation yield of 72% (5 mg) (Fig. S9†). The bis-pyrazolone product was observed when this reaction was run at the peptide concentration of 300 μM. When the aldehyde-bearing CCK8 18 was used instead of formaldehyde, a similar condition offered a CCK8-conjugated bicyclic GLP1 analog 22 with an isolation yield of 66% (6.2 mg) (Fig. 5b and e). Interestingly, this ligation was set up with both peptides at 330 μM while not observing any formation of the bis-pyrazolone product. In both bioconjugation reactions, the cyclization proceeded cleanly and achieved full conversion within 60 min.
We next introduced an aldehyde group to the N-terminus of a pyrazolone-stapled GLP-1 analog. A Ser residue was installed at the N-terminus of the GLP analog via one OEG linker resulting in peptide 23 (Fig. S27†), which was found to be smoothly converted to 2-oxoacetamide by the treatment with 1.5 eq. sodium periodate (NaIO4) at 0 °C.27 Within one pot, the newly generated aldehyde group immediately cyclized onto the pyrazolone core through an intramolecular Knoevenagel reaction generating bicyclic peptide 24 with an isolation yield of 54% (2.7 mg) (Fig. 5c and S10†). We initially tested this conversion on a peptide with the Ser residue attached directly to the N-terminal His of GLP-1 analog 19, despite the NaIO4 treatment proceeded efficiently, the cyclization was very slow, presumably due to the unfavorable conformational constraint during this macrocyclization. We also introduced a Cys residue to the C-terminus of peptide 23 (peptide S-6) (Fig. S28†) aiming to construct a tricyclic peptide. We expect that once the N-terminal Ser is converted to the 2-oxoacetamide group, the formation of the second macrocycle will take place via an intramolecular Knoevenagel reaction followed by the third cyclization through an intramolecular thiol Michael addition. After the treatment of NaIO4, the product with mass corresponding to the sulfone form of the desired tricyclic peptide product was observed in the reaction mixture; however, the majority of the products were found to be associated with the direct oxidation of the C-terminal Cys thiol group (Fig. S11†). Alternative design of a synthetic scheme to introduce both aldehyde and thiol groups into the pyrazolone-stapled peptide are required to achieve higher quality synthesis of tricyclic peptides.
Conclusion
We have reported an unprecedent sequential bioconjugation strategy that can quickly assemble four individual segments into a quadri-functional single construct. When applied intramolecularly, it serves as a unique and versatile peptide poly-macrocyclization technology. Furthermore, the combined employment of intermolecular conjugation and intramolecular macrocyclization offers a set of diverse biomolecule scaffolds. The process starts with the pyrazolone ligation between a β-ketoester and a hydrazine, followed by Knoevenagel condensation with an aldehyde and subsequent Michael addition with a thiol. Although the intermolecular pyrazolone ligation requires catalysts to enhance the reaction rate, the intramolecular pyrazolone macrocyclization can be completed instantly. The following Knoevenagel condensation and Michael addition both possess fast kinetics producing the respective products within 60 min. We envision such new bioconjugation strategy will provide quick accesses to polyfunctional biomolecule constructs enabling quick proof of concept assessment of various polypharmacy hypothesis. We hope the pyrazolone-mediated sequential conjugation chemistry presented here will serve the first demonstration and spark more innovative method development in this emerging multiple-component bioconjugation field.Conflicts of interest
F. L. is currently a full-time employee and shareholder of Novo Nordisk.References
- (a) O. Boutureira and G. J. L. Bernardes, Advances in Chemical Protein Modification, Chem. Rev., 2015, 115(5), 2174–2195 CrossRef CAS PubMed; (b) E. M. Sletten and C. R. Bertozzi, Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality, Angew. Chem., Int. Ed., 2009, 48(38), 6974–6998 CrossRef CAS PubMed.
- P. Khongorzul, C. J. Ling, F. U. Khan, A. U. Ihsan and J. Zhang, Antibody-drug conjugates: a comprehensive review, Mol. Cancer Res., 2020, 18(1), 3–19 CrossRef CAS PubMed.
- J. N. deGruyter, L. R. Malins and P. S. Baran, Residue-Specific Peptide Modification: A Chemist's Guide, Biochemistry, 2017, 56(30), 3863–3873 CrossRef CAS PubMed.
- (a) I. Presolski Stanislav, P. Hong Vu and M. G. Finn, Copper-Catalyzed Azide-Alkyne Click Chemistry for Bioconjugation, Curr. Protoc. Chem. Biol., 2011, 3(4), 153–162 CrossRef CAS PubMed; (b) V. Hong, S. I. Presolski, C. Ma and M. G. Finn, Analysis and Optimization of Copper-Catalyzed Azide-Alkyne Cycloaddition for Bioconjugation, Angew. Chem., Int. Ed., 2009, 48(52), 9879–9883 CrossRef CAS PubMed.
- D. K. Kolmel and E. T. Kool, Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis, Chem. Rev., 2017, 117(15), 10358–10376 CrossRef CAS PubMed.
- E. Saxon and C. R. Bertozzi, Cell surface engineering by a modified Staudinger reaction, Science, 2000, 287(5460), 2007–2010 CrossRef CAS PubMed.
- M. L. Blackman, M. Royzen and J. M. Fox, Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels-Alder Reactivity, J. Am. Chem. Soc., 2008, 130(41), 13518–13519 CrossRef CAS PubMed.
- (a) K. Lang and J. W. Chin, Bioorthogonal Reactions for Labeling Proteins, ACS Chem. Biol., 2014, 9(1), 16–20 CrossRef CAS PubMed; (b) F. Saito, H. Noda and J. W. Bode, Critical Evaluation and Rate Constants of Chemoselective Ligation Reactions for Stoichiometric Conjugations in Water, ACS Chem. Biol., 2015, 10(4), 1026–1033 CrossRef CAS PubMed; (c) N. K. Devaraj, The Future of Bioorthogonal Chemistry, ACS Cent. Sci., 2018, 4(8), 952–959 CrossRef CAS PubMed.
- (a) B. Finan, B. Yang, N. Ottaway, D. L. Smiley, T. Ma, C. Clemmensen, J. Chabenne, L. Zhang, K. M. Habegger, K. Fischer, J. E. Campbell, D. Sandoval, R. J. Seeley, K. Bleicher, S. Uhles, W. Riboulet, J. Funk, C. Hertel, S. Belli, E. Sebokova, K. Conde-Knape, A. Konkar, D. J. Drucker, V. Gelfanov, P. T. Pfluger, T. D. Mueller, D. Perez-Tilve, R. D. Di Marchi and M. H. Tschoep, A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents, Nat. Med., 2015, 21(1), 27–36 CrossRef CAS PubMed; (b) T. J. Albin, J. K. Tom, S. Manna, A. P. Gilkes, S. A. Stetkevich, B. B. Katz, M. Supnet, J. Felgner, A. Jain, R. Nakajima, A. Jasinskas, A. Zlotnik, E. Pearlman, D. H. Davies, P. L. Felgner, A. M. Burkhardt and A. P. Esser-Kahn, Linked Toll-Like Receptor Triagonists Stimulate Distinct, Combination-Dependent Innate Immune Responses, ACS Cent. Sci., 2019, 5(7), 1137–1145 CrossRef CAS PubMed; (c) Y. Wang, J. Du, H. Zou, Y. Liu, Y. Zhang, J. Gonzalez, E. Chao, L. Lu, P. Yang, H. Parker, V. Nguyen-Tran, W. Shen, D. Wang, P. G. Schultz and F. Wang, Multifunctional Antibody Agonists Targeting Glucagon-like Peptide-1, Glucagon, and Glucose-Dependent Insulinotropic Polypeptide Receptors, Angew. Chem., Int. Ed., 2016, 55(40), 12475–12478 CrossRef CAS PubMed; (d) S. M. Rangwala, K. D'Aquino, Y.-M. Zhang, L. Bader, W. Edwards, S. Zheng, A. Eckardt, A. Lacombe, R. Pick, V. Moreno, L. Kang, W. Jian, E. Arnoult, M. Case, C. Jenkinson, E. Chi, R. V. Swanson, P. Kievit, K. Grove, M. Macielag, M. D. Erion, R. SinhaRoy and J. N. Leonard, A Long-Acting PYY3-36 Analog Mediates Robust Anorectic Efficacy with Minimal Emesis in Nonhuman Primates, Cell Metab., 2019, 29(4), 837–843 e5 CrossRef CAS PubMed; (e) V. R. Doppalapudi, J. Huang, D. Liu, P. Jin, B. Liu, L. Li, J. Desharnais, C. Hagen, N. J. Levin, M. J. Shields, M. Parish, R. E. Murphy, J. Del Rosario, R. D. Oates, J.-Y. Lai, M. J. Matin, Z. Ainekulu, A. Bhat, C. W. Bradshaw, G. Woodnutt, R. A. Lerner and R. W. Lappe, Chemical generation of bispecific antibodies, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(52), 22611–22616 CrossRef CAS PubMed.
- S. Hoelder, P. A. Clarke and P. Workman, Discovery of small molecule cancer drugs: Successes, challenges and opportunities, Mol. Oncol., 2012, 6(2), 155–176 CrossRef CAS PubMed.
- S. Singh, N. K. Kumar, P. Dwiwedi, J. Charan, R. Kaur, P. Sidhu and V. K. Chugh, Monoclonal Antibodies: A Review, Curr. Clin. Pharmacol., 2018, 13(2), 85–99 CrossRef PubMed.
- B. Leader, Q. J. Baca and D. E. Golan, Protein therapeutics: a summary and pharmacological classification, Nat. Rev. Drug Discovery, 2008, 7(1), 21–39 CrossRef CAS PubMed.
- J. L. Lau and M. K. Dunn, Therapeutic peptides: Historical perspectives, current development trends, and future directions, Bioorg. Med. Chem., 2018, 26(10), 2700–2707 CrossRef CAS PubMed.
- C. I. E. Smith and R. Zain, Therapeutic Oligonucleotides: State of the Art, Annu. Rev. Pharmacol. Toxicol., 2019, 59, 605–630 CrossRef CAS PubMed.
- S. Guedan, M. Ruella and C. H. June, Emerging Cellular Therapies for Cancer, Annu. Rev. Immunol., 2019, 37, 145–171 CrossRef CAS PubMed.
- (a) R. Kudirka, R. M. Barfield, J. McFarland, A. E. Albers, G. W. de Hart, P. M. Drake, P. G. Holder, S. Banas, L. C. Jones, A. W. Garofalo and D. Rabuka, Generating Site-Specifically Modified Proteins via a Versatile and Stable Nucleophilic Carbon Ligation, Chem. Biol., 2015, 22(2), 293–298 CrossRef CAS PubMed; (b) R. A. Kudirka, R. M. Barfield, J. M. McFarland, P. M. Drake, A. Carlson, S. Banas, W. Zmolek, A. W. Garofalo and D. Rabuka, Site-Specific Tandem Knoevenagel Condensation-Michael Addition To Generate Antibody-Drug Conjugates, ACS Med. Chem. Lett., 2016, 7(11), 994–998 CrossRef CAS PubMed; (c) J. Yu, D. Shen, H. Zhang and Z. Yin, Rapid, Stoichiometric, Site-Specific Modification of Aldehyde-Containing Proteins Using a Tandem Knoevenagel-Intra Michael Addition Reaction, Bioconjugate Chem., 2018, 29(4), 1016–1020 CrossRef CAS PubMed.
- (a) M. Arnost, A. Pierce, E. ter Haar, D. Lauffer, J. Madden, K. Tanner and J. Green, 3-Aryl-4-(arylhydrazono)-1H-pyrazol-5-ones: Highly ligand efficient and potent inhibitors of GSK3β, Bioorg. Med. Chem. Lett., 2010, 20(5), 1661–1664 CrossRef CAS PubMed; (b) I. J. Bakanas and G. Moura-Letts, Synthesis of Tetrasubstituted Pyrazoles from Substituted Hydrazines and β-Keto Esters, Eur. J. Org. Chem., 2016, 2016(32), 5345–5349 CrossRef CAS; (c) K. Karrouchi, S. Radi, Y. Ramli, J. Taoufik, Y. N. Mabkhot, F. A. Al-Aizari and M. Ansar, Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review, Molecules, 2018, 23, 134 CrossRef PubMed.
- (a) P. Agarwal, R. Kudirka, A. E. Albers, R. M. Barfield, G. W. de Hart, P. M. Drake, L. C. Jones and D. Rabuka, Hydrazino-Pictet-Spengler Ligation as a Biocompatible Method for the Generation of Stable Protein Conjugates, Bioconjugate Chem., 2013, 24(6), 846–851 CrossRef CAS PubMed; (b) P. Agarwa, J. van der Weijden, E. M. Sletten, D. Rabuka and C. R. Bertozzi, Pictet-Spengler ligation for protein chemical modification, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(1), 46–51 CrossRef CAS PubMed.
- S. A. Jacobs, A. C. Gibbs, M. Conk, F. Yi, D. Maguire, C. Kane and K. T. O'Neil, Fusion to a highly stable consensus albumin binding domain allows for tunable pharmacokinetics, Protein Eng., Des. Sel., 2015, 28(10), 385–393 CrossRef CAS PubMed.
- J. Lau, P. Bloch, L. Schaffer, I. Pettersson, J. Spetzler, J. Kofoed, K. Madsen, L. B. Knudsen, J. McGuire, D. B. Steensgaard, H. M. Strauss, D. X. Gram, S. M. Knudsen, F. S. Nielsen, P. Thygesen, S. Reedtz-Runge and T. Kruse, Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide, J. Med. Chem., 2015, 58(18), 7370–7380 CrossRef CAS PubMed.
- (a) A. Dirksen, S. Dirksen, T. M. Hackeng and P. E. Dawson, Nucleophilic Catalysis of Hydrazone Formation and Transimination: Implications for Dynamic Covalent Chemistry, J. Am. Chem. Soc., 2006, 128(49), 15602–15603 CrossRef CAS PubMed; (b) P. Crisalli and E. T. Kool, Water-Soluble Organocatalysts for Hydrazone and Oxime Formation, J. Org. Chem., 2013, 78(3), 1184–1189 CrossRef CAS PubMed.
- S. Wang, G. N. Nawale, S. Kadekar, O. P. Oommen, N. K. Jena, S. Chakraborty, J. Hilborn and O. P. Varghese, Saline Accelerates Oxime Reaction with Aldehyde and Keto Substrates at Physiological pH, Sci. Rep., 2018, 8(1), 2193 CrossRef PubMed.
- D. Larsen, A. M. Kietrys, S. A. Clark, H. S. Park, A. Ekebergh and E. T. Kool, Exceptionally rapid oxime and hydrazone formation promoted by catalytic amine buffers with low toxicity, Chem. Sci., 2018, 9(23), 5252–5259 RSC.
- C. S. D'Santos, G. C. Nicholson, J. M. Moseley, T. Evans, T. J. Martin and B. E. Kemp, Biologically active, derivatizable salmon calcitonin analog: design, synthesis, and applications, Endocrinology, 1988, 123(3), 1483–1488 CrossRef PubMed.
- S. Honda, E. Akao, S. Suzuki, M. Okuda, K. Kakehi and J. Nakamura, High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl-5-pyrazolone derivatives, Anal. Biochem., 1989, 180(2), 351–357 CrossRef CAS PubMed.
- J. F. Rehfeld, L. Friis-Hansen, J. P. Goetze and T. V. O. Hansen, The biology of cholecystokinin and gastrin peptides, Curr. Top. Med. Chem., 2007, 7(12), 1154–1165 CrossRef CAS PubMed.
- J. Huang, H. Qin, Z. Sun, G. Huang, J. Mao, K. Cheng, Z. Zhang, H. Wan, Y. Yao, J. Dong, J. Zhu, F. Wang, M. Ye and H. Zou, A peptide N-terminal protection strategy for comprehensive glycoproteome analysis using hydrazide chemistry based method, Sci. Rep., 2015, 5, 10164 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc02466j |
| This journal is © The Royal Society of Chemistry 2020 |