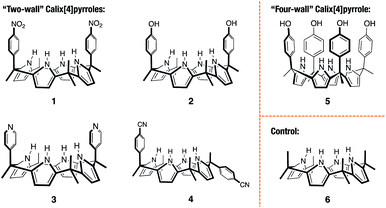
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Molecular recognition of pyrazine N,N′-dioxide using aryl extended calix[4]pyrroles†
Chenxing
Guo‡
 a,
Hu
Wang‡
a,
Hu
Wang‡
 a,
Vincent M.
Lynch
a,
Xiaofan
Ji
a,
Vincent M.
Lynch
a,
Xiaofan
Ji
 *b,
Zachariah A.
Page
*b,
Zachariah A.
Page
 *a and
Jonathan L.
Sessler
*a and
Jonathan L.
Sessler
 *a
*a
aDepartment of Chemistry, The University of Texas at Austin, 105 East 24th Street, Stop A5300, Austin, Texas 78712, USA. E-mail: zpage@cm.utexas.edu; sessler@cm.utexas.edu
bSchool of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), Wuhan 430074, China. E-mail: xiaofanji@hust.edu.cn
First published on 20th April 2020
Abstract
Calix[4]pyrrole (C4P)-based systems have been extensively explored as binding agents for anions and ion pairs. However, their capacity to act as molecular containers for neutral species remains underexplored. We report here the molecular recognition of pyrazine N,N′-dioxide (PZDO) using a series of aryl extended C4Ps including three α,α-diaryl substituted C4Ps (receptors 1–3), an α,β-diaryl substituted C4P (receptor 4) and an α,α,α,α-tetraaryl substituted C4P (receptor 5). Single crystal structural analyses of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes between receptors 1–3 and PZDO revealed that the C4P subunits exist in an unusual partial cone conformation and that the PZDO guest is held within electron-rich cavities formed by the lower rims of the individual C4P macrocycle. In contrast, receptor 5 was seen to adopt the cone conformation in the solid state, allowing one PZDO molecule to be accommodated inside the upper-rim cavity. Evidence for guest-directed self-assembly is also seen in the solid state. Evidence for C4P–PZDO interactions in CD3CN/CD3OD solution came from 1H NMR spectroscopic titrations. Electrostatic potential maps created by means of density functional theory calculations were constructed. Density functional theory calculations were also performed to analyse the energetics of various limiting binding modes. On the basis of these studies, it is inferred that interactions between the ‘two-wall’ C4P derivatives (i.e. receptors 1–4) and PZDO involve a complex binding mode that differs from what has been seen in previous host–guest complexes formed between C4Ps and N-oxides. The present study thus paves the way for the further design of C4P-based receptors with novel recognition features.
1 host–guest complexes between receptors 1–3 and PZDO revealed that the C4P subunits exist in an unusual partial cone conformation and that the PZDO guest is held within electron-rich cavities formed by the lower rims of the individual C4P macrocycle. In contrast, receptor 5 was seen to adopt the cone conformation in the solid state, allowing one PZDO molecule to be accommodated inside the upper-rim cavity. Evidence for guest-directed self-assembly is also seen in the solid state. Evidence for C4P–PZDO interactions in CD3CN/CD3OD solution came from 1H NMR spectroscopic titrations. Electrostatic potential maps created by means of density functional theory calculations were constructed. Density functional theory calculations were also performed to analyse the energetics of various limiting binding modes. On the basis of these studies, it is inferred that interactions between the ‘two-wall’ C4P derivatives (i.e. receptors 1–4) and PZDO involve a complex binding mode that differs from what has been seen in previous host–guest complexes formed between C4Ps and N-oxides. The present study thus paves the way for the further design of C4P-based receptors with novel recognition features.
Introduction
Heterocyclic N-oxides have emerged as promising therapeutic drugs or prodrugs thanks to their, inter alia, antimicrobial, antitumoral, anti-inflammatory and antiviral activities.1 In this context, efforts have been made to synthesise derivatives of quinoxaline 1,4-di-N-oxides and phenazine N,N′-dioxides and study their biological activities.2,3 Notably, pyrazine N,N′-dioxide (PZDO) is the redox-active motif embedded in these two types of heterocyclic N-oxides and is thought to account for the antitumoral activities towards hypoxic cells. Creating artificial receptors for PZDO and its analogues might inform the development of improved supramolecular drug delivery systems and sensors for this class of biologically active species.4,5 As a first step towards realising this promise, we report here a series of calix[4]pyrrole-based receptors that allow for the molecular recognition of PZDO in CD3CN/CD3OD solution and in the solid state.Calix[4]pyrroles (C4Ps) are a class of easy-to-prepare nonaromatic macrocycles that have been extensively studied for their ability to stabilise complexes with anions and ion pairs, particularly in non-competitive solvents.6–10 Although an ability to form complexes with simple uncharged solvents was noted early on,11 much less effort has been devoted to the recognition of neutral guests using unfunctionalised C4Ps.§ In 2009, however, Ballester et al. made a seminal contribution to the field by showing that water-soluble C4P derivatives could be used to bind effectively pyridine N-oxides (PNOs) in aqueous media.12 Subsequently, a number of C4P-based receptors and sensors for neutral guests were reported.13–18 Ballester and co-workers have also exploited appropriately sized bis-N-oxides (e.g. 4,4′-bipyridine N,N′-dioxide) to stabilise C4P dimers or direct the self-assembly of C4P-based capsules.19–24 Inspired by these efforts, our group recently prepared a stimuli-responsive supra-amphiphile in water that relies on host–guest interactions between a water-soluble C4P derivative and a tetraphenylethene (TPE)-derived pyridine bis-N-oxide.25
In principle, PZDO is one of the smallest aromatic bis-N-oxides. To date, PZDO and its derivatives have been extensively studied in the context of crystal engineering and have proved to be useful precursors for the fabrication of functional materials.26–31 On the other hand, to our knowledge, only one recent study by Mateo-Alonso and co-workers has targeted PZDO as a substrate for supramolecular complex formation, wherein the formation of a host–guest complex between an anthracene-linked tetralactam receptor and phenazine N,N′-dioxides was reported.32 As detailed below, we have now prepared several ‘two-wall’ C4P-based molecular receptors (1–4) that allow for the encapsulation of PZDO in the form of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes in the solid state (Scheme 1).
1 host–guest complexes in the solid state (Scheme 1).
Results and discussion
Preparation and characterisation of the receptors
The cis-‘two-wall’ C4Ps 1–3 were prepared in accord with literature methods.33–35 The trans-‘two-wall’ C4P 4 was prepared via a simple two-step synthetic strategy. Briefly, commercially available 4-acetylbenzonitrile was converted to the corresponding dipyrromethane 7 (4-(1,1-di(1H-pyrrol-2-yl)ethyl)benzonitrile; cf. ESI† for structure) via the condensation reaction with pyrrole in the presence of trifluoroacetic acid (TFA). The key intermediate 7 was, in turn, converted to compound 4 by reacting with acetone in the presence of boron trifluoride diethyl etherate (BF3·OEt2). The desired trans-‘two-wall’ C4P 4 was then separated from its cis-congener, which also formed under conditions of the condensation, by means of column chromatography. The ‘four-wall’ C4P 5 and control compound 6 were also made according to reported procedures.36,37 Compounds 4 and 7 were fully characterised by 1H NMR and 13C NMR spectroscopies, as well as high-resolution mass spectrometry (HRMS) (see ESI† for details).Solid-state complex formation
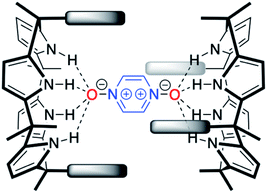
With C4Ps 1–6 in hand, efforts turned to studying them as possible receptors for PZDO. On the basis of prior studies by Ballester and co-workers involving C4P-based receptors and aromatic bis-N-oxides, we envisaged an analogous binding mode would pertain in the case of α,α-diaryl substituted C4Ps and PZDO giving rise to the generic structure shown in Fig. 1. Specifically, we expected that two C4P hosts would combine to create a dimer that would encapsulate a single PZDO guest within the central cavity. Support for this expectation came from a study by Cafeo et al., wherein a solid-state structure revealed a host–guest complex between the ‘two-wall’ C4P 1 and isophthalate dianion similar to that shown in Fig. 1.38 However, as detailed below, a different binding mode was seen when PZDO was allowed to crystallise in the presence of 1.Single crystals suitable for X-ray diffraction analyses were obtained by allowing PZDO to crystallise in the presence of 1. This was done by dissolving 1 and PZDO in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of 1
1 molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform
1 chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
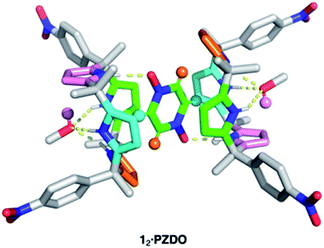
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol, and allowing n-heptane to diffuse into the resulting solution. The resulting crystal structure is shown in Fig. 2. In contrast to the proposal presented in Fig. 1, each C4P subunit (i.e. receptor 1) was seen to adopt the partial cone conformation rather than the expected cone conformation. Nevertheless, an overall 2
methanol, and allowing n-heptane to diffuse into the resulting solution. The resulting crystal structure is shown in Fig. 2. In contrast to the proposal presented in Fig. 1, each C4P subunit (i.e. receptor 1) was seen to adopt the partial cone conformation rather than the expected cone conformation. Nevertheless, an overall 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–substrate complex (12·PZDO) is seen with the individual PZDO molecules encapsulated inside the cavity defined by the lower rims of the two constituent C4P moieties, albeit offset from the C4P–C4P axis. Methanol molecules are bound to the upper rim of each C4P subunit.
1 receptor–substrate complex (12·PZDO) is seen with the individual PZDO molecules encapsulated inside the cavity defined by the lower rims of the two constituent C4P moieties, albeit offset from the C4P–C4P axis. Methanol molecules are bound to the upper rim of each C4P subunit.
On the basis of the metric parameters, we infer that intermolecular hydrogen bonding interactions serve to stabilise the methanol-solvated 12·PZDO complex. Specifically, each oxygen atom of PZDO is bound to one pyrrolic NH moiety via inferred N–H⋯O hydrogen bonds based on the observed N⋯O distance of 2.79 Å. Hydrogen bonds between the methanol oxygen atom and the three other pyrrolic NH moieties are reflected in an average N⋯O distance of 3.08 Å. Evidence for nonclassical hydrogen bonds involving both C–H⋯π and O–H⋯π interactions39 came from the fact that each aromatic hydrogen atom of PZDO resides in close proximity to an adjacent pyrrole moiety (ca. 2.4 Å H-to-centroid). One of the pyrrole rings also lies parallel to the bound PZDO at centroid-to-centroid distance of 3.6 Å, indicating possible donor–acceptor π–π interactions between these two moieties.40 The net result is an overall binding mode that differs from that seen for the recognition of other N-oxide moieties, which are typically accommodated by C4Ps in their cone conformations via predominantly pyrrolic NH hydrogen bonding interactions in analogy to what is seen in the case of most C4P anion complexes. In contrast, the binding of PZDO to 1 resembles the binding motif seen for small cations in C4P-based ion pair complexes.
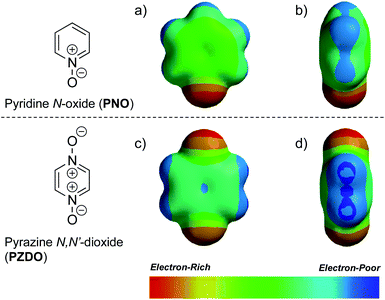
To gain insight into the disparate binding behaviour observed for PNO and PZDO, so-called electrostatic potential (ELPOT) maps of these two ostensibly similar substrates were generated by means of density functional theory (DFT) calculations carried out in the gas phase at the B3LYP/6-31G* level. As shown in Fig. 3, all oxygen atoms on the N-oxide moieties of PNO and PZDO are relatively electron-rich. This is consistent with the finding that these oxygen atoms form hydrogen bonds with the pyrrolic NH protons. On the other hand, the C–H hydrogen atoms of PZDO, as well as the associated π-system, were found to be more electron-deficient than those of PNO. Presumably, these features allow the hydrogen atoms and π electrons of PZDO to stabilise C–H⋯π and donor–acceptor π–π interactions with the pyrrole moieties of 1 more readily than PNO, thus facilitating encapsulation of PZDO within the lower-rim cavity of 1.¶
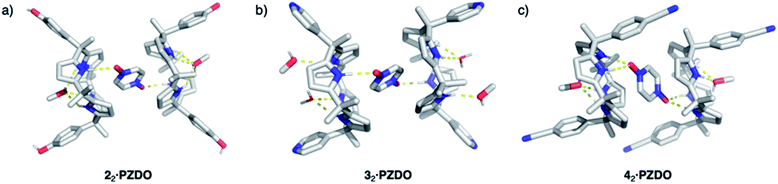
To test the extent to which the C4P might serve to regulate the binding of PZDO, studies analogous to the above were carried out using a C4P derivative, i.e. receptor 2, bearing more electron-rich ‘walls’. A single-crystal X-ray diffraction analysis of the resulting complex, 22·PZDO complex (cf.Fig. 4a), revealed a binding mode nearly identical to that seen for 12·PZDO complex; again, evidence of N–H⋯O hydrogen bonding, C–H⋯π, O–H⋯π and donor–acceptor π–π interactions was seen (see Table S2† for a summary of the bond lengths).
Further support for the notion that it is the substrate, PZDO, rather than the electronics of the diaryl ‘walls’ that dictates the binding mode seen for the present ‘two-wall’ C4P receptors came from the X-ray diffraction analysis of single crystals of 32·PZDO obtained by vapour diffusion of n-heptane into the CHCl3/CH3OH solution containing 3 and PZDO (cf.Fig. 4b). Again, a non-classical binding mode analogous to what was seen for 12·PZDO and 22·PZDO was observed in the solid state (cf.Fig. 2 and 4a). However, in the case of 32·PZDO, one molecule of water and one molecule of methanol, rather than just one molecule of methanol, were found inside the upper-rim cavity, giving rise to a slightly different geometry than that seen in the complexes based on 1 and 2.
Receptors 1, 2 and 3 all contain two ‘walls’ that are cis to one another. The present study was thus extended to include the trans-‘two-wall’ system 4. In this latter case, analysis of single crystals grown from a CHCl3/CH3OH solution containing 4 and PZDO revealed the formation of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–substrate complex wherein receptor 4 was found to adopt the 1,2-alternate conformation (cf.Fig. 4c). Although unsubstituted C4Ps typically adopt the 1,3-alternate conformation in the absence of guests (e.g. anions), several trans-‘two-wall’ C4Ps have been reported to adopt the 1,2-alternate conformation in the solid state in the absence of a guest.33,35,41–43 These observations led us to suggest that the 1,2-alternate form may be the most stable conformation for trans-‘two-wall’ diaryl C4Ps. To the extent this inference is true, we conclude that the interaction with PZDO does not induce a conformational change in the case of receptor 4. Nevertheless, as above, evidence for N–H⋯O hydrogen bonding, C–H⋯π, O–H⋯π and donor–acceptor π–π interactions is seen in the solid state. A short contact between the oxygen atom of PZDO and the 4-cyanophenyl moieties present in 4 was also observed, as evidenced by the 2.98 Å separation between the oxygen atom of PZDO and the centroid of the phenyl ring.
1 receptor–substrate complex wherein receptor 4 was found to adopt the 1,2-alternate conformation (cf.Fig. 4c). Although unsubstituted C4Ps typically adopt the 1,3-alternate conformation in the absence of guests (e.g. anions), several trans-‘two-wall’ C4Ps have been reported to adopt the 1,2-alternate conformation in the solid state in the absence of a guest.33,35,41–43 These observations led us to suggest that the 1,2-alternate form may be the most stable conformation for trans-‘two-wall’ diaryl C4Ps. To the extent this inference is true, we conclude that the interaction with PZDO does not induce a conformational change in the case of receptor 4. Nevertheless, as above, evidence for N–H⋯O hydrogen bonding, C–H⋯π, O–H⋯π and donor–acceptor π–π interactions is seen in the solid state. A short contact between the oxygen atom of PZDO and the 4-cyanophenyl moieties present in 4 was also observed, as evidenced by the 2.98 Å separation between the oxygen atom of PZDO and the centroid of the phenyl ring.
Taken in concert, the above structural findings lead us to conclude that PZDO, in spite of being a neutral species, behaves more like a small cation rather than an anion, in terms of its interaction with ‘two-wall’ diaryl C4Ps. Moreover, cation–π and donor–acceptor π–π interactions, rather than just pyrrolic NH-based hydrogen bonds, appear to control the interactions between the PZDO guest and the C4P receptors in the case of complexes 12·PZDO–42·PZDO.
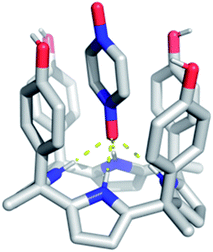
In spite of what was observed with the ‘two-wall’ diaryl C4Ps 1–4, we wondered if PZDO could act as an anion surrogate and bind to C4Ps in a manner analogous to PNO. With such considerations in mind, PZDO was tested in conjunction with receptor 5, i.e. a so-called ‘four-wall’ C4P derivative bearing electron-rich moieties at the meso-positions.36 In this case, analysis of single crystals grown from a CHCl3/CH3OH solution containing 5 and PZDO revealed that receptor 5 adopts a cone conformation in the solid state. This choice of conformation allows PZDO to be accommodated within the upper-rim cavity via four convergent hydrogen bonds between one oxygen atom of PZDO and all four pyrrolic NH protons with an average length of 2.97 Å. As the result, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex (i.e.5·PZDO; Fig. 5) was formed in analogy to what was seen in Ballester's seminal study.12 In addition, evidence of C–H⋯π and donor–acceptor π–π interactions was also found in the 5·PZDO complex, as inferred from average bond lengths of 2.44 Å and 3.94 Å, respectively (cf. Table S2†).
1 host–guest complex (i.e.5·PZDO; Fig. 5) was formed in analogy to what was seen in Ballester's seminal study.12 In addition, evidence of C–H⋯π and donor–acceptor π–π interactions was also found in the 5·PZDO complex, as inferred from average bond lengths of 2.44 Å and 3.94 Å, respectively (cf. Table S2†).
Unfortunately, attempts to grow diffraction grade single crystals of the putative PZDO complex of 6, an unsubstituted C4P used as a control, using the above procedures returned only mixtures of single crystals of methanol-solvated 6 and PZDO.||
Extended solid-state interactions
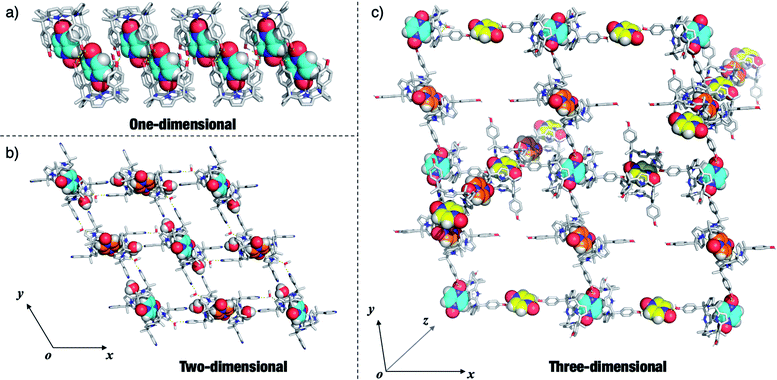
Given the interest in PZDO as a building block in crystal engineering studies, the packing diagrams of the above systems were considered in an effort to determine whether any guest-mediated self-assembled architectures are stabilised in the solid state.44,45 These analyses revealed that whereas only finite ensembles constructed via hydrogen bonds were found in the case of 12·PZDO and 42·PZDO, infinite ensembles were found in the case of 5·PZDO, 32·PZDO and 22·PZDO. Coincidentally, these structures were found to exist in the form of one-, two- and three-dimensional hydrogen bonding networks, respectively (vide infra).As shown in Fig. 6a, a pair of adjacent 5·PZDO complexes was seen to constitute a [5·PZDO]2 dimeric pseudo-capsule, in which the oxygen atom of PZDO not directly bound to the pyrrolic NHs forms a presumed intermolecular hydrogen bond with one hydroxyl group of the other host molecule; adjacent [5·PZDO]2 pseudo-capsules were further linked by two intermolecular hydrogen bonds between the hydroxyl groups. As a result, a formal one-dimensional supramolecular polymer is formed.
In the case of the two-dimensional hydrogen bonding network seen in the extended structure of 32·PZDO (Fig. 6b), both CH3OH and H2O act as bridges that link individual 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 32·PZDO complexes via presumed hydrogen bonding interactions. Here, the pyridine nitrogen atoms act as hydrogen bond acceptors.
1 32·PZDO complexes via presumed hydrogen bonding interactions. Here, the pyridine nitrogen atoms act as hydrogen bond acceptors.
In contrast to receptors 3 and 5, 22·PZDO complex is engaged in two distinctive one-dimensional networks that orientated along two of the xyz coordination axes as illustrated in Fig. 6c. The PZDO molecules, rendered with cyan, yellow and orange, are located in the xy, xz and yz planes, respectively. This complexity is thought to reflect the fact that C4P 2 contains two relatively accessible hydroxyl groups that can act as either hydrogen bond donors or hydrogen bond acceptors. Including interactions with both the bound substrate and the disordered methanol molecules (not shown in Fig. 6c but inferred from the structural analysis), both kinds of limiting hydrogen bonding interactions are seen in the extended crystal structure of 22·PZDO. Presumably, this multiplicity of interactions plays a key role in stabilising the extended array seen in the solid state.
Solution-phase analyses
1H NMR spectral titrations were carried out in an effort to quantify the binding interactions between hosts 1–5 and PZDO in solution. Again, the unmodified C4P-based receptor 6 was used as a control compound. Since it and several of the other receptors were found to possess low solubilities in the original solvent system used to grow the crystals for the solid-state analyses (i.e. CHCl3/CH3OH), the 1H NMR spectral titrations were carried out in an alternative and better solubilising solvent system, namely a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CD3CN/CD3OD. The results of these binding studies are summarised in Fig. 7 and S1–S28 (ESI†).
1 mixture of CD3CN/CD3OD. The results of these binding studies are summarised in Fig. 7 and S1–S28 (ESI†).
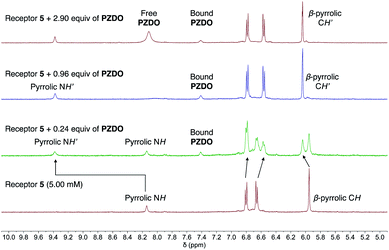
Not surprisingly, perhaps given the presence of four, rather than two, ‘walls’ and the end-on binding seen in the solid state (vide supra), receptor 5 was found to possess the highest affinity for PZDO. Initial evidence for a strong interaction between 5 and PZDO came from the observation of slow exchange kinetics on the NMR time scale. Specifically, as can be seen from an inspection of Fig. 7, the singlet peak assigned to the pyrrolic NH protons of the free receptor at 8.14 ppm gradually disappears upon the addition of increasing quantities of PZDO. Concurrently, a new peak at 9.38 ppm assigned to the pyrrolic NH protons of the PZDO complex was seen to grow in. The aromatic hydrogens of PZDO were found to undergo an upfield shift from 8.14 ppm to 7.41 ppm. Furthermore, after the addition of ca. 1.0 equiv. of PZDO, no further changes in the chemical shifts were seen other than a continuous increase of the peak at 8.11 ppm ascribed to the aromatic hydrogens of the free PZDO guest. These observations were taken as evidence for not only a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry (as seen in the solid state) but also an association constant (Ka) > 104 M−1 for this binding event in this solvent system (CD3CN/CD3OD 1
1 binding stoichiometry (as seen in the solid state) but also an association constant (Ka) > 104 M−1 for this binding event in this solvent system (CD3CN/CD3OD 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v).
1 v/v).
The relatively high affinity inferred from the 1H NMR spectral titrations was confirmed by means of isothermal titration calorimetry (ITC). As shown in Fig. S23,† the binding isotherm could be fitted to a one-site binding mode, allowing an association constant of (1.53 ± 0.22) × 104 M−1 to be determined.
In marked contrast to the slow exchange kinetics seen in the 1H NMR spectral titration of 5 with PZDO, evidence for fast exchange kinetics was seen when analogous titrations were carried out using receptors 1–4 or 6. Although not a proof, fast exchange is often associated with weak binding interactions. In addition, although the crystal structures of 12·PZDO–42·PZDO revealed 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometries in the solid state, fitting the binding data to a 2
1 binding stoichiometries in the solid state, fitting the binding data to a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model gave poor fits and returned unreasonable stepwise binding constants (cf. ESI†). On the other hand, good fits were obtained when the data were fitted to a 1
1 model gave poor fits and returned unreasonable stepwise binding constants (cf. ESI†). On the other hand, good fits were obtained when the data were fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm. We thus believe that a 1
1 binding isotherm. We thus believe that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 interaction dominates in solution. The corresponding mole ratio plots also proved consistent with a 1
1 interaction dominates in solution. The corresponding mole ratio plots also proved consistent with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode. The calculated 1
1 binding mode. The calculated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 association constants were found to be (1.4 ± 0.3) × 102 M−1, 51 ± 5 M−1, 66 ± 7 M−1, <10 M−1 and 17 ± 0.2 M−1 for the host–guest complexes of 1, 2, 3, 4 and 6 with PZDO, respectively, in CD3CN/CD3OD (1
1 association constants were found to be (1.4 ± 0.3) × 102 M−1, 51 ± 5 M−1, 66 ± 7 M−1, <10 M−1 and 17 ± 0.2 M−1 for the host–guest complexes of 1, 2, 3, 4 and 6 with PZDO, respectively, in CD3CN/CD3OD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). Although these values are all low compared to what is seen for most C4P–anion interactions, they do serve to underscore the expectation that it is possible to create modified C4P systems, such as 1–3, that act as receptors for appropriately chosen neutral substrates and that are more effective in this regard than the parent C4P system, 6. However, it is also important to note that the electronic variations in the ‘walls’ in these ‘two-wall’ systems do not directly correlate with the binding affinities for PZDO complexation. Presumably, this reflects the fact that substrate recognition in solution as in the solid state (vide supra) involves the lower rims of the C4P subunits, rather than more classic pyrrolic NH–O− interactions as seen in the case of previous studies involving PNO.
1 v/v). Although these values are all low compared to what is seen for most C4P–anion interactions, they do serve to underscore the expectation that it is possible to create modified C4P systems, such as 1–3, that act as receptors for appropriately chosen neutral substrates and that are more effective in this regard than the parent C4P system, 6. However, it is also important to note that the electronic variations in the ‘walls’ in these ‘two-wall’ systems do not directly correlate with the binding affinities for PZDO complexation. Presumably, this reflects the fact that substrate recognition in solution as in the solid state (vide supra) involves the lower rims of the C4P subunits, rather than more classic pyrrolic NH–O− interactions as seen in the case of previous studies involving PNO.
In an attempt to elucidate the binding mode between the ‘two-wall’ C4Ps and PZDO in solution and per the suggestion of a referee, an effort was made to analyse the complexes by two-dimensional nuclear Överhauser effect spectroscopy (2D NOESY). Specifically, the 2D NOESY spectra of PZDO (5.0 mM) were recorded in CD3CN/CD3OD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) in the presence of 1.0 molar equiv. of receptors 2, 3 and 6, respectively. No clear crosspeak between PZDO and these receptors was found in the NOESY spectra (cf. Fig. S29–S31†). Moreover, the chemical shifts seen in the 1D NMR spectrum as a function of added guest proved difficult to interpret in terms of the limiting binding modes illustrated in Fig. 4 and 5.
1 v/v) in the presence of 1.0 molar equiv. of receptors 2, 3 and 6, respectively. No clear crosspeak between PZDO and these receptors was found in the NOESY spectra (cf. Fig. S29–S31†). Moreover, the chemical shifts seen in the 1D NMR spectrum as a function of added guest proved difficult to interpret in terms of the limiting binding modes illustrated in Fig. 4 and 5.
Computational analyses
To gain further insight into the interactions between the ‘two-wall’ C4Ps and PZDO, preliminary DFT calculations were performed (see ESI† for more details). Here, CAM-B3LYP was chosen as the density functional since it would allow for more accurate estimations of long-range interactions,46 thus allowing account of the putative donor–acceptor π–π interactions between the pyrrole moieties and the bound PZDO. Towards this end, ‘two-wall’ C4Ps 2 and 3, and the control compound 6 were analysed as the case studies. Two plausible 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding modes—a ‘hypothetical mode’ and the ‘experimental mode’—were investigated, wherein the C4P receptor adopts a cone or partial cone conformation, respectively.
1 binding modes—a ‘hypothetical mode’ and the ‘experimental mode’—were investigated, wherein the C4P receptor adopts a cone or partial cone conformation, respectively.
DFT-optimised structures of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes and their relative energies are shown in Fig. S32.† It was found that the 1
1 host–guest complexes and their relative energies are shown in Fig. S32.† It was found that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes of 2 and 3 with PZDO seen by experiment [i.e. (2·PZDO)exp and (3·PZDO)exp] proved less stable than their respective hypothetical counterparts [i.e. (2·PZDO)hyp and (3·PZDO)hyp] by ca. 4.6 kcal mol−1 in the gas phase, whereas the (6·PZDO)exp complex proved more stable than its hypothetical counterpart (6·PZDO)hyp, by 8.2 kcal mol−1. These findings provide support for the contention that the C4P subunit per se favours the binding of PZDO along the lower bowl-like rim, whereas introducing aryl ‘walls’ serves to create cavities into which PZDO is bound, with the effect scaling with the number of ‘walls’ (i.e., 4 aryl substituents > 2). However, it is worth noting that contributions from solvation were not considered in these preliminary calculations.47 Thus, a clear determination of which limiting binding mode, if any, dominates in solution for the ‘two-wall’ C4Ps 2 and 3 cannot be made at the present time. In contrast, the limits provided by the ‘no-wall’ and ‘four-wall’ receptors (6 and 5, respectively) appear much more clearly defined.
1 host–guest complexes of 2 and 3 with PZDO seen by experiment [i.e. (2·PZDO)exp and (3·PZDO)exp] proved less stable than their respective hypothetical counterparts [i.e. (2·PZDO)hyp and (3·PZDO)hyp] by ca. 4.6 kcal mol−1 in the gas phase, whereas the (6·PZDO)exp complex proved more stable than its hypothetical counterpart (6·PZDO)hyp, by 8.2 kcal mol−1. These findings provide support for the contention that the C4P subunit per se favours the binding of PZDO along the lower bowl-like rim, whereas introducing aryl ‘walls’ serves to create cavities into which PZDO is bound, with the effect scaling with the number of ‘walls’ (i.e., 4 aryl substituents > 2). However, it is worth noting that contributions from solvation were not considered in these preliminary calculations.47 Thus, a clear determination of which limiting binding mode, if any, dominates in solution for the ‘two-wall’ C4Ps 2 and 3 cannot be made at the present time. In contrast, the limits provided by the ‘no-wall’ and ‘four-wall’ receptors (6 and 5, respectively) appear much more clearly defined.
DFT analyses were also used to probe the stability of the hypothetical capsule shown in Fig. 1. However, no energy-minimised structures could be found even after extensive computational time. Although not a proof, these findings lead us to infer that the hypothetical capsules formed by the cis-‘two-wall’ C4Ps with PZDO shown in Fig. 1 might be intrinsically unstable, presumably due to the steric clashes that would be present in such a tightly coupled system.
In contrast, DFT-optimised structures of the experimental 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes (22·PZDO)exp and (32·PZDO)exp converged readily (cf. Fig. S33 and S34†). Moreover, as shown in Fig. S35,† these calculated structures are in good agreement with their corresponding single crystal structures. The same proved true for (62·PZDO)exp (cf. Fig. S36†). Taken in aggregate, these results lead us to suggest that the 2
1 host–guest complexes (22·PZDO)exp and (32·PZDO)exp converged readily (cf. Fig. S33 and S34†). Moreover, as shown in Fig. S35,† these calculated structures are in good agreement with their corresponding single crystal structures. The same proved true for (62·PZDO)exp (cf. Fig. S36†). Taken in aggregate, these results lead us to suggest that the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes observed in the solid state are energetically reasonable.
1 host–guest complexes observed in the solid state are energetically reasonable.
Conclusions
In summary, we report the molecular recognition of PZDO using a series of C4P-based receptors (systems 1–6), both in the solid state and in mixed organic media. The solid-state binding modes between these receptors and PZDO were unequivocally determined by means of single-crystal X-ray diffraction analyses. It was found that PZDO, unlike PNO, was capable of acting as either a pseudo-cation or pseudo-anion in terms of its interactions with the C4Ps receptors. In many cases, a non-axially symmetric binding mode was seen, which has little precedent in the case of C4P·PNO complexes. Moreover, in the present study a number of extended structures are seen in the solid state whose specifics depend on the choice of the ‘two-wall’ receptors. 1H NMR spectral titrations, NOESY studies and DFT calculations were performed in an effort to gain greater insight into the binding events in solution. It was found that the ‘four-wall’ system bearing four electron-rich ‘walls’, namely receptor 5, had the highest affinity for PZDO, but that the ‘two-wall’ derivatives, 1–3, were able to stabilise complexes with PZDO in CD3CN/CD3OD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The present study thus serves to extend our understanding of neutral substrate recognition mediated by C4P-type receptors, while expanding the lexicon of binding modes that this class of well-studied macrocycles is able to support.
1 v/v). The present study thus serves to extend our understanding of neutral substrate recognition mediated by C4P-type receptors, while expanding the lexicon of binding modes that this class of well-studied macrocycles is able to support.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work in Austin was supported by the National Institutes of Health (grant RO1 GM 103790 to J. L. S.) and the Robert A. Welch Foundation (F-0018 to J. L. S. and F-2007 to Z. A. P.). X. J. acknowledges initial funding from the Huazhong University of Science and Technology for support through the Fundamental Research Funds for the Central Universities (grant 2020kfyXJJS013). Researchers in Austin also acknowledge use of a Bruker AVIII HD 500 with Prodigy liquid nitrogen cryoprobe supported by NIH grant 1 S10 OD021508. C. G. also acknowledges the Texas Advanced Computing Centre (TACC) at the University of Texas at Austin for providing high performance computing resources (Stampede2) that have contributed to the computational results reported within this paper.Notes and references
- A. M. Mfuh and O. V. Larionov, Curr. Med. Chem., 2015, 22, 2819–2857 CrossRef CAS PubMed.
- G. Cheng, W. Sa, C. Cao, L. Guo, H. Hao, Z. Liu, X. Wang and Z. Yuan, Front. Pharmacol., 2016, 7, 64 Search PubMed.
- A. Sharma, J. F. Arambula, S. Koo, R. Kumar, H. Singh, J. L. Sessler and J. S. Kim, Chem. Soc. Rev., 2019, 48, 771–813 RSC.
- L. You, D. Zha and E. V. Anslyn, Chem. Rev., 2015, 115, 7840–7892 CrossRef CAS PubMed.
- X. Ma and Y. Zhao, Chem. Rev., 2015, 115, 7794–7839 CrossRef CAS PubMed.
- S. K. Kim and J. L. Sessler, Acc. Chem. Res., 2014, 47, 2525–2536 CrossRef CAS PubMed.
- D. S. Kim and J. L. Sessler, Chem. Soc. Rev., 2015, 44, 532–546 RSC.
- I. Saha, J. T. Lee and C.-H. Lee, Eur. J. Org. Chem., 2015, 2015, 3859–3885 CrossRef CAS.
- I. A. Rather, S. A. Wagay, M. S. Hasnain and R. Ali, RSC Adv., 2019, 9, 38309–38344 RSC.
- S. Peng, Q. He, G. I. Vargas-Zúñiga, L. Qin, I. Hwang, S. K. Kim, N. J. Heo, C.-H. Lee, R. Dutta and J. L. Sessler, Chem. Soc. Rev., 2020, 49, 865–907 RSC.
- W. E. Allen, P. A. Gale, C. T. Brown, V. M. Lynch and J. L. Sessler, J. Am. Chem. Soc., 1996, 118, 12471–12472 CrossRef CAS.
- B. Verdejo, G. Gil-Ramírez and P. Ballester, J. Am. Chem. Soc., 2009, 131, 3178–3179 CrossRef CAS PubMed.
- A. Galán, E. C. Escudero-Adán, A. Frontera and P. Ballester, J. Org. Chem., 2014, 79, 5545–5557 CrossRef PubMed.
- G. Aragay, D. Hernández, B. Verdejo, E. C. Escudero-Adán, M. Martínez and P. Ballester, Molecules, 2015, 20, 16672–16686 CrossRef CAS PubMed.
- L. Escobar, A. Díaz-Moscoso and P. Ballester, Chem. Sci., 2018, 9, 7186–7192 RSC.
- G. Peñuelas-Haro and P. Ballester, Chem. Sci., 2019, 10, 2413–2423 RSC.
- L. Escobar and P. Ballester, Org. Chem. Front., 2019, 6, 1738–1748 RSC.
- A. F. Sierra, D. Hernández-Alonso, M. A. Romero, J. A. González-Delgado, U. Pischel and P. Ballester, J. Am. Chem. Soc., 2020, 142, 4276–4284 CrossRef CAS PubMed.
- G. Gil-Ramírez, M. Chas and P. Ballester, J. Am. Chem. Soc., 2010, 132, 2520–2521 CrossRef PubMed.
- V. Valderrey, E. C. Escudero-Adán and P. Ballester, J. Am. Chem. Soc., 2012, 134, 10733–10736 CrossRef CAS PubMed.
- M. Espelt and P. Ballester, Org. Lett., 2012, 14, 5708–5711 CrossRef CAS PubMed.
- A. Galán, G. Aragay and P. Ballester, Chem. Sci., 2016, 7, 5976–5982 RSC.
- A. Galan, M. Espelt and P. Ballester, Supramol. Chem., 2016, 28, 455–463 CrossRef CAS.
- A. Galán, E. C. Escudero-Adán and P. Ballester, Chem. Sci., 2017, 8, 7746–7750 RSC.
- X. Chi, H. Zhang, G. I. Vargas-Zúñiga, G. M. Peters and J. L. Sessler, J. Am. Chem. Soc., 2016, 138, 5829–5832 CrossRef CAS PubMed.
- H.-L. Sun, Z.-M. Wang and S. Gao, Inorg. Chem., 2005, 44, 2169–2176 CrossRef CAS PubMed.
- H.-L. Sun, S. Gao, B.-Q. Ma, G. Su and S. R. Batten, Cryst. Growth Des., 2005, 5, 269–277 CrossRef CAS.
- J. M. Shi, X. Zhang, H. Y. Xu, C. J. Wu and L. D. Liu, J. Coord. Chem., 2007, 60, 647–654 CrossRef CAS.
- R. Podgajny, D. Pinkowicz, B. Czarnecki, M. Kozieł, S. Chorąży, M. Wis, W. Nitek, M. Rams and B. Sieklucka, Cryst. Growth Des., 2014, 14, 4030–4040 CrossRef CAS.
- C. B. Aakeröy, T. K. Wijethunga and J. Desper, Chem.–Eur. J., 2015, 21, 11029–11037 CrossRef PubMed.
- M. K. Bellas and A. J. Matzger, Angew. Chem., Int. Ed., 2019, 58, 17185–17188 CrossRef CAS PubMed.
- C. Gozalvez, J. L. Zafra, A. Saeki, M. Melle-Franco, J. Casado and A. Mateo-Alonso, Chem. Sci., 2019, 10, 2743–2749 RSC.
- G. Bruno, G. Cafeo, F. H. Kohnke and F. Nicolò, Tetrahedron, 2007, 63, 10003–10010 CrossRef CAS.
- L. Adriaenssens, G. Gil-Ramírez, A. Frontera, D. Quiñonero, E. C. Escudero-Adán and P. Ballester, J. Am. Chem. Soc., 2014, 136, 3208–3218 CrossRef CAS PubMed.
- D. S. Kim, V. M. Lynch, J. S. Park and J. L. Sessler, J. Am. Chem. Soc., 2013, 135, 14889–14894 CrossRef CAS PubMed.
- P. Anzenbacher, K. Jursíková, V. M. Lynch, P. A. Gale and J. L. Sessler, J. Am. Chem. Soc., 1999, 121, 11020–11021 CrossRef CAS.
- A. Baeyer, Ber. Dtsch. Chem. Ges., 1886, 19, 2184–2185 CrossRef.
- G. Cafeo, F. H. Kohnke, L. Valenti and A. J. P. White, Chem.–Eur. J., 2008, 14, 11593–11600 CrossRef CAS PubMed.
- E. A. Meyer, R. K. Castellano and F. Diederich, Angew. Chem., Int. Ed., 2003, 42, 1210–1250 CrossRef CAS PubMed.
- C. R. Martinez and B. L. Iverson, Chem. Sci., 2012, 3, 2191–2201 RSC.
- J. S. Park, K. Y. Yoon, D. S. Kim, V. M. Lynch, C. W. Bielawski, K. P. Johnston and J. L. Sessler, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 20913–20917 CrossRef CAS PubMed.
- Y. Han, J.-J. Sun, G.-L. Wang and C.-G. Yan, J. Mol. Struct., 2015, 1083, 300–310 CrossRef CAS.
- J. Lee, N. W. Waggoner, L. Polanco, G. R. You, V. M. Lynch, S. K. Kim, S. M. Humphrey and J. L. Sessler, Chem. Commun., 2016, 52, 8514–8517 RSC.
- G. R. Desiraju, J. Am. Chem. Soc., 2013, 135, 9952–9967 CrossRef CAS PubMed.
- C. A. Gunawardana and C. B. Aakeröy, Chem. Commun., 2018, 54, 14047–14060 RSC.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- Y.-D. Wu, D.-F. Wang and J. L. Sessler, J. Org. Chem., 2001, 66, 3739–3746 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and characterisation data for all new compounds. CCDC 1976059–1976064. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc01496f |
| ‡ These authors contributed equally to this work. |
| § An important class of C4Ps that act as receptors for neutral guests are those bearing fused tetrathiafulvalene (TTF)-subunits. Much of the substrate recognition capability of these systems is ascribed to the presence of the TTF moieties, rather than to the C4Ps per se. However, the C4P scaffolds were recognised for providing allosteric control over the recognition processes in question. See: J. S. Park and J. L. Sessler, Acc. Chem. Res., 2018, 51, 2400–2410; S. Bähring, H. D. Root, J. L. Sessler and J. O. Jeppesen, Org. Biomol. Chem., 2019, 17, 2594–2613. |
| ¶ Support for the notion that the aromatic hydrogen atoms of PZDO are relatively electron-deficient and capable of forming nonclassical hydrogen bonds came from the early work by Babu and Nangia involving the study of C–H⋯O hydrogen bonding patterns within two distinct PZDO polymorphs. See: N. J. Babu and A. Nangia, CrystEngComm, 2007, 9, 980–983. |
| || The single crystals of PZDO obtained in the context of the present study appear to be one of the previously reported polymorphs (CCDC No.: 648204), although with slightly different metric parameters. Data for this crystal structure was thus deposited to the Cambridge Structural Database (CSD) and assigned a CCDC number of 1976061. |
| This journal is © The Royal Society of Chemistry 2020 |