Open Access Article
Open Access ArticleCatalytic asymmetric multiple dearomatizations of phenols enabled by a cascade 1,8-addition and Diels–Alder reaction†
Xihong
Liu
,
Jingying
Zhang
,
Lutao
Bai
,
Linqing
Wang
,
Dongxu
Yang
* and
Rui
Wang
 *
*
Key Laboratory of Preclinical Study for New Drugs of Gansu Province, Institute of Drug Design & Synthesis, School of Basic Medical Sciences, Lanzhou University, Lanzhou 730000, China. E-mail: yangdx@lzu.edu.cn; wangrui@lzu.edu.cn
First published on 6th December 2019
Abstract
A direct catalytic asymmetric multiple dearomatization reaction of phenols was disclosed, which provides expedient access to a series of architecturally complex polycyclic compounds bearing four stereogenic centers in high enantiopurity. The key to achieve such a transformation is the combination of a dearomative 1,8-addition of β-naphthols to para-quinone methides generated in situ from propargylic alcohols and a subsequent intramolecular dearomative Diels–Alder reaction. Noteworthily, this protocol enrichs not only the diversity of dearomatized products but also the toolbox of dearomatization strategies.
Introduction
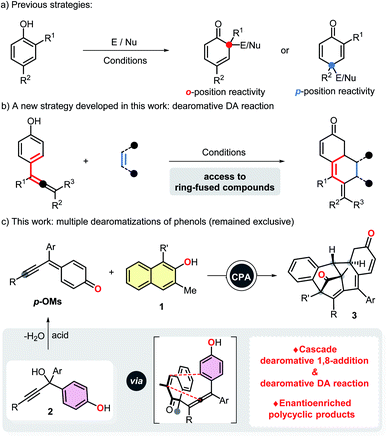
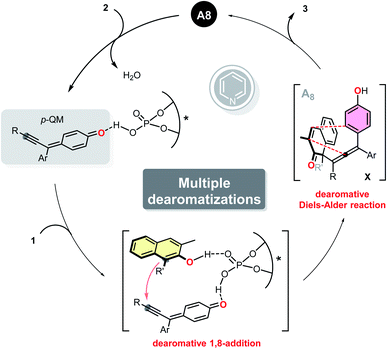
The catalytic asymmetric dearomatization (CADA) reactions of arenes offer direct access to highly functionalized ring systems of versatility and flexibility.1 The great practicality of such transformations have been elegantly highlighted by their extensive applications in the synthesis of numerous natural products and biologically active compounds.2 It is precisely because of the above reasons, dearomatization reactions have attracted tremendous attention from synthetic chemists and pharmaceutical chemists in recent years. However, although great achievements have been made in this research field, there still remain some unresolved issues. Of particular note is that to date, there are almost no examples which documented the multiple dearomatization processes of at least two different aromatic molecules via a single operation, even in a nonstereoselective version. Thus, the development of additional novel and efficient cross dearomatization strategies enabling the construction of architecturally complex building blocks seems to be a goal of high challenge and priority.As a kind of widespread and readily available chemical feedstock, phenols can be routinely dearomatized to deliver highly functionalized cyclohexadienones, which feature prominently in a variety of natural products and pharmaceutical agents. Considering the fundamental role and extensive use of α,β-unsaturated ketone fragments in organic synthesis,3 we hypothesized that a subsequent intramolecular dearomatization event could be realized by utilizing the unsaturated double bond of the dearomatized products (cyclohexadienones).4 However, despite the fact that impressive dearomatization approaches of phenols, such as asymmetric oxidation,5 alkylation,6 allylation,7 arylation,8 alkenylation,9 halogenation10 and amination11 have been developed, all of the cases rely heavily on the reactivity of ortho or para-position of phenol hydroxyl group, usually resulting in only structurally simple products (Scheme 1a). Moreover, although catalytic dearomative carbon–carbon bond forming conjugate additions of β-naphthols to nitroethylene and propargylic ketones have been realized, Michael acceptors with an aryl or alkyl group at the β-positions were not compatible with these protocols, probably due to the steric effect.6c,12a For above reasons, it seems that the development of an alternative dearomative cycloaddition strategy is essential for the rapid assembly of previously inaccessible molecular complexity, and also realizing a multiple dearomatization process of phenols. To the best of our knowledge, as one of the most prominent and highly efficient methods for accessing highly functionalized six-membered rings, Diels–Alder reaction have been successfully employed to disrupt the conjugated π-systems of arenes and heteroarenes.13–16 Among which, the dearomative Diels–Alder reactions of styrenes15 and vinylheteroarenes16 with electron-deficient dienophiles have been proved to be promising protocols for the efficient synthesis of polycyclic compounds with multiple stereogenic centers. Inspired by these elegant works, we envisioned that 4-hydroxystyrenes could also undergo the similar dearomatization processes. With this in mind, we developed herein an intramolecular dearomative Diels–Alder reaction of 4-(propa-1,2-dien-1-yl)phenols with α,β-unsaturated ketones to fulfill our original intention (Scheme 1b).
Compared with 1,4- and 1,6-conjugate additions, catalytic asymmetric 1,8-additions received far less attention and still remains underdeveloped.17 In conjunction with our interests in dearomatization reaction, we previously reported a stereocontrolled dearomative addition of β-naphthols to indol-2-ones generated in situ from 3-bromooxindoles,6f which led us to suspect that conjugate 1,8-additions of phenols to trienyl acceptors could also be achievable, and would not be influenced by the terminal substituents. To further explore the methodologies of 1,8-addition, we reported herein a catalytic asymmetric dearomative 1,8-addition of β-naphthols to para-quinone methides generated in situ from propargylic alcohols 2.17c,e Additionally, we also anticipated that tetrasubstituted allenes generated from the 1,8-addition can further undergo an intramolecular dearomative Diels–Alder reaction, thus allowing the efficient construction of stereochemically complex polycyclic compounds via a multiple dearomatization process of phenols (Scheme 1c).
Results and discussion
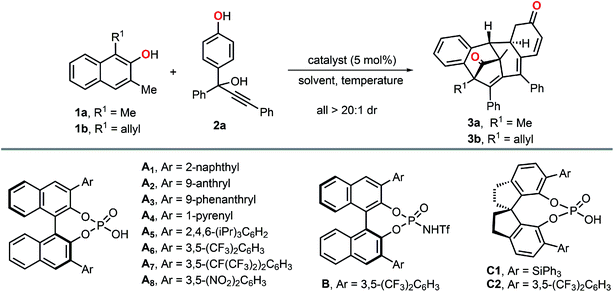
Initial studies towards our envisioned scenario began with a model reaction of 1,3-dimethyl-2-naphthol 1a and propargylic alcohol 2a in the presence of 5 mol% of chiral phosphoric acid A1 (Table 1, entry 1). As expected, β-naphthol 1a and p-QM generated in situ from 2a were simultaneously activated by A1 to provide tetrasubstituted allene smoothly at room temperature. More interestingly, the allene intermediate could successfully undergo a subsequent intramolecular dearomative Diels–Alder reaction to provide polycyclic compound 3a with four stereogenic centers in 44% yield, albeit with only 9% ee. Encouraged by this result, some other BINOL-derived chiral phosphoric acids with different substituents were evaluated (Table 1, entries 2–8). To our delight, chiral phosphoric acid A8 with relatively stronger acidity exhibited significantly enhanced catalytic activity and afforded the corresponding polycyclic product 3a in 90% yield with 57% ee (Table 1, entry 8). Then, B, C1 and C2 were further examined, and all of them offered inferior reaction outcomes (Table 1, entries 9–11). Subsequently, some other variables including solvent and temperature were screened. The solvent evaluation indicated that toluene was the best choice (Table 1, entries 12–14). It should be noted that with benzene or THF as the solvent, the reaction did not occur (entries 12 and 14). Lowing the temperature of the first step of the reaction to −60 °C, the dearomatized product 3a was obtained in 65% ee without erosion of the yield (Table 1, entry 15). Besides, the addition of 2.5 mol% pyridine could further improve the enantioselectivity to 83% (Table 1, entry 16). It was worth noting that when an allyl group was installed to the C1 position of β-naphthol, the corresponding dearomatized product 3b was obtained with even more excellent enantioselectivity (Table 1, entry 17, 98% ee).| Entry | Catalyst | Solvent | T (°C) | t (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: β-naphthol 1a (0.12 mmol, 1.2 equiv.), propargylic alcohol 2a (0.1 mmol, 1 equiv.), catalyst (5 mol%) in 1.0 mL of solvent. b Isolated yield. c Enantiomeric excess was determined by chiral HPLC. d With 10 mol% A8. e 2.5 mol% pyridine was added. f Conducted with 1b. | ||||||
| 1 | A1 | Toluene | rt | 36 | 44 | 9 |
| 2 | A2 | Toluene | rt | 36 | 36 | 26 |
| 3 | A3 | Toluene | rt | 36 | 38 | 30 |
| 4 | A4 | Toluene | rt | 36 | 46 | 29 |
| 5 | A5 | Toluene | rt | 36 | 38 | 20 |
| 6 | A6 | Toluene | rt | 36 | 61 | 65 |
| 7 | A7 | Toluene | rt | 36 | 79 | 16 |
| 8 | A8 | Toluene | rt | 36 | 90 | 57 |
| 9 | B | Toluene | rt | 36 | 46 | 2 |
| 10 | C1 | Toluene | rt | 36 | Trace | n.d. |
| 11 | C2 | Toluene | rt | 36 | 48 | 30 |
| 12 | A8 | Benzene | rt | 36 | Trace | n.d. |
| 13 | A8 | CH2Cl2 | rt | 36 | 81 | 34 |
| 14 | A8 | THF | rt | 36 | Trace | n.d. |
| 15d | A8 | Toluene | −60 °C to rt | 24, 24 | 92 | 65 |
| 16d,e | A8 | Toluene | −60 °C to rt | 48, 48 | 92 | 83 |
| 17d,e,f | A8 | Toluene | −60 °C to rt | 72, 72 | 80 | 98 |
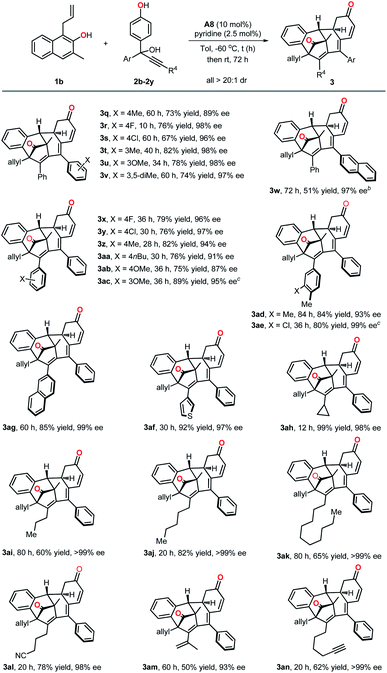
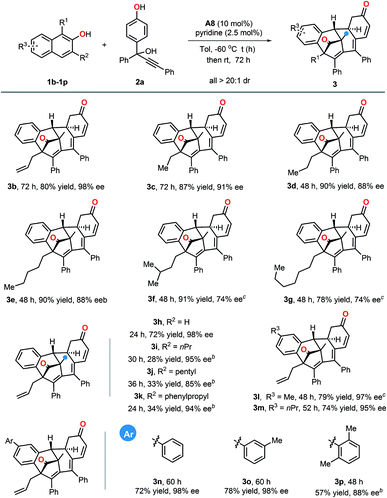
With the optimized reaction conditions in hand, we first investigated the substrate scope with respect to β-naphthols. As indicated in Table 2, β-naphthols with different alkyl groups, such as n-propyl, n-pentyl or n-heptyl at its C1 position, were all well tolerated and gave the corresponding polycyclic products in high yields and enantioselectivities (3c–3g, 78–91% yield, 74–91% ee). In addition, the effect of substitutions at the C3 position of β-naphthols was examined. β-Naphthol 1h bearing no substituent at its 3-position worked well and delivered the desired product 3h in 72% yield with 98% ee. Relatively bulkier substituents (n-propyl, n-pentyl and phenylpropyl) at 3-position of β-naphthols led to the corresponding products with high ee values but lower yields (3i–3k, 28–34% yields, 85–95% ee). Additionally, both alkyl and aryl substituents on the aromatic ring of β-naphthols were all compatible in the current protocol (3l–3p, 57–79% yield, 88–98% ee).
| a Reaction conditions: 1 (0.12 mmol), 2a (0.10 mmol), A8 (10 mol%) and pyridine (2.5 mol%) in toluene (1 mL). Isolated yields based on 2a. The ee values were determined by chiral HPLC. The reaction time at −60 °C was given. b Conducted at −50 °C. c Conducted at −45 °C. For the cases of 3c–3g, 2.0 mol% pyridine was used. |
|---|
|
Subsequently, we further explored the generality of this multiple dearomatization process by employing various substituted propargylic alcohols (Table 3). In general, aromatic rings of Ar with either electron-withdrawing or electron-donating groups at different positions were all well tolerated, and the corresponding dearomatized products were obtained in high yields and excellent enantioselectivities (3q–3v, 67–82% yields, 89–98% ee). It should be noted that 2h with a polyarene naphthalene ring also worked well. In addition, different substituents including alkyl, methoxyl and halogen on the phenyl ring of R4 were all compatible with this reaction (3x–3ac). In particular, the reactions of propargylic alcohols with either a disubstituted phenol ring or a heteroaromatic ring occurred in satisfactory yields and enantioselectivities (3ad–3af). It was especially noteworthy that alkyl-substituted propargylic alcohols were also suitable substrates for this transformation and afforded the corresponding polycyclic products with excellent outcomes (3ah–3ak). Furthermore, functional groups such as nitriles (3al), olefins (3am) and alkynes (3an), were all compatible substituents. The absolute configuration of the dearomatized product 3a was determined by X-ray crystallographic analysis, and those of others were inferred accordingly.18
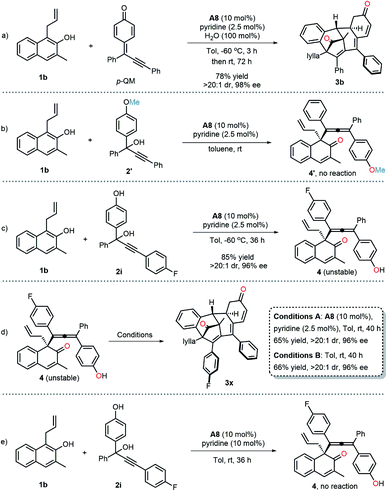
To gain insight into the mechanism of this multiple dearomatization process, some related control experiments were conducted. The pre-prepared p-QM successfully underwent the current protocol, furnishing the corresponding polycyclic product 3b with essentially the same yield and enantioselectivity as that achieved with 2a (Scheme 2a). Methyl-protected substrate 2′ failed to give any product, which highlights the in situ formation of the para-quinone methide intermediate (Scheme 2b). The dearomatized product 4, featuring a vicinal all-carbon quaternary stereocenter and an axially chiral tetrasubstituted allene, was isolated with precisely the same ee value as 3x, suggesting that the stereochemical outcome was determined in the first step of this protocol (Scheme 2c). In addition, the isolated axially chiral tetrasubstituted allene 4 could smoothly undergo an intramolecular dearomative Diels–Alder reaction in the absence of A8, giving the bridged polycyclic compound 3x with almost the same yield as that obtained from 4 under the standard conditions (Scheme 2d). Which indicated that A8 did not have significant effect on the current dearomative Diels–Alder reaction. Furthermore, when 10 mol% of pyridine was added, the reaction failed to afford corresponding axially tetrasubstituted allene 4 at room temperature, indicating that pyridium phosphate salt could not promote the dearomative 1,8-addition independently (Scheme 2e). So we hypothesized that traces of pyridine might improve the enantioselectivity of the dearomatized products via regulating the pH of the reaction medium. Based on the above experiment results and previous studies,17c,e a working mechanism was proposed, as illustrated in Scheme 3. The para-quinone methide intermediate was first generated in situ from propargylic alcohol 2 under acidic conditions. Subsequently, β-naphthol 1 and p-QM were simultaneously activated by chiral phosphoric acid A8via a dual hydrogen-bonding activation mode, enabling the stereocontrolled conjugate 1,8-addition. Then, the resulting unstable tetrasubstituted allene underwent an intramolecular dearomative Diels–Alder reaction to deliver the desired polycyclic product 3.
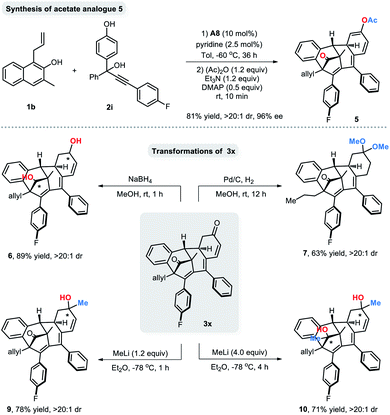
To demonstrate the synthetic practicality of this newly developed protocol, some representative transformations of the dearomatized products were carried out (Scheme 4). An acetyl protection procedure of the axially tetrasubstituted allene 4 led to the cyclized acetate analogue 5 in 81% yield with 96% ee. Besides, both of the ketone groups of 3x could be reduced with NaBH4 in methanol to afford diol 6 in excellent yield and diastereoselectivity (89% yield, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). 3x could also be converted to 7 in 63% yield via a Pd/C-mediated hydrogenation reaction. Moreover, 1,2-addition reactions of methyllithium to 3x were also achievable and led to monomethylated 9 and dimethylated 10.
1 dr). 3x could also be converted to 7 in 63% yield via a Pd/C-mediated hydrogenation reaction. Moreover, 1,2-addition reactions of methyllithium to 3x were also achievable and led to monomethylated 9 and dimethylated 10.
Conclusions
In summary, a new dearomatization strategy based on a Diels–Alder reaction was developed and successfully applied to an enantioselective multiple dearomatization process of phenols. This methodology, involving a conjugate 1,8-addition and a subsequent intramolecular Diels–Alder reaction, provides a facile and step-economical access to a variety of structurally and stereochemically complex polycyclic compounds in good to excellent yields and stereoselectivities. Traces of pyridine was proved to be an efficient additive to improve the enantioselectivities. To the best of our knowledge, this protocol is the first to document a catalytic asymmetric multiple dearomatizations of two different aromatic molecules.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the NSFC (21432003, 81473095, 21602091), the Program for Chang-jiang Scholars and Innovative Research Team in University (PCSIRT: No. IRT_15R27), and the Fundamental Research Funds for the Central Universities (lzujbky-2019-68, lzujbky-2018-kb11, lzujbky-2017-19 and lzujbky-2017-118).Notes and references
- For selected reviews, see: (a) F. L. Ortiz, M. J. Iglesias, I. Fernández, C. M. Andújar Sánchez and G. Ruiz Gómez, Chem. Rev., 2007, 107, 1580 CrossRef PubMed; (b) C.-X. Zhuo, W. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 12662 CrossRef PubMed; (c) C.-X. Zhuo, C. Zheng and S.-L. You, Acc. Chem. Res., 2014, 47, 2558 CrossRef CAS PubMed; (d) Q. Ding, X. Zhou and R. Fan, Org. Biomol. Chem., 2014, 12, 4807 RSC; (e) S. P. Roche, J.-J. Youte Tendoung and B. Tréguier, Tetrahedron, 2015, 71, 3549 CrossRef CAS; (f) W.-T. Wu, L. Zhang and S.-L. You, Chem. Soc. Rev., 2016, 45, 1570 RSC; (g) W. Sun, G. Li, L. Hong and R. Wang, Org. Biomol. Chem., 2016, 14, 2164 RSC; (h) X.-W. Liang, C. Zheng and S.-L. You, Chem. –Eur. J., 2016, 22, 11918 CrossRef CAS PubMed; (i) C. Zheng and S.-L. You, Chem, 2016, 1, 830 CrossRef CAS; (j) J.-B. Chen and Y.-X. Jia, Org. Biomol. Chem., 2017, 15, 3550 RSC; (k) W. C. Wertjes, E. H. Southgate and D. Sarlah, Chem. Soc. Rev., 2018, 47, 7996 RSC; (l) G. Huang and B. Yin, Adv. Synth. Catal., 2019, 361, 405 CrossRef CAS.
- For a review, see: S. P. Roche and J. A. Porco Jr, Angew. Chem., Int. Ed., 2011, 50, 4068 CrossRef CAS PubMed ; for selected examples, see: (a) S. Dong, E. Hamel, R. Bai, D. G. Covell, J. A. Beutler and J. A. Porco Jr, Angew. Chem., Int. Ed., 2009, 48, 1494 CrossRef CAS PubMed; (b) J. Qi, A. B. Beeler, Q. Zhang and J. A. Porco Jr, J. Am. Chem. Soc., 2010, 132, 13642 CrossRef CAS PubMed; (c) J. C. Green and T. R. R. Pettus, J. Am. Chem. Soc., 2011, 133, 1603 CrossRef CAS PubMed; (d) H. Zhang, L. Hong, H. Kang and R. Wang, J. Am. Chem. Soc., 2013, 135, 14098 CrossRef CAS PubMed; (e) K. Douki, H. Ono, T. Taniguchi, J. Shimokawa, M. Kitamura and T. Fukuyama, J. Am. Chem. Soc., 2016, 138, 14578 CrossRef CAS PubMed; (f) G. Zhao, G. Xu, C. Qian and W. Tang, J. Am. Chem. Soc., 2017, 139, 3360 CrossRef CAS PubMed; (g) Y. Zhang, Y. Liao, X. Liu, X. Xu, L. Lin and X. Feng, Chem. Sci., 2017, 8, 6645 RSC.
- (a) S. Reymond and J. Cossy, Chem. Rev., 2008, 108, 5359 CrossRef CAS PubMed; (b) K. Zheng, X. Liu and X. Feng, Chem. Rev., 2018, 118, 7586 CrossRef CAS PubMed.
- Up to date, dearomative 1,2-addition reactions of phenols to carbonyl compounds have not yet been realized.
- (a) J. Zhu, N. P. Grigoriadis, J. P. Lee and J. A. Porco Jr, J. Am. Chem. Soc., 2005, 127, 9342 CrossRef CAS PubMed; (b) S. Dong, J. Zhu and J. A. Porco Jr, J. Am. Chem. Soc., 2008, 130, 2738 CrossRef CAS PubMed; (c) T. Dohi, A. Maruyama, N. Takenaga, K. Senami, Y. Minamitsuji, H. Fujioka, S. B. Caemmerer and Y. Kita, Angew. Chem., Int. Ed., 2008, 47, 3787 CrossRef CAS PubMed; (d) M. Uyanik, T. Yasui and K. Ishihara, Angew. Chem., Int. Ed., 2010, 49, 2175 CrossRef CAS PubMed; (e) A. Rudolph, P. H. Bos, A. Meetsma, A. J. Minnaard and B. L. Feringa, Angew. Chem., Int. Ed., 2011, 50, 5834 CrossRef CAS PubMed; (f) T. Oguma and T. Katsuki, J. Am. Chem. Soc., 2012, 134, 20017 CrossRef CAS PubMed; (g) T. Dohi, N. Takenaga, T. Nakae, Y. Toyoda, M. Yamasaki, M. Shiro, H. Fujioka, A. Maruyama and Y. Kita, J. Am. Chem. Soc., 2013, 135, 4558 CrossRef CAS PubMed; (h) M. Uyanik, T. Yasui and K. Ishihara, Angew. Chem., Int. Ed., 2013, 52, 9215 CrossRef CAS PubMed.
- (a) J. Qi and J. A. Porco Jr, J. Am. Chem. Soc., 2007, 129, 12682 CrossRef CAS PubMed; (b) D. Yang, L. Wang, F. Han, D. Li, D. Zhao and R. Wang, Angew. Chem., Int. Ed., 2015, 54, 2185 CrossRef CAS PubMed; (c) S.-G. Wang, X.-J. Liu, Q.-C. Zhao, C. Zheng, S.-B. Wang and S.-L. You, Angew. Chem., Int. Ed., 2015, 54, 14929 CrossRef CAS PubMed; (d) H. Nakayama, S. Harada, M. Kono and T. Nemoto, J. Am. Chem. Soc., 2017, 139, 10188 CrossRef CAS PubMed; (e) G. Zhu, Y. Li, G. Bao, W. Sun, L. Huang, L. Hong and R. Wang, ACS Catal., 2018, 8, 1810 CrossRef CAS; (f) X. Liu, P. Wang, L. Bai, D. Li, L. Wang, D. Yang and R. Wang, ACS Catal., 2018, 8, 10888 CrossRef CAS.
- (a) Q.-F. Wu, W.-B. Liu, C.-X. Zhuo, Z.-Q. Rong, K.-Y. Ye and S.-L. You, Angew. Chem., Int. Ed., 2011, 50, 4455 CrossRef CAS PubMed; (b) C.-X. Zhuo and S.-L. You, Angew. Chem., Int. Ed., 2013, 52, 10056 CrossRef CAS PubMed; (c) Q. Cheng, Y. Wang and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 3496 CrossRef CAS PubMed; (d) H.-F. Tu, C. Zheng, R.-Q. Xu, X.-J. Liu and S.-L. You, Angew. Chem., Int. Ed., 2017, 56, 3237 CrossRef CAS PubMed; (e) D. Shen, Q. Chen, P. Yan, X. Zeng and G. Zhong, Angew. Chem., Int. Ed., 2017, 56, 3242 CrossRef CAS PubMed; (f) B. Yang, X. Zhai, S. Feng, D. Hu, Y. Deng and Z. Shao, Org. Lett., 2019, 21, 330 CrossRef CAS PubMed.
- (a) R.-Q. Xu, Q. Gu, W.-T. Wu, Z.-A. Zhao and S.-L. You, J. Am. Chem. Soc., 2014, 136, 15469 CrossRef CAS PubMed; (b) K. Du, P. Guo, Y. Chen, Z. Cao, Z. Wang and W. Tang, Angew. Chem., Int. Ed., 2015, 54, 3033 CrossRef CAS PubMed; (c) X.-Q. Li, H. Yang, J.-J. Wang, B.-B. Gou, J. Chen and L. Zhou, Chem. –Eur. J., 2017, 23, 5381 CrossRef CAS PubMed.
- (a) L. Yang, H. Zheng, L. Luo, J. Nan, J. Liu, Y. Wang and X. Luan, J. Am. Chem. Soc., 2015, 137, 4876 CrossRef CAS PubMed; (b) J. Zheng, S.-B. Wang, C. Zheng and S.-L. You, J. Am. Chem. Soc., 2015, 137, 4880 CrossRef CAS PubMed.
- (a) R. J. Phipps and F. D. Toste, J. Am. Chem. Soc., 2013, 135, 1268 CrossRef CAS PubMed; (b) Q. Yin, S.-G. Wang, X.-W. Liang, D.-W. Gao, J. Zheng and S.-L. You, Chem. Sci., 2015, 6, 4179 RSC; (c) Y.-F. Wang, J.-J. Shao, B. Wang, M.-M. Chu, S.-S. Qi, X.-H. Du and D.-Q. Xu, Adv. Synth. Catal., 2018, 360, 2285 CrossRef CAS; (d) P. Wang, J. Wang, L. Wang, D. Li, K. Wang, Y. Liu, H. Zhu, X. Liu, D. Yang and R. Wang, Adv. Synth. Catal., 2018, 360, 401 CrossRef CAS.
- (a) S.-G. Wang, Q. Yin, C.-X. Zhuo and S.-L. You, Angew. Chem., Int. Ed., 2015, 54, 647 CAS; (b) J. Nan, J. Liu, H. Zheng, Z. Zuo, L. Hou, H. Hu, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2015, 54, 2356 CrossRef CAS PubMed; (c) X. Lian, L. Lin, G. Wang, X. Liu and X. Feng, Chem. –Eur. J., 2015, 21, 17453 CrossRef CAS PubMed; (d) Z.-L. Xia, C. Zheng, R.-Q. Xu and S.-L. You, Nat. Commun., 2019, 10, 3150 CrossRef PubMed.
- (a) D. Yang, L. Wang, M. Kai, D. Li, X. Yao and R. Wang, Angew. Chem., Int. Ed., 2015, 54, 9523 CrossRef CAS PubMed; (b) L. Wang, D. Yang, D. Li, P. Wang, K. Wang, J. Wang, X. Jiang and R. Wang, Chem. –Eur. J., 2016, 22, 8483 CrossRef CAS PubMed; (c) G. Zhu, G. Bao, Y. Li, J. Yang, W. Sun, J. Li, L. Hong and R. Wang, Org. Lett., 2016, 18, 5288 CrossRef CAS.
- For reviews, see: (a) S. Reymond and J. Cossy, Chem. Rev., 2008, 108, 5359 CrossRef CAS PubMed; (b) J.-L. Li, T.-Y. Liu and Y.-C. Chen, Acc. Chem. Res., 2012, 45, 1491 CrossRef CAS PubMed; (c) X. Jiang and R. Wang, Chem. Rev., 2013, 113, 5515 CrossRef CAS PubMed; (d) B. Yang and S. Gao, Chem. Soc. Rev., 2018, 47, 7926 RSC.
- For selected examples, see: (a) G. Himbert and L. Henn, Angew. Chem., Int. Ed., 1982, 21, 620 CrossRef; (b) Y. Schmidt, J. K. Lam, H. V. Pham, K. N. Houk and C. D. Vanderwal, J. Am. Chem. Soc., 2013, 135, 7339 CrossRef CAS PubMed; (c) L. W. Hernandez, U. Klöckner, J. Pospech, L. Hauss and D. Sarlah, J. Am. Chem. Soc., 2018, 140, 4503 CrossRef CAS PubMed; (d) W. C. Wertjes, M. Okumura and D. Sarlah, J. Am. Chem. Soc., 2019, 141, 163 CrossRef CAS PubMed; (e) J. Ma, F. Strieth-Kalthoff, T. Dalton, M. Freitag, J. L. Schwarz, K. Bergander, C. Daniliuc and F. Glorius, Chem, 2019, 5, 2854 CrossRef CAS.
- (a) J. K. Williams, D. W. Wiley and B. C. Mckusick, J. Am. Chem. Soc., 1962, 84, 2210 CrossRef CAS; (b) E. Ciganek, J. Org. Chem., 1969, 34, 1923 CrossRef CAS; (c) R. Brückner, R. Huisgen and J. Schmid, Tetrahedron Lett., 1990, 31, 7129 CrossRef; (d) S. P. Kolis, M. D. Chordia, R. Liu, M. E. Kopach and W. D. Harman, J. Am. Chem. Soc., 1998, 120, 2218 CrossRef CAS; (e) L. Shen, K. Zhao, K. Doitomi, R. Ganguly, Y.-X. Li, Z.-L. Shen, H. Hirao and T.-P. Loh, J. Am. Chem. Soc., 2017, 139, 13570 CrossRef CAS.
- (a) W. H. Cherry, J. T. Craig and Q. N. Porter, Aust. J. Chem., 1979, 32, 133 CrossRef CAS; (b) H. Kotsuki, A. Kondo, H. Nishizawa, M. Ochi and K. Matsuoka, J. Org. Chem., 1981, 46, 5455 CrossRef; (c) A. Benítez, F. R. Herrera, M. Romero and F. X. Talamás, J. Org. Chem., 1996, 61, 1487 CrossRef; (d) M. G. B. Drew, A. Jahans, L. M. Harwood and S. A. B. H. Apoux, Eur. J. Org. Chem., 2002, 3589 CrossRef CAS; (e) F. L. Strat, H. Vallette, L. Toupet and J. Maddaluno, Eur. J. Org. Chem., 2005, 5296 CrossRef; (f) C. Gioia, A. Hauville, L. Bernardi, F. Fini and A. Ricci, Angew. Chem., Int. Ed., 2008, 47, 9236 CrossRef CAS; (g) B. Tan, G. Hernández-Torres and C. F. Barbas III, J. Am. Chem. Soc., 2011, 133, 12354 CrossRef CAS; (h) Y.-C. Xiao, C.-Z. Yue, P.-Q. Chen and Y.-C. Chen, Org. Lett., 2014, 16, 3208 CrossRef CAS; (i) B.-X. Xiao, W. Du and Y.-C. Chen, Adv. Synth. Catal., 2017, 359, 1018 CrossRef CAS.
- For a review, see: (a) N. Krause and S. Thorand, Inorg. Chim. Acta, 1999, 296, 1 CrossRef CAS ; for examples, see:; (b) D. Uraguchi, K. Yoshioka, Y. Ueki and T. Ooi, J. Am. Chem. Soc., 2012, 134, 19370 CrossRef CAS; (c) D. Qian, L. Wu, Z. Lin and J. Sun, Nat. Commun., 2017, 8, 567 CrossRef; (d) C. Yue, F. Na, X. Fang, Y. Cao and J. C. Antilla, Angew. Chem., Int. Ed., 2018, 57, 11004 CrossRef CAS; (e) P. Zhang, Q. Huang, Y. Cheng, R. Li, P. Li and W. Li, Org. Lett., 2019, 21, 503 CrossRef CAS; (f) M. Chen, D. Qian and J. Sun, Org. Lett., 2019, 21, 8127 CrossRef CAS.
- CCDC 1901924 contains the supplementary crystallographic data for this paper.†.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details and characterisation. CCDC 1901924. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc05320d |
| This journal is © The Royal Society of Chemistry 2020 |