Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rational synthesis of interpenetrated 3D covalent organic frameworks for asymmetric photocatalysis†
Xing
Kang
,
Xiaowei
Wu
,
Xing
Han
,
Chen
Yuan
,
Yan
Liu
and
Yong
Cui
 *
*
School of Chemistry and Chemical Engineering, State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: yongcui@sjtu.edu.cn
First published on 19th December 2019
Abstract
Covalent organic frameworks (COFs) show great promise as heterogeneous photocatalysts, but they have not yet been explored for asymmetric photocatalysis, which is important for the sustainable production of pharmaceuticals and fine chemicals. We report here a pair of twofold interpenetrated 3D COFs adopting a rare (3,4)-connected ffc topology for photocatalytic asymmetric reactions by imine condensation of rectangular and trigonal building blocks. Both COFs containing a photoredox triphenylamine moiety are efficient photocatalysts for the cross-dehydrogenative coupling reactions and asymmetric α-alkylation of aldehydes integrated with a chiral imidazolidinone catalyst. Under visible-light irradiation, the targeted chiral products are produced in satisfactory yields with up to 94% enantiomeric excess, which are comparable to those of reported reactions using molecular metal complexes or organic dyes as photosensitizers. Whereas the COFs became amorphous after catalysis, they can be recrystallized through solvent-assisted linker exchange and reused without performance loss. This is the first report utilizing COFs as photocatalysts to promote enantioselective photochemical reactions.
Introduction
Photocatalytic methods have shown great promise for bulk production and are widely accepted as convenient strategies in the field of asymmetric catalysis.1,2 Since MacMillan et al., in 2001, reported the combination of organo- with photoredox catalysis to promote the asymmetric α-alkylation of aldehydes via a process catalyzed by a ruthenium complex,3 much attention has been devoted to the development of more environment friendly reaction conditions and to the extension to more meaningful reactions.4–7 Remarkable recent advances involve the development of hybrid catalysts composed of inorganic semiconductor photocatalysts including PbBiO2Br and Bi2O3 and chiral organocatalysts, which display outstanding photocatalytic performance in stereoselectively promoting carbon–carbon bond formation reactions.8 This approach combines the advantages of heterogeneous catalysis (robust, simple, and easy to separate) with the high stereoselectivity of organocatalysis.2,9 Nonetheless, related reports are still very limited and it is challenging to design new hybrid catalyst systems for asymmetric photochemical reactions.10 Solid organic photocatalysts offer numerous advantages over inorganic semiconductors such as wide spectral absorption, tunability of porous textures, and high processibility, thereby providing a more environmentally friendly alternative to metal-based photocatalysts.11–13 However, to the best of our knowledge, organic solids have not yet been explored for asymmetric photocatalytic reactions. In this work, we demonstrated a new metal-free photocatalytic system for asymmetric catalysis based on 3D covalent organic frameworks (COFs) combined with a chiral imidazolidinone catalyst.COFs are a new class of highly tunable, porous crystalline organic polymers with 2D or 3D network topologies.14,15 By judicious choices of constituent building blocks, COFs have provided a powerful platform for engineering functional materials and hold promise for many applications such as molecule storage and separation,16 catalysis,17 energy storage,18 and optoelectronics.19 From a structural perspective, this area is dominated by 2D COFs,20,21 which generally have eclipsed stacking structures with unidirectional channels. In contrast, 3D COFs are far less explored21 and, with few exceptions,22 they have only been reported for nets based on building blocks with the tetrahedral geometry,23 presumably because of their limited availability of building blocks and the difficulty of their crystallization. Compared to 2D COFs, 3D COFs can characteristically possess high surface areas and numerous open sites and fascinating confinement effects,23 which provide many opportunities for expanding COFs' potential applications. Therefore, the targeted synthesis of 3D COFs with novel topologies and functions is highly desirable. Here we reported imine condensations of rectangular and trigonal monomers to lead to 3D porous COFs with a rare twofold-interpenetrated ffc topology. We selected triphenylamine as the functional molecule, since it represents a type of important hole-conducting molecule with unique photophysical and redox properties.24 This led to the conclusion that the as-prepared 3D COFs can be used as photocatalysts for the cross-dehydrogenative coupling (CDC) reaction and the asymmetric α-alkylation of aldehydes integrated with a chiral imidazolidinone as the organocatalyst.3
Results and discussion
Synthesis and characterization
The targeted 3D COFs were designed based on triangular and rectangular precursors connected by [3 + 4] condensation reactions. As shown in Fig. 1, COF-1 was prepared through the imine condensation of tetraamine ETTA and trialdehyde NBC in a mixture of o-DBC/n-butanol/6 M acetic acid (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v/v) at 120 °C for 3 days, which afforded yellow crystalline powders in 78% yield. COF-2 was synthesized from the imine condensation of 4′,4′′′,4′′′′′,4′′′′′′-tetraaldehyde ETBC and triamine BADA in a mixture of o-DBC/n-butanol/9 M acetic acid (3
2, v/v/v) at 120 °C for 3 days, which afforded yellow crystalline powders in 78% yield. COF-2 was synthesized from the imine condensation of 4′,4′′′,4′′′′′,4′′′′′′-tetraaldehyde ETBC and triamine BADA in a mixture of o-DBC/n-butanol/9 M acetic acid (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v/v) at 120 °C for 3 days, which produced yellow powders in 70% yield.
2, v/v/v) at 120 °C for 3 days, which produced yellow powders in 70% yield.
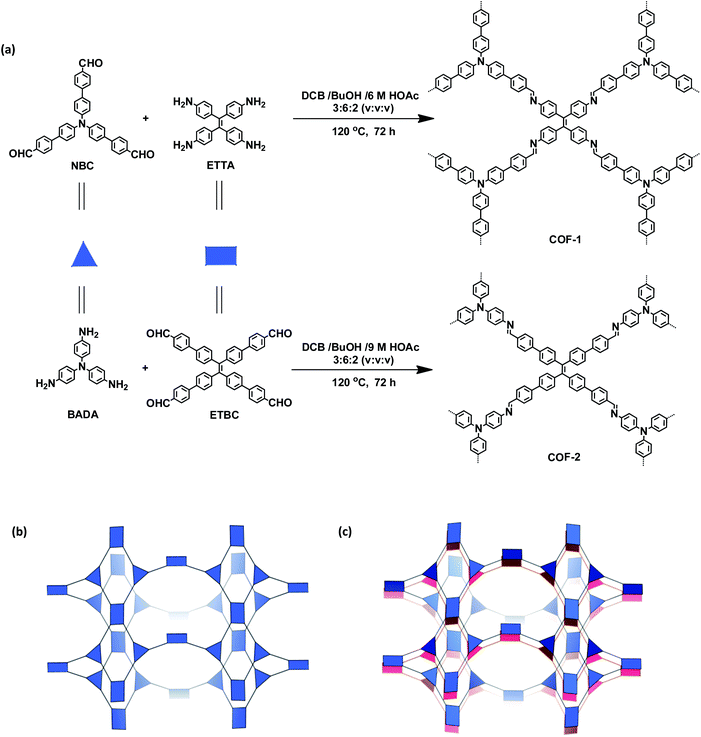 | ||
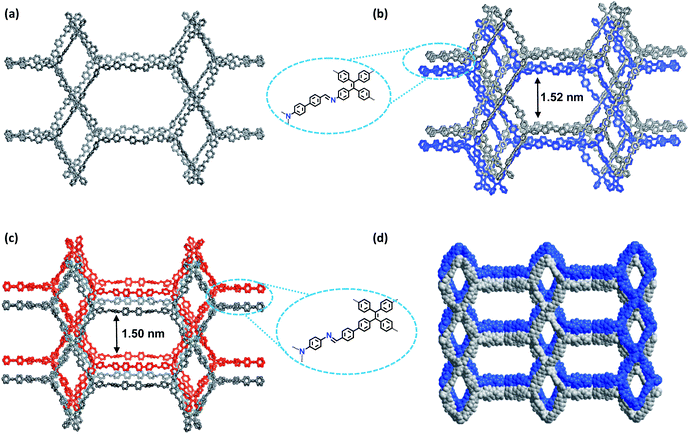
| Fig. 1 (a) Synthesis of the COFs. (b) Scheme showing the (3,4)-connected network with the ffc topology. (c) Interpenetration of two independent (3,4)-connected networks in the COFs. | ||
Both COFs are insoluble in water and common organic solvents such as THF, DCM, MeOH, EtOH and DMF. The FT-IR spectra of the COFs show the nearly complete disappearance of the characteristic aldehyde and amino stretching bands of the starting materials. Stretching vibration bands attributed to the generation of new C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N linkages were observed at 1621 and 1623 cm−1, respectively (Fig. S1†). In the 13C CP-MAS NMR spectra, the characteristic signals due to C
N linkages were observed at 1621 and 1623 cm−1, respectively (Fig. S1†). In the 13C CP-MAS NMR spectra, the characteristic signals due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds were observed at 160 and 157 ppm, respectively. The aldehyde carbon peaks were no longer present (Fig. S2†). In addition, scanning electron microscopy (SEM) images showed that both COFs possess a uniform spherical morphology (Fig. S4†) and were small and highly aggregated. The submicrometer-sized crystals from COFs were studied by 3D electron diffraction tomography (3D-EDT). The EDT data set collected from COF-1 suggested that it maintained good crystallinity and high stability for electron diffraction (Fig. S5†). However, COF-2 was not stable under the electron beam and failed to give electron diffraction.
N bonds were observed at 160 and 157 ppm, respectively. The aldehyde carbon peaks were no longer present (Fig. S2†). In addition, scanning electron microscopy (SEM) images showed that both COFs possess a uniform spherical morphology (Fig. S4†) and were small and highly aggregated. The submicrometer-sized crystals from COFs were studied by 3D electron diffraction tomography (3D-EDT). The EDT data set collected from COF-1 suggested that it maintained good crystallinity and high stability for electron diffraction (Fig. S5†). However, COF-2 was not stable under the electron beam and failed to give electron diffraction.
Crystal structure
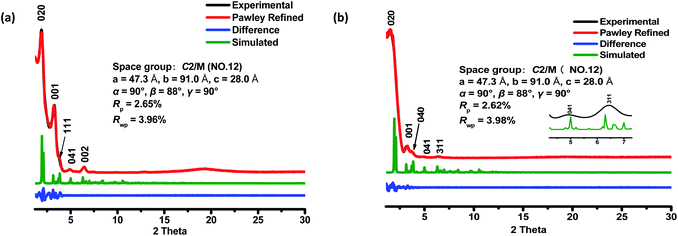
The crystalline structures of the two COFs were determined by powder X-ray diffraction (PXRD) analysis with Cu Kα radiation (Fig. 2). As revealed from PXRD analyses, COF-1 exhibited two strong peaks at 1.89° and 3.22° and relatively weak signals at 3.82°, 4.84°, and 6.44°, which can be attributed to the (020), (001), (111), (041) and (002) facets, respectively. For COF-2, the first and most intense peak corresponding to the (020) reflection plane appears at 1.88°, with other minor peaks at 3.37°, 3.68°, 4.89° and 6.29°, which can be attributed to the (001), (040), (041) and (311) facets, respectively (Fig. 2b). The crystal models were then generated using the Materials Studio software package. According to Reticular Chemistry Structure Resource (RCSR), only a few nets (e.g., tbo, pto, ffc, fjh, ptd, etc.) are reasonable for COF-1/2. After considering these possible nets with different space groups, the detailed simulations (Fig. S6–S9†) clearly suggested that both COFs are proposed to adopt a twofold interpenetrated ffc topology with the C2/m space group. The space-filling models of COFs exhibit a 3D extended framework by linking the triangular and rectangle building blocks through imine condensations generating a 2-fold interpenetrated ffc net (Fig. 3). Full profile pattern matching (Pawley) refinements for both 3D COFs were carried out and the refinement results yielded unit cell parameters nearly equivalent to the predictions with good agreement factors (a = 47.3 Å, b = 91.0 Å, c = 28.0 Å, α = 90°, β = 88°, γ = 90°, Rp = 2.65% and Rwp = 3.96% for COF-1; a = 47.3 Å, b = 91.0 Å, c = 28.0 Å, α = 90°, β = 88°, γ = 90°, Rp = 2.62 and Rwp = 3.98 for COF-2).As shown in Fig. 1c, in the present 3D COFs, two sets of independent (3,4)-networks are interwoven to form 1D tubular channels with an opening of 15.2 × 33.3 Å2 for 1 and 15.0 × 34.8 Å2 for 2. It should be noted that the networks reported for other 3D COFs are generally based on organic building blocks with the tetrahedral geometry.22,23 There are only two examples of (3,4)-connected COFs with square planar four-connected motifs reported thus far, in which framework interpenetration was suppressed by using short organic linkers.23d It is likely that the interpenetration of theses COFs is controlled by the lengths of the building blocks. The ability to manipulate framework interpenetration is key to the future synthesis of new porous 3D COFs, which hold great promise in heterogeneous catalysis and molecule storage and separation.15,22,23 It is noted that a (3,4)-connected 3D metal–organic framework with a two-fold interpenetrated ffc topology has been reported in the literature.25
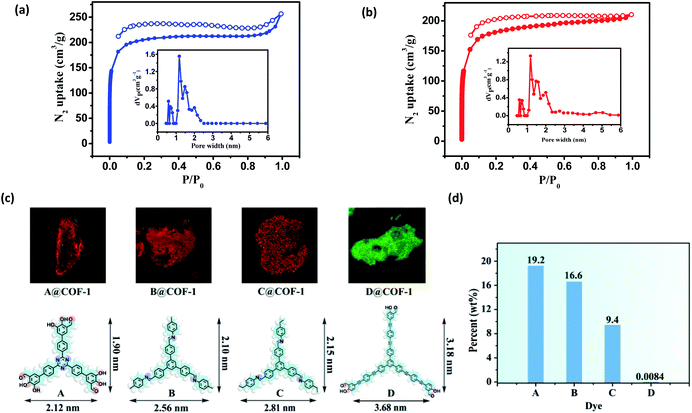
Nitrogen sorption isotherms were measured at 77 K to evaluate the porosity of the two COFs. Prior to the measurement, the samples were degassed at 120 °C at 1 × 10−5 Torr for 12 h. As shown in Fig. 4, both 3D COFs exhibited a type I isotherm displaying a sharp increase under low relative pressures (P/P0 < 0.01), which is characteristics of microporous materials. The Brunauer–Emmett–Teller (BET) surface areas were calculated to be 624 m2 g−1 for COF-1 and 570 m2 g−1 for COF-2, respectively (Fig. S10c†). By using the model of nonlocal density functional theory (NLDFT), the pore size distributions were also calculated (Fig. 4a and b). They displayed a major peak centered at 1.18 nm, corresponding to the simulated values of the smaller pores (Fig. 3). However, the larger pore diameter obtained from the crystal structures was 2.7 nm for both COF-1 and COF-2. For the low adsorption of N2, it may not be strictly correct with such a large open pore.
We have developed a dye uptake assay to evaluate the pore size distribution of the COFs.26 We carried out dye-uptake studies by soaking the COFs in a solution of dyes with different sizes for 24 hours. The dye solution was decanted and the COFs were washed several times to remove dye molecules adsorbed on the external surfaces of the solids. Dye molecules and COFs after spectral separation can be assigned to red and green fluorescence by confocal fluorescence microscopy (CFM), respectively (Fig. 4c and S12†). The CFM result showed the uniform distributions of dyes A–C with molecular sizes ranging from 1.90 × 2.12 nm2 to 2.15 × 2.81 nm2. However, the sterically bulky dye D (3.18 × 3.68 nm2) was only attached to the surfaces of the COFs and cannot enter the pores from the open channels, probably due to its larger size (3.68 × 3.18 nm2). The dye absorption amount was determined by measuring the UV-vis spectra in THF. As shown in Fig. 4d and S20,† remarkable size selectivity was observed for the dye uptake: the COFs had very significant uptake of dyes A–C (9.4–19.2% of the COF weight), but had only negligible uptake of dye D. By carefully adjusting the CFM, we obtained the cross sectional fluorescence images across the crystals in different Z wide positions, which reflects the spatial arrangement of incubated dye molecules within the COF. Dye A and COF-1 after spectral separation can be assigned to red and green fluorescence by CFM, respectively (Fig. S13†). In all cases, the inclusion adducts gave almost the same PXRD patterns as the pristine sample (Fig. S21†), indicating that the structural integrity and open channels of the two COFs are maintained in solution. Notably, during the dye-uptake experiments, no free NBC monomer was detected, indicating that no ligand exchange occurred. This was also supported by the almost identical IR spectra of the as-treated samples and the pristine COF-1 (Fig. S14†). Moreover, the 1H NMR spectra showed the digested dye-uptake of COF-1 contained only the aldehyde peaks of NBC (Fig. S15†). The dye uptake experiment indicated that both the maximum pore diameter and opening of the two COFs were in the range of 2.2 nm to 3.2 nm, consistent with the crystal structures and BET results.
The stability of the COFs was examined under various conditions. Thermogravimetric analysis (TGA) showed that both COFs have excellent thermal stabilities up to 350 °C under a nitrogen atmosphere (Fig. S3†). The chemical stability of the COFs was assessed by PXRD after 24 h of treatment in common organic solvents including DMF and MeOH, water, HCl(aq), and NaOH(aq) (Fig. S11†). Both COFs displayed good stability in organic solvents and water, although slightly decreased crystallinity was observed for the water-treated samples. Both COFs lost crystallinity and became amorphous in 1 M HCl. However, the COFs were capable of retaining crystallinity in 2 M NaOH. The BET surface areas of the as-treated COFs 1 and 2 were 381 and 242 m2 g−1, respectively (Fig. S10†), further indicative of the stability of the framework.
Photocatalysis
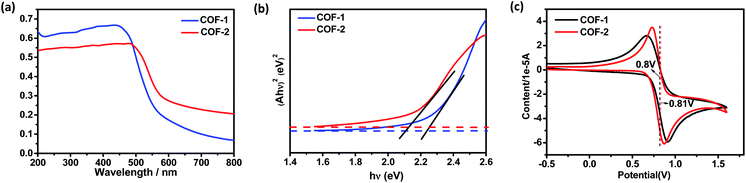
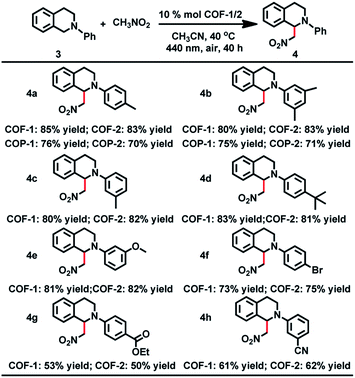
As a start for the photocatalytic study, the optical properties of the two COFs with the triphenylamine moiety were studied. Diffuse reflectance UV/vis spectra of the COFs and their monomers are shown in Fig. 5 and S17.† Obviously, both COFs 1 and 2 can absorb light in the UV and visible regions, with absorption edges at about 554 and 579 nm, respectively. These values are red-shifted by 46–147 nm in comparison to the solid state absorption spectra of the parent monomers, which might be attributed to a higher degree of conjugation in the extended structures. Based on the Kubelka–Munk formula, the optical band gaps of COFs 1 and 2 were calculated to be 2.24 and 2.14 eV, respectively, smaller than those of the building blocks such as NBC (2.44 eV) and BADA (2.87 eV) (Fig. S24†). Thus, the two 3D COFs may serve as new candidates for metal free photocatalysts in visible-light-driven reactions.The CDC reaction is one of the most efficient synthetic strategies for the construction of carbon–carbon bonds by oxidative coupling of two distinct C–H bonds.27 To test the photocatalytic activity of COF-1, the CDC reaction between N-phenyl tetrahydroisoquinoline and CH3NO2 was chosen as the model reaction. Initially different reaction conditions were screened and the results are shown in Table S3.† When 1-(nitromethyl)-2-(p-tolyl)-1,2,3,4-tetrahydro isoquinoline (3a) was reacted with nitromethane in CH3CN at 40 °C for 40 h, the product (4a) was obtained in good yield. Under the optimized reaction conditions, a series of substituted tetrahydroisoquinoline derivatives can react with nitromethane, affording the products in 53–85% yield. Notably, substituted tetrahydroisoquinoline derivatives with electron-rich groups gave higher yields than substrates with electron-withdrawing substituents. For instance, when 1,2,3,4-tetrahydroisoquinoline with a methyl group (3a–c) was reacted under standard conditions, the desired products were isolated in 85%, 80% and 80% yields, respectively. However, substrates with electron-withdrawing substituents (3g and 3h) only gave the targeted products in low yield. In addition, COF-2 was also capable of promoting the CDC reactions, generating the products in 50–83% yield, close to those obtained with COF-1.
The carbonyls as powerful building blocks play an important role in broad areas of organic synthesis.28 Compared with α-carbonyl substitution, direct β-activation of saturated carbonyls has demonstrated to be a more cumbersome and challenging task owing to the typically unreactive β-C(sp3)–H bonds and other competitive reactions. Recent studies indicated that the combination of organocatalysis and photoredox methods may provide a catalytic solution to this problem.3,7 Therefore, the enantioselective α-alkylation of aldehydes was selected as a test reaction to apply COF-based heterogeneous photocatalysts.
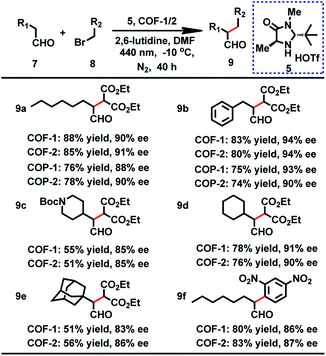
From the outset, the product 9a was obtained in 73% yield with 36% ee in this reaction under a white LED (Table S7,† entry 1). On the basis of the above result, we initiated our investigation with the reaction of 7a and 8a as a model reaction to explore the optimal reaction conditions. After screening the solvents of the reaction, we found that the substrate showed the highest activity for this reaction in DMF. Moreover, further improvement was achieved when a 440 nm LED instead of white light was used. Nevertheless, the ee values were still not desirable. It is worth mentioning that when 7a and 8a were employed at −10 °C with a 440 nm LED, the desired product was obtained in 90% ee, but with longer reaction time for high yield (Table S7†). When the α-alkylation of 7a with 8a was carried out in air or oxygen, the product 9a was obtained in lower yield (45% or 39%) and ee (73% or 69%). Fluorescence quenching titration showed that COF-1 and Macmillan catalyst 5 can form a stable host–guest adduct with association constants (Ka) of 3700 M−1 (Fig. S16†). The uniform distribution of 5 in COF-1 was further confirmed by CFM (Fig. S17†). When 10 mol% host–guest adduct was used to promote the α-alkylation of aldehydes, the product 9a was obtained in 65% yield with 89% ee (Scheme S2†).
With the optimized conditions in hand, we then extended the scope of this reaction, and the results are illustrated in Table 2. When benzenepropanal 7b and 8a participated in this reaction, the product 9b was obtained in 83% yield with 94% ee. From the reactions of 8a with alicyclic aldehydes, the products 9e and 9d were obtained in 51 and 78% yields with 83 and 91% ee, respectively. It is likely that alicyclic aldehydes made the reaction sluggish, leading to a decreased yield. tert-Butyl 4-(2-oxoethyl)piperidine-1-carboxylate can also proceed smoothly in this reaction, and the product 9c was isolated in 55% yield with 85% ee. When 1-bromo-2,4-dinitrobenzene was subjected to the transformation, the expected products 9f were isolated in 80% yield with 86% ee. Besides, COF-2 can also act as a photosensitizer for these catalytic reactions, affording the products in 51–85% yield and 85–94% ee (Table 2). The observed yields and ee values are comparable to those of previously reported homogeneous reactions using transition metal complexes,3 organic dyes29 or inorganic semiconductors8 as photosensitizers.
To probe the role of the pore aperture of COFs in photocatalysis, we studied the ability of the COF to encapsulate the substrates and organocatalyst by 1H NMR. The result showed that the activated COFs had significant uptake of the reactants 7b and 8a and the Macmillan catalyst 5 (35%, 20% and 15% of the COF weight, respectively) (Fig. S23†), indicating that the catalytic reaction may occur within the COF. It is thus likely that the photocatalytic reaction can occur both inside and outside the COF. However, attempts to prove that the reaction can occur in the COF cavities by using different sterically aromatic aldehydes (Scheme S3†) as substrates have failed so far.
After CDC and α-alkylation of aldehydes, both COFs 1 and 2 lost their crystallinity, as revealed by PXRD. However, after heating the amorphous covalent organic polymers (COPs) in a mixture of o-DBC/n-butanol/6 M HOAc (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v/v) in the presence of NBC or ETBC (Scheme 1), the crystallinity of the COF can be fully restored, as confirmed by PXRD (Fig. S25†). The BET surface areas were 499 and 467 m2 g−1 for the regenerated COFs 1 and 2, respectively. The pore size distributions also corresponded to the values of the parent COFs. Therefore, the two COFs went through a structural distortion that was recoverable via solvent-assisted linker exchange. This phenomenon is often observed in highly porous MOFs.30 Moreover, the reconstructed COF-1 exhibited similar catalytic activities to the pristine sample (conversions for 4a are 86%, 81%, 85%, 83% and 83% for 1–5 runs, respectively, and conversions/ee's for 9b are 80/93%, 81/91%, 85/93%, 83/93% and 83/94% for 1–5 run).
2, v/v/v) in the presence of NBC or ETBC (Scheme 1), the crystallinity of the COF can be fully restored, as confirmed by PXRD (Fig. S25†). The BET surface areas were 499 and 467 m2 g−1 for the regenerated COFs 1 and 2, respectively. The pore size distributions also corresponded to the values of the parent COFs. Therefore, the two COFs went through a structural distortion that was recoverable via solvent-assisted linker exchange. This phenomenon is often observed in highly porous MOFs.30 Moreover, the reconstructed COF-1 exhibited similar catalytic activities to the pristine sample (conversions for 4a are 86%, 81%, 85%, 83% and 83% for 1–5 runs, respectively, and conversions/ee's for 9b are 80/93%, 81/91%, 85/93%, 83/93% and 83/94% for 1–5 run).
In order to further understand the photocatalytic process, multiple control experiments were performed with the reaction of 3a and CH3NO2 (or 7a and 8a). The careful exclusion of light completely suppressed the reaction process, confirming the photochemical nature of the reaction. The inhibition of the reactivity was also observed under an aerobic atmosphere for the CDC reaction or in the presence of the radical scavenger DMPO or TEMP (1 equiv.), with the latter experiment being indicative of a radical mechanism. An EPR spin-trapping technique was employed to confirm the general radical and superoxide anion process (Fig. S26†).
Electrochemical measurements showed that COF-1 had a redox potential at 0.80 V, due to the redox potential of the COF-1+/COF-1 couple (Fig. 5c). The redox potential of the excited-state COF-1+/COF-1* couple was calculated as −1.60 V depending on a free energy change (E0–0) between the ground state and the vibrationally related excited state of 2.40 eV (Fig. S27†). Based on the above findings and a literature report,31 a proposed reaction pathway is shown in Fig. S22.† The CDC reaction is initiated by photoinduced electron-transfer from COF-1 to oxygen [Ered(O2/O2˙−) = −0.75]32 to generate COF-1+, which is rapidly reduced by the amine substrate 3 (Eox = 0.83 V)27c affording the amine radical cation THIQ+. The nucleophile attacks the intermediate 10 to obtain the target product 4 (Fig. S28†). In contrast, α-alkylation of aldehyde catalyzed by the COF is similar to the photocatalytic reaction catalyzed by fac-IrIII(ppy)3.4 The excited state of triphenylamine could initiate a photoinduced single electron transfer from the COF to diethyl 2-bromomalonate (E1/2 = −0.49 V),3 rendering a radical anion that undergoes σ-bond cleavage to give an electrophilic radical. Meantime, the chiral organocatalyst serves as cooperative active sites, where a π-nucleophilic enamine combines with the electrophilic radical to forge a crucial reaction center that drives the reaction in an asymmetric manner. The electron-rich amino radical 13 is rapidly oxidized by COF-1+, which closes the redox cycle while regenerating the photocatalyst COF-1. Furthermore, subsequent hydrolysis of iminium 14 would regenerate the organocatalyst 5 while offering the enantioenriched product 9 (Fig. S29†).4a
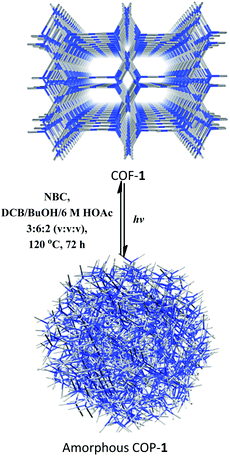 | ||
| Scheme 1 Recrystallization of COF-1 from the amorphous COP-1 through solvent-assisted linker exchange. | ||
The crystallinity and porosity of COFs play a vital role in determining their catalytic performances. In catalyzing CDC and α-alkylation of aldehyde, COFs 1 and 2 displayed similar enantioselectivities to the amorphous COPs 1 and 2, but with high efficiency. For example, the reaction of CH3NO2 with 3a or 3b catalyzed by the COPs afforded 5–13% lower yields of the products than those by the COFs, as shown in Table 1 (4a and 4b). Similarly, the COPs promoted the reaction of 7a with 8a or 8b generating the products in 10% lower yields than COF-1 (Table 2, 9a and 9b). The improved catalytic performance of the COFs is probably a result of their crystallinity and permanent porosity, which may optimize substrate adsorption/activation and facilitate electron transfer for efficient reduction of the α-bromocarbonyl substrate and α-amino radical.
| a 7 (0.769 mmol), 8 (0.385 mmol), 2,6-lutidine (0.769 mmol), 5 (0.0769 mmol), COF (10 mol% based on 8), DMF (2 mL), LED as the light source. The reactions were performed in Pyrex glassware, and the reaction mixture was degassed before irradiation. b Isolated yield. c Determined by 1H NMR of the diastereomeric acetals obtained by derivatization. |
|---|
|
Conclusions
We have designed and synthesized a pair of twofold interpenetrated 3D COFs with the ffc topology through condensation of rectangular and trigonal building blocks by imine linkages. The structure assignment was supported by PXRD analyses, modeling study, pore size distribution and dye-uptake experimental data. The 3D COFs were shown to be efficient photocatalysts for the CDC reaction and the asymmetric α-alkylation of aldehydes integrated with a MacMillan imidazolidinone as the chiral catalyst under visible light. The observed enantioselectivities are comparable to those of reported reactions using molecular metal complexes or organic dyes as photosensitizers. The COF materials that lost crystallinity after catalysis can be readily recrystallized and reused without performance loss. This work thus paves the way for future applications of COFs in visible-light-driven photoredox asymmetric catalysis and will promote the design of more 3D COFs with novel topologies and functions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Science Foundation of China (Grants 21431004, 21620102001, 21875136 and 91856204) and the Key Project of Basic Research of Shanghai (17JC1403100 and 18JC1413200).References
- (a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (b) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527–532 CrossRef CAS PubMed.
- Y. Zou, F. M. Hörmann and T. Bach, Chem. Soc. Rev., 2018, 47, 278–290 RSC.
- D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS PubMed.
- (a) H.-W. Shih, M. N. Vander Wal, R. L. Grange and D. W. C. MacMillan, J. Am. Chem. Soc., 2010, 132, 13600–13603 CrossRef CAS PubMed; (b) A. G. Capacci, J. T. Malinowski, N. J. McAlpine, J. Kuhne and D. W. C. MacMillan, Nat. Chem., 2017, 9, 1073–1077 CrossRef CAS PubMed.
- (a) Y. Yin, Y. Dai, H. Jia, J. Li, L. Bu, B. Qiao, X. Zhao and Z. Jiang, J. Am. Chem. Soc., 2018, 140, 6083–6087 CrossRef CAS PubMed; (b) K. Cao, S. M. Tan, R. Lee, S. Yang, H. Jia, X. Zhao, B. Qiao and Z. Jiang, J. Am. Chem. Soc., 2019, 141, 5437–5443 CrossRef CAS PubMed.
- (a) Z. D. Miller, B. J. Lee and T. P. Yoon, Angew. Chem., Int. Ed., 2017, 56, 11891–11895 CrossRef CAS PubMed; (b) W. Ding, L. Lu, Q. Zhou, Y. Wei, J. Chen and W. Xiao, J. Am. Chem. Soc., 2017, 139, 63–66 CrossRef CAS PubMed.
- (a) Y. Zhang, J. Guo, L. Shi, Y. Zhu, K. Hou, Y. Zheng and Z. Tang, Sci. Adv., 2017, 3, 1701162–1701168 CrossRef PubMed; (b) P. Wu, C. He, J. Wang, X. Peng, X. Li, Y. An and C. Duan, J. Am. Chem. Soc., 2012, 134, 14991–14999 CrossRef CAS PubMed.
- (a) M. Cherevatskaya, M. Neumann, S. Füldner, C. Harlander, S. Kümmel, S. Dankesreiter, A. Pfitzner, K. Zeitler and B. König, Angew. Chem., Int. Ed., 2012, 51, 4062–4066 CrossRef CAS PubMed; (b) P. Riente, A. M. Adams, J. Albero, E. Palomares and M. A. Pericàs, Angew. Chem., Int. Ed., 2014, 53, 9613–9616 CrossRef CAS PubMed.
- (a) J. Liang, Z. B. Liang, R. Q. Zou and Y. L. Zhao, Adv. Mater., 2017, 29, 1701139–1701159 CrossRef PubMed; (b) Y. Liu, W. Xuan and Y. Cui, Adv. Mater., 2010, 22, 4112–4135 CrossRef CAS PubMed.
- (a) L. Deiana, Y. Jiang, C. Palo-Nieto, S. Afewerki, C. A. Incerti-Pradillos, O. Verho, C.-W. Tai, E. V. Johnston and A. Córdova, Angew. Chem., Int. Ed., 2014, 53, 3447–3451 CrossRef CAS PubMed; (b) X.-H. Ho, M. Kang, S. Kim, E. D. Park and H.-Y. Jang, Catal. Sci. Technol., 2011, 1, 923–926 RSC.
- (a) C. Su, R. Tandiana, B. Tian, A. Sengupta, W. Tang, J. Su and K. P. Loh, ACS Catal., 2016, 6, 3594–3599 CrossRef CAS; (b) J. D. Smith, A. M. Jamhawi, J. B. Jasinski, F. Gallou, J. Ge, R. Advincula, J. Liu and S. Handa, Nat. Commun., 2019, 10, 1837–1846 CrossRef PubMed; (c) R. S. Sprick, J.-X. Jiang, B. Bonillo, S. Ren, T. Ratvijitvech, P. Guiglion, M. A. Zwijnenburg, D. J. Adams and A. I. Cooper, J. Am. Chem. Soc., 2015, 137, 3265–3270 CrossRef CAS PubMed; (d) N. Kang, J. H. Park, K. C. Ko, J. Chun, E. Kim, H.-W. Shin, S. M. Lee, H. J. Kim, T. K. Ahn, J. Y. Lee and S. U. Son, Angew. Chem., Int. Ed., 2013, 52, 6228–6232 CrossRef CAS PubMed; (e) X. Ding and B. Han, Angew. Chem., Int. Ed., 2015, 54, 6536–6539 CrossRef CAS PubMed; (f) X. Chen, M. Addicoat, E. Jin, L. Zhai, H. Xu, N. Huang, Z. Guo, L. Liu, S. Irle and D. Jiang, J. Am. Chem. Soc., 2015, 137, 3241–3247 CrossRef CAS PubMed; (g) Z. J. Wang, S. Ghasimi, K. Landfester and K. A. I. Zhang, Chem. Mater., 2015, 27, 1921–1924 CrossRef CAS; (h) W. Zhang, B. Aguila and S. Ma, J. Mater. Chem. A, 2017, 5, 8795–8824 RSC.
-
(a) P.-F. Wei, M.-Z. Qi, Z. Wang, S. Ding, W. Yu, Q. Liu, L. Wang, H. Wang, W. An and W. Wang, J. Am. Chem. Soc., 2018, 140, 4623–4631 CrossRef CAS PubMed;
(b) R. Chen, J.-L. Shi, Y. Ma, G. Lin, X. Lang and C. Wang, Angew. Chem., Int. Ed., 2019, 58, 6430–6434 CrossRef CAS PubMed;
(c) M. Bhadra, S. Kandambeth, M. K. Sahoo, M. Addicoat, E. Balaraman and R. Banerjee, J. Am. Chem. Soc., 2019, 141, 6152–6156 CrossRef CAS PubMed;
(d) S. Yang, W. Hu, X. Zhang, P. He, B. Pattengale, C. Liu, M. Cendejas, I. Hermans, X. Zhang, J. Zhang and J. Huang, J. Am. Chem. Soc., 2018, 140, 14614–14618 CrossRef CAS PubMed;
(e) X. Wang
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) , L. Chen, S. Y. Chong, M. A.
, L. Chen, S. Y. Chong, M. A. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Little, Y. Wu, W.-H. Zhu, R. Clowes, Y. Yan, M. A. Zwijnenburg, R. S. Sprick and A. I. Cooper, Nat. Chem., 2018, 10, 1180–1189 CrossRef PubMed.
Little, Y. Wu, W.-H. Zhu, R. Clowes, Y. Yan, M. A. Zwijnenburg, R. S. Sprick and A. I. Cooper, Nat. Chem., 2018, 10, 1180–1189 CrossRef PubMed. - (a) H. Kisch, Angew. Chem., Int. Ed., 2013, 52, 812–847 CrossRef CAS PubMed; (b) L. Wang, R. Fernandez-Teran, L. Zhang, D. L. A. Fernandes, L. Tian, H. Chen and H. Tian, Angew. Chem., Int. Ed., 2016, 55, 12306–12310 CrossRef CAS PubMed.
- (a) A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed; (b) S. Kandambeth, A. Mallick, B. Lukose, M. V. Mane, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2012, 134, 19524–19527 CrossRef CAS PubMed; (c) E. Jin, M. Asada, Q. Xu, S. Dalapati, M. A. Addicoat, M. A. Brady, H. Xu, T. Nakamura, T. Heine, Q. Chen and D. Jiang, Science, 2017, 357, 673–676 CrossRef CAS PubMed; (d) H. Li, A. D. Chavez, H. Li, H. Li, W. R. Dichtel and J.-L. Bredas, J. Am. Chem. Soc., 2017, 139, 16310–16318 CrossRef CAS PubMed; (e) H. Lyu, C. S. Diercks, C. Zhu and O. M. Yaghi, J. Am. Chem. Soc., 2019, 141, 6848–6852 CrossRef CAS PubMed.
- (a) T. Ma, E. A. Kapustin, S. X. Yin, L. Liang, Z. Zhou, J. Niu, L. Li, Y. Wang, J. Su, J. Li, X. Wang, W. D. Wang, W. Wang, J. Sun and O. M. Yaghi, Science, 2018, 361, 48–52 CrossRef CAS PubMed; (b) C. Gao, J. Li, S. Yin, G. Lin, T. Ma, Y. Meng, J. Sun and C. Wang, Angew. Chem., Int. Ed., 2019, 58, 9770–9775 CrossRef CAS PubMed; (c) X. Han, J. Huang, C. Yuan, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2018, 140, 892–895 CrossRef CAS PubMed; (d) T. Sun, L. Wei, Y. Chen, Y. Ma and Y.-B. Zhang, J. Am. Chem. Soc., 2019, 141, 10962–10966 CrossRef CAS PubMed; (e) S. Yan, X. Guan, H. Li, D. Li, M. Xue, Y. Yan, V. Valtchev, S. Qiu and Q. Fang, J. Am. Chem. Soc., 2019, 141, 2920–2924 CrossRef CAS PubMed; (f) Y. Liu, C. S. Diercks, Y. Ma, H. Lyu, C. Zhu, S. A. Alshmimri, S. Alshihri and O. M. Yaghi, J. Am. Chem. Soc., 2019, 141, 677–683 CrossRef CAS PubMed.
- (a) Y. Yang, M. Faheem, L. Wang, Q. Meng, H. Sha, N. Yang, Y. Yuan and G. Zhu, ACS Cent. Sci., 2018, 4, 748–754 CrossRef CAS PubMed; (b) R. W. Tilford, S. J. Mugavero III, P. J. Pellechia and J. Lavigne, Adv. Mater., 2008, 20, 2741–2746 CrossRef CAS PubMed; (c) J. Huang, X. Han, S. Yang, Y. Cao, C. Yuan, Y. Liu, J. Wang and Y. Cui, J. Am. Chem. Soc., 2019, 141, 8996–9003 CrossRef CAS PubMed; (d) L. A. Baldwin, J. W. Crowe, D. A. Pyles and P. L. McGrier, J. Am. Chem. Soc., 2016, 138, 15134–15137 CrossRef CAS PubMed; (e) K. Dey, M. Pal, K. C. Rout, H. S. Kunjattu, A. Das, R. Mukherjee, U. K. Kharul and R. Banerjee, J. Am. Chem. Soc., 2017, 139, 13083–13091 CrossRef CAS PubMed.
- (a) L. Li, X. Feng, X. Cui, Y. Ma, S. Ding and W. Wang, J. Am. Chem. Soc., 2017, 139, 6042–6045 CrossRef CAS PubMed; (b) X. Han, Q. Xia, J. Huang, Y. Liu, C. Tan and Y. Cui, J. Am. Chem. Soc., 2017, 139, 8693–8697 CrossRef CAS PubMed; (c) J. Zhang, X. Han, X. Wu, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2017, 139, 8277–8285 CrossRef CAS PubMed; (d) H. Li, Q. Pan, Y. Ma, X. Guan, M. Xue, Q. Fang, Y. Yan, V. Valtchev and S. Qiu, J. Am. Chem. Soc., 2016, 138, 14783–14788 CrossRef CAS PubMed.
- (a) Y. Du, H. Yang, J. M. Whiteley, S. Wan, Y. Jin, S. Lee and W. Zhang, Angew. Chem., Int. Ed., 2016, 55, 1737–1741 CrossRef CAS PubMed; (b) A. Halder, M. Ghosh, M. A. Khayum, S. Bera, M. Addicoat, H. S. Sasmal, S. Karak, S. Kurungot and R. Banerjee, J. Am. Chem. Soc., 2018, 140, 10941–10945 CrossRef CAS PubMed; (c) H. Wei, J. Ning, X. Cao, X. Li and L. Hao, J. Am. Chem. Soc., 2018, 140, 11618–11622 CrossRef CAS PubMed.
- (a) G. H. V. Bertrand, V. K. Michaelis, T. Ong, R. G. Griffin and M. Dincă, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 4923–4928 CrossRef CAS PubMed; (b) D. Bessinger, L. Ascherl, F. Auras and T. Bein, J. Am. Chem. Soc., 2017, 139, 12035–12042 CrossRef CAS PubMed; (c) S. Haldar, D. Chakraborty, B. Roy, G. Banappanavar, K. Rinku, D. Mullangi, P. Hazra, D. Kabra and R. Vaidhyanathan, J. Am. Chem. Soc., 2018, 140, 13367–13374 CrossRef CAS PubMed; (d) X. Wu, X. Han, Q. Xu, Y. Liu, C. Yuan, S. Yang, Y. Liu, J. Jiang and Y. Cui, J. Am. Chem. Soc., 2019, 141, 7081–7089 CrossRef CAS PubMed.
- (a) X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC; (b) S. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC.
- (a) S. Das, P. Heasman, T. Ben and S. Qiu, Chem. Rev., 2017, 117, 1515–1563 CrossRef CAS PubMed; (b) M. S. Lohse and T. Bein, Adv. Funct. Mater., 2018, 28, 170553–170623 Search PubMed; (c) T. Zhou, S. Xu, Q. Wen, Z.-F. Pang and X. Zhao, J. Am. Chem. Soc., 2014, 136, 15885–15888 CrossRef CAS PubMed.
- (a) F. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klöck, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 4570–4571 CrossRef CAS PubMed; (b) Y. Liu, Y. Ma, Y. Zhao, X. Sun, F. Gándara, H. Furukawa, Z. Liu, H. Zhu, C. Zhu, K. Suenaga, P. Oleynikov, A. S. Alshammari, X. Zhang, O. Terasaki and O. M. Yaghi, Science, 2016, 351, 365–369 CrossRef CAS PubMed; (c) D. Beaudoin, T. Maris and J. D. Wuest, Nat. Chem., 2013, 5, 830–834 CrossRef CAS PubMed; (d) Q. Fang, J. Wang, S. Gu, R. B. Kaspar, Z. Zhuang, J. Zheng, H. Guo, S. Qiu and Y. Yan, J. Am. Chem. Soc., 2015, 137, 8352–8355 CrossRef CAS PubMed; (e) T. Ma, J. Li, J. Niu, L. Zhang, A. S. Etman, C. Lin, D. Shi, P. Chen, L. Li, X. Du, J. Sun and W. Wang, J. Am. Chem. Soc., 2018, 140, 6763–6766 CrossRef CAS PubMed.
- (a) H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortés, A. P. Côté, R. E. Taylor, M. O'Keeffe and O. M. Yaghi, Science, 2007, 316, 268–272 CrossRef CAS PubMed; (b) G. Lin, H. Ding, R. Chen, Z. Peng, B. Wang and C. Wang, J. Am. Chem. Soc., 2017, 139, 8705–8709 CrossRef CAS PubMed; (c) Y. Lan, X. Han, M. Tong, H. Huang, Q. Yang, D. Liu, X. Zhao and C. Zhong, Nat. Commun., 2018, 9, 5274–5283 CrossRef PubMed; (d) O. Yahiaoui, A. N. Fitch, F. Hoffmann, M. Fröba, A. Thomas and J. Roeser, J. Am. Chem. Soc., 2018, 140, 5330–5333 CrossRef CAS PubMed; (e) Y. Zhang, J. Duan, D. Ma, P. Li, S. Li, H. Li, J. Zhou, X. Ma, X. Feng and B. Wang, Angew. Chem., Int. Ed., 2017, 56, 16313–16317 CrossRef CAS PubMed; (f) Q. Fang, S. Gu, J. Zheng, Z. Zhuang, S. Qiu and Y. Yan, Angew. Chem., Int. Ed., 2014, 53, 2878–2882 CrossRef CAS PubMed; (g) D. N. Bunck and W. R. Dichtel, Chem. Commun., 2013, 49, 2457–2459 RSC.
- (a) Z. Ning and H. Tian, Chem. Commun., 2009, 0, 5483–5495 RSC; (b) T. Zhang, X. Guo, Y. Shi, C. He and C. Duan, Nat. Commun., 2018, 9, 4024–4032 CrossRef PubMed.
- S. Hong, Y. Zou, D. Moona and M. S. Lah, Chem. Commun., 2007, 1707–1709 RSC.
- S. M. Shin, M. S. Lee, J. H. Han and N. Jeong, Chem. Commun., 2014, 50, 289–291 RSC.
- (a) A. Noble and J. C. Anderson, Chem. Rev., 2013, 113, 2887–2939 CrossRef CAS PubMed; (b) A. G. Condie, J. C. González-Gómez and C. R. J. Stephenson, J. Am. Chem. Soc., 2010, 132, 1464–1465 CrossRef CAS PubMed; (c) H. Bartling, A. Eisenhofer, B. Konig and R. M. Gschwind, J. Am. Chem. Soc., 2016, 138, 11860–11871 CrossRef CAS PubMed.
- J. R. Hummel, J. A. Boerth and J. A. Ellman, Chem. Rev., 2017, 117, 9163–9227 CrossRef CAS PubMed.
- (a) K. Fidaly, C. Ceballos, A. Falguières, M. S. Veitia, A. Guy and C. Ferroud, Green Chem., 2012, 14, 1293–1297 RSC; (b) M. Neumann and K. Zeitler, Org. Lett., 2012, 14, 2658–2661 CrossRef CAS PubMed.
- W. Xuan, C. Ye, M. Zhang, Z. Chen and Y. Cui, Chem. Sci., 2013, 4, 3154–3159 RSC.
- A. Casado-Sánchez, M. Uygur, D. Gonázlez-Muñoz, F. Aguilar-Galindo, J. L. Nova-Fernández, J. Arranz-Plaza, S. Díaz-Tendero, S. Cabrera, O. G. Mancheño and J. Alemán, J. Org. Chem., 2019, 84, 6437–6447 CrossRef PubMed.
- D. T. Sawyer, M. J. Gibian, M. M. Morrison and E. T. Seo, J. Am. Chem. Soc., 1978, 100, 627–628 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc04882k |
| This journal is © The Royal Society of Chemistry 2020 |