Developing technological pedagogical science knowledge through educational computational chemistry: a case study of pre-service chemistry teachers’ perceptions†
Jorge
Rodríguez-Becerra
 *ab,
Lizethly
Cáceres-Jensen
*ab,
Lizethly
Cáceres-Jensen
 ab,
Tatiana
Díaz
c,
Sofía
Druker
ab,
Tatiana
Díaz
c,
Sofía
Druker
 b,
Víctor
Bahamonde Padilla
b,
Víctor
Bahamonde Padilla
 a,
Johannes
Pernaa
a,
Johannes
Pernaa
 d and
Maija
Aksela
d and
Maija
Aksela
 d
d
aPhysical & Analytical Chemistry Laboratory (PahchemLab), Departamento de Química, Facultad de Ciencias Básicas, Universidad Metropolitana de Ciencias de la Educación, Santiago, Chile. E-mail: jorge.rodriguez@umce.cl; Tel: +56-22-241-2495
bPrograma de Doctorado en Educación, Universidad Metropolitana de Ciencias de la Educación, UMCE, Santiago, Chile
cDepartamento de Educación Diferencial, Facultad de Filosofía y Educación, Universidad Metropolitana de Ciencias de la Educación, Santiago, Chile
dThe Unit of Chemistry Teacher Education, Department of Chemistry, Faculty of Science, University of Helsinki, A.I. Virtasen aukio 1 (P.O. Box 55), 00014 Helsinki, Finland
First published on 24th February 2020
Abstract
The purpose of this descriptive case study was to develop pre-service chemistry teachers’ Technological Pedagogical Science Knowledge (TPASK) through novel computational chemistry modules. The study consisted of two phases starting with designing a computational chemistry based learning environment followed by a case study where students’ perceptions towards educational computational chemistry were explored. First, we designed an authentic research-based chemistry learning module that supported problem-based learning through the utilisation of computational chemistry methods suitable for pre-service chemistry education. The objective of the learning module was to promote learning of specific chemistry knowledge and development of scientific skills. Systematic design decisions were made through the TPASK framework. The learning module was designed for a third-year physical chemistry course taken by pre-service chemistry teachers in Chile. After the design phase, the learning module was implemented in a course, and students’ perceptions were gathered using semi-structured group interviews. The sample consisted of 22 pre-service chemistry teachers. Data were analysed through qualitative content analysis using the same TPASK framework employed in the learning module design. Based on our findings, pre-service chemistry teachers first acquired Technological Scientific Knowledge (TSK) and then developed some elements of their TPASK. Besides, they highly appreciated the combination of student-centred problem-based learning and the use of computational chemistry tools. Students felt the educational computational learning environment supported their own knowledge acquisition and expressed an interest in applying similar learning environments in their future teaching careers. This case study demonstrates that learning through authentic real-world problems using educational computational methods offers great potential in supporting pre-service teachers’ instruction in the science of chemistry and pedagogy. For further research in the TPASK framework, we propose there would be significant benefit from developing new learning environments of this nature and evaluating their utility in pre-service and in-service chemistry teacher's education.
Introduction
It is difficult to imagine the progress of science without the use of instruments and computers. Specialised software has facilitated the processing and analysis of data and contributed significantly to the understanding of scientific phenomena through visual representations. Viewed as the intersection of applied mathematics, computer science, and applied sciences, Computational Science (CSc) is essential for chemistry research (Yasar, et al., 2000; Yasar and Landau, 2003). In chemistry, this intersection is called Computational Chemistry (CC). CC is a field of chemistry that uses mathematical algorithms, statistics, and large databases to integrate chemical theory and modelling with experimental observations. Today, advances in computer visualisation capabilities facilitate the illustration of complex analyses in an easily understandable form. These are widely used in designing experiments and new materials and validating results.According to chemistry education research, contemporary research perspective and cutting-edge chemistry knowledge should be included in all levels of chemistry education. This would provide students with up-to-date scientific information in addition to chemistry-specific content knowledge (Blonder and Mamlok-Naaman, 2019). As a widely used method in chemistry research, CC offers excellent possibilities for integrating modern research methods into chemistry education.
In chemistry education research, the most studied applications of CC are 3D Molecular Visualization (3DMV) tools and data processing tools. Dozens of research publications show examples of how 3DMV and data processing tools have been integrated into chemistry curriculums worldwide. The possibilities and challenges that they offer for science education have also been studied extensively (Pfennig and Frock, 1999; Mahaffy, 2004; Jones et al., 2005; Ramos and Fernandes, 2005; Xie and Tinker, 2006; Geldenhuys et al., 2007; Burkholder et al., 2008; José and Williamson, 2008; Toplis, 2008; Angeli and Valanides, 2009; Tofan, 2009; Venkataraman, 2009; Abraham et al., 2010; Tuvi-Arad and Blonder, 2010; Battle et al., 2011; Evans and Moore, 2011; Kang and Kang, 2011; Kim et al., 2011; Linenberger et al., 2011; Milner-Bolotin, 2012; Ruddick et al., 2012; Wedler et al., 2012; Avramiotis and Tsaparlis, 2013; Krause et al., 2013; Ziegler, 2013; Al-Balushi and Al-Hajri, 2014; Ochterski, 2014; Springer, 2014; Lukas et al., 2019).
3DMV tools and animations improve conceptual understanding and spatial abilities of students. The development of visual representations supports chemistry-related communication benefitting chemistry learning and teaching significantly. In this sense, 3DMV improves the understanding of some chemical phenomena of high abstraction and at the same time, promotes the acquisition of dynamic mental images of molecular processes, helping to better understand the molecular structure and chemical reactivity (Mahaffy, 2004). Additionally, Venkataraman (2009) concludes that 3DMV is a useful aid in both underlying learning concepts and understanding the role of molecules in the observed phenomena. This study showed that students appreciated and valued the interactive nature of using 3DMV, which promoted the development of molecular-level mental models of chemical systems and processes (Venkataraman, 2009). Waddington (2001) proposed four key areas that need to be addressed to understand the possibilities of 3DMV: visual subtlety, complexity, abstractness, and conceptual depth. These critical areas must be considered in educational research and curriculum development because they pose significant challenges to learners and science teachers in the field of technology integration (Waddington, 2001).
José and Williamson (2008) studied the effects of 3DMV on teachers’ attitudes, content knowledge, and spatial ability. They observed no changes in content knowledge and only a few significant changes in attitudes, but they reported a significant increase in teachers' spatial abilities. Additionally, Tasker and Dalton (2006) analysed molecular-level animations, concluding that for the effective use of animations, it is necessary to direct the students’ attention to key features of the animation, avoid overloading working memory, and promote meaningful integration with prior content knowledge.
The majority of studies reported in the literature over the last decade have focused on incorporating CC courses or CC tools into scientific education at the undergraduate or postgraduate level (see the summary from Appendix 1, ESI†). In this regard, some of these studies highlight the use of CC tools to promote learning of chemistry at introductory college-level courses (Jones, 2001; Paselk and Zoellner, 2002; Cody and Wiser, 2003; Feller et al., 2004). However, despite the summarised potential benefits of CC tools for teaching and learning chemistry and the advances achieved through its use into scientific research, we have not found studies documenting the integration of CC tools, e.g., authentic Research-Grade Computational Chemistry Software (RGCCS), into pre-service chemistry teacher education.
In the case of in-service chemistry teachers’ some work has been done in supporting necessary knowledge, experience, and technology access to improve their teaching through the use of RGCCS (North Carolina Science, n.d.; Sendlinger and Metz, 2010; Royal Society of Chemistry, 2017). In general, the attitude among chemistry teachers towards RGCCS is positive. At the same time, teachers say that it is difficult to use new tools effectively in their teaching because of the lack of skills, training, and learning materials. Moreover, there is a specific need for supporting material in their mother tongue (Aksela and Lundell, 2008).
In light of the clear benefits of integrating CC tools into chemistry curricula, two objectives were set for this research. The first objective was to design a model for how to integrate CC into the curriculum of pre-service chemistry teachers training, which, according to our literature review, has not been done before. To achieve this goal, we designed a CC-based learning module that supports the professional development of future chemistry teachers' by training them on how to utilise computational chemistry practices in a pedagogically sound manner. To ensure a holistic view of professional development through the module, we used the Technological Pedagogical Science Knowledge (TPASK) model as the design framework (Jimoyiannis, 2010). The second objective was to explore pre-service chemistry teachers’ perceptions of chemistry learning and teaching through novel Educational Computational Chemistry (ECC) tools. This case study was designed to provide new knowledge for filling the gap regarding perceptions of pre-service teachers.
The aims of the study allowed the formulation of two research questions that guided the research:
• RQ1: What kind of learning environment is suitable for implementing novel computational chemistry practices into pre-service chemistry education?
• RQ2: What possibilities do pre-service chemistry teachers think computational chemistry offers for learning and teaching chemistry analysed through the TPASK framework?
Answers for the RQ1 were generated through the educational computational chemistry module design report. The resulting module was implemented in the Physical Chemistry I course in a bachelor's degree in Chemical Education at a Chilean university, and pre-service chemistry teachers’ perceptions (RQ2) were studied through a qualitative assessment after the implementation.
In this paper, first, we introduce the theoretical framework behind the TPASK model, which is needed to understand the design decisions made in ECC module development. Next, we describe the designed ECC learning module designed within the TPASK framework. Finally, we report student's perceptions of possible applications of ECCs to chemistry learning and teaching.
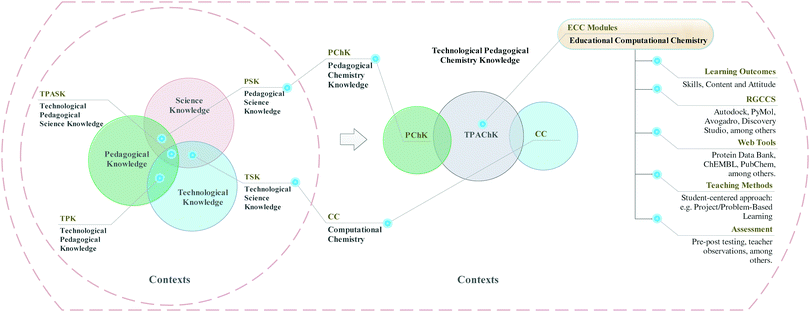
Technological pedagogical science knowledge (TPASK)
In this research, we used the TPASK framework for both the design and analysis of the ECC module. We chose TPASK as a theoretical tool because it is an application of the Technological Pedagogical Content Knowledge (TPACK) framework designed for science education (Jimoyiannis, 2010). We reasoned that TPASK was more suitable for content-specific work than TPACK.TPACK
The original TPACK model represents Pedagogical Content Knowledge (PCK) through a Venn diagram that represents PCK as the intersection of content knowledge and pedagogical knowledge (see Fig. 1) (Mishra and Koehler, 2006). PCK was originally Shulman's (1986, 1987) idea, who described PCK as the category most likely to distinguish the understanding of the content specialist from the pedagogical expert. This kind of knowledge goes beyond a simple consideration of content and pedagogy in isolation from one another (Shulman, 1986; Shulman, 1987).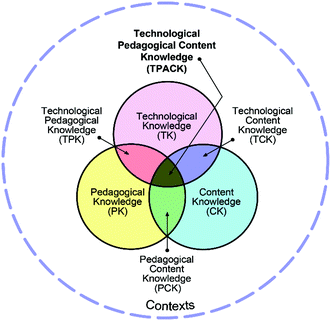 | ||
| Fig. 1 The classic visualization of TPACK framework. Reproduced with permission of the publisher, © 2012 by tpack.org. | ||
The conceptualisation of TPACK has been inspiring researchers. For example, Voogt et al. (2013) suggested based on their literature review that there are three different understandings of the TPACK concept: (1) T(PCK) is an extension of PCK, (2) TPCK is a unique isolated knowledge domain, and (3) TP(A)CK is an integrated knowledge domain combining all three domains of knowledge and their intersections as seen in Fig. 1. Multiple definitions indicate that there is no clear consensus of what TPACK should be. One reason for this may be that there is no consensus for the definition of PCK either. However, they emphasised that there is at least some consistency, in that earlier research agrees that TPACK stems from Shulman's PCK model (Voogt et al., 2013).
Cox (2008) also reviewed TPACK definitions. Based on her review, she suggests that TPACK is a construct of “knowledge of the technology-pedagogy-content interaction in the context of content-specific instructional strategies and topic-specific representations.” Subsequently, Cox and Graham (2009) proposed that “based on the elaborated model of the framework, TPACK refers to a teacher's knowledge of how to coordinate the use of subject-specific activities or topic-specific activities with topic-specific representations using emerging technologies to facilitate student learning.” Chai et al. (2013) synthesised their literature reviewed defining TPACK as “Knowledge of using various technologies to teach and/represent and/facilitate knowledge creation of specific subject content.”
From TPACK into TPASK
Arguments behind the development of TPASK are that science teachers develop a knowledge that “is different from knowledge of a disciplinary expert (a physicist, chemist, or biologist), or a technology expert, and from the general pedagogical knowledge shared by teachers across disciplines. TPASK represents what science teachers need to know about ICT in science education” (Jimoyiannis, 2010).Based on the definitions of TPACK and TPASK and our experience in training pre-service chemistry teachers, we support the perspective that TPASK should be understood as a distinct body of knowledge. Moreover, we believe that the TPASK framework implies a teacher's knowledge about the simultaneous interaction of three knowledge domains: emergent technology, pedagogy, and science, in the context of science-specific instructional strategies and topic-specific representations using emerging technologies aimed at understanding subject matters in effective learning environments. Within TPASK framework (Jimoyiannis, 2010), the seven knowledge areas constituents are:
(1) Technology knowledge (TK) is knowledge about how to use emerging technologies in a specific science domain
(2) Science knowledge (SK) is subject-specific knowledge like chemical bonding
(3) Pedagogical knowledge (PK) is generic knowledge about learning and teaching
(4) Pedagogical science knowledge (PSK) is knowledge about how to combine pedagogy and science effectively
(5) Technological science knowledge (TSK) is knowledge about how emerging technology may be used to provide new ways of representing and applying topic-specific in a given science domain.
(6) Technological pedagogical knowledge (TPK) is knowledge about the possibilities and challenges implied on different ways to teach and learn
(7) TPASK is the understanding of simultaneous interaction between SP, PK, and TK.
Fig. 2 displays the diagrammatic depiction of the TPASK as an adaptation of the TPACK framework proposed by Cox and Graham (2009). They emphasised that different bodies of knowledge are represented in the TPACK framework and proposed an elaborate model for classifying the seven constructs (Cox, 2008; Cox and Graham, 2009). For instance, TSK is practical and widespread in a way that forms knowledge about how to represent and apply concepts with technology. Accordingly, this knowledge about science representations and applications can exist independently of knowledge about their use in a pedagogical context.
We agree with Cox and Graham that “as the technologies used in the representations become mainstream, which knowledge transforms into content knowledge.” For instance, the knowledge of how the standard laboratory equipment of electronic types, such as balances, pH-meters, colorimeters and voltmeters, among others, facilitate chemical representations is now part of the content of chemistry itself. Indeed, the use of traditional laboratory equipment is an integral part of the subject of chemistry in chemistry teacher education. In this regard, how traditional laboratory equipment facilitates those chemical representations would be classified as SK. On the other hand, computational chemistry software for molecular modelling corresponds to emerging technology. The knowledge of how it facilitates chemical representation and concept applications would be considered as TSK (see Fig. 2).
Pedagogical possibilities behind educational computational chemistry
Computational chemistry – a technological science knowledge
CC is a chemistry field that offers theoretical knowledge and specialised software for modelling and visualising complex chemical topics (Nature.com, 2017). Current scientific and technological-computing development has allowed CC to reach a state of solid discipline maturity. CC-based simulating and modelling help to predict, understand and explain the structure and properties of molecules, materials, and chemical reactivity. Moreover, chemical principles, such as conformational analysis, acid–base equilibria, physical organic chemistry, molecular structure, thermodynamics, and stereochemistry, are implicit in this technological field. These contents are necessary for selecting and effectively applying CC tools and performing an insightful analysis of the results.A review of literature integrating CC into the Chemistry Curriculum constitutes a valuable reference base from which to orient pre-service chemistry teachers’ education (Jones, 2001; Paselk and Zoellner, 2002; Cody and Wiser, 2003; Feller et al., 2004; Jones et al., 2005; Tsai, 2007; Sendlinger et al., 2008; Metz and Sendlinger, 2009; Olvera and Bedolla, 2009; Sendlinger and Metz, 2010; Akaygun and Jones, 2013; Jones, 2013; Levy, 2013; Kelly, 2014; Ochterski, 2014). It also supports the value of in-service chemistry teachers’ training in the integration of CC in their classrooms as a means to develop their TPASK. In other words, CC represents knowledge about how TK and SK are reciprocally related, allowing an emergent form of knowledge described as TCK by Mishra and Koehler (2006) or TSK by Jimoyiannis (2010). The possible ways in which the integration of CC knowledge components into pre-service or in-service chemistry teacher training could support their TSK are summarised in Table 1. This table presents the main knowledge components of CC within the TSK framework.
| Knowledge components | Descriptive components |
|---|---|
| Theoretical principles used | • Quantum chemistry, molecular mechanics, molecular dynamics, Monte Carlo methods, Brownian dynamics, continuum electrostatics, reaction dynamics, numerical analysis methods, artificial intelligence, chemometrics, cheminformatics and others. (Dekock et al., 2007) |
| Problem-solving skills | • Formulating hypotheses based on antecedent and test |
| • Modelling and designing based on available data (molecular, biologic, environmental) | |
| • Drawing conclusions through analysis and interpreting of data results | |
| Skills in integrating computational methods to solve problems | • Extracting information from large data sets |
| • Applying software for molecular data collection and analysis. | |
| • Making visual representations of molecular phenomena and datasets | |
| • Applying computational methods to chemical, physicochemical and biochemical processes | |
Problem-based learning: learning environments in chemistry education
Problem-Based Learning (PBL) is a learning methodology that supports the use of problems as a starting point for the acquisition and integration of new knowledge (Barrows, 1986). This methodology uses a student-centred approach, where the student must learn to solve complex situations based on real, relevant and significant problems (Prince, 2004).In the last decade, different studies published used PBL learning environment to implement activities into the chemistry curriculum effectively. The findings demonstrated that PBL led to an increase in the students' achievement in chemistry knowledge (Günter et al., 2017; Gunter and Alpat, 2017; Baran and Sozbilir, 2018). Besides, these studies also found that students had positive opinions regarding PBL activities. These activities allow students to associate chemistry with real-world problems and helps them to build interdisciplinary connections (Cowden and Santiago, 2016; Günter et al., 2017; Gunter and Alpat, 2017; Strollo and Davis, 2017).
Educational computational chemistry in supporting TPASK
TPACK “represents a class of knowledge that is central to teachers’ work with technology. This knowledge would not typically be held by technologically proficient subject matter experts, or by technologists who know little of the subject or of pedagogy, or by teachers who know little of that subject or about technology.’’ (Mishra and Koehler, 2006). In the case of science education, Jimoyiannis (2010) argues that TPASK represents a class of knowledge needed by science teachers to allow productive technology integration in science education. Consequently, CC integration into pre-service chemistry teachers’ education constitutes an essential basis for teaching chemistry with technology that is adequate for the discipline.Chemistry and science education are suitable subject matters for technology integration due to implicit technology development in science. Thus, technology integration can help teachers to provide a learning environment that encourages the development of student-centred learning environments by requiring students to learn independently (Agapova et al., 2002).
The Venn diagram in Fig. 2 (right), represent the technological pedagogical chemistry knowledge (TPAChK) adapted from the TPASK framework. This construct emerges from the natural integration of CC (TSK) and pedagogical chemistry knowledge (PChK). In this sense, the knowledge and skills necessary to produce 3DMV and graphical representations of data using a collection of computational tools and methods, which can be applied effectively in teaching and learning of chemistry subject-matters, it goes beyond PChK and CC (TSK) in themselves. According to the transformative view (Angeli and Valanides, 2009), we can understand TPAChK as a body of knowledge that contains core elements of its own. These core elements can be related to how science topics that are difficult to be understood by learners or challenging to teach by teachers can be transformed and taught more effectively with learning environments that integrate CC or also CSc.
We propose that integrating emerging technologies in science education requires contextualisation of knowledge of these new technologies, considering their use in generating scientific knowledge and the significance of this knowledge to society. In this regard, advances in computational methods in chemistry (or CC) represent an example of emerging technology in science that can be used in the teaching of chemistry. The contextualisation of this emerging technology in the teaching of chemistry is related to the use of real-world problems and examples of scientific development.
As an attempt to integrate computational chemistry and contextualised chemistry teaching using a real-world problem with social and environmental projections, we have developed an ECC module aimed to (a) promote the learning of chemical concepts, allowing students to establish their individual learning needs and priorities (the instructor does not establish priorities); (b) promote meaningful and contextualised learning of chemical concepts based on a real-world problem that integrates computational chemistry with knowledge of other areas of science; (c) promote the analysis of CC model approximations, evaluating their applicability in a real-world problem.
The ECC module can be defined as a practical model that reveals the affordances of computational chemistry to integrate technological knowledge of emerging technologies and chemistry knowledge, thus constituting technology chemistry knowledge (or TSK). This module shows how CC can be used with the PBL teaching method, considering the specific chemistry topics that the pre-service chemistry teachers will be expected to teach when working as teachers themselves. In this regard, the ECC model implicitly opens a range of opportunities for innovation in the development of learning activities. This model of CC integration in teaching and learning of chemistry requires concurrent interweaving of all three sources of knowledge: chemistry, pedagogy, and computational chemistry. For the last source of knowledge, we should note that computational chemistry represents the interdisciplinary blend of applied mathematics, computer science, and applied chemistry.
Similarly, Yasar et al. (2000) initially defined the CSc field as the intersection of applied mathematics, computer science, and applied sciences. However, today CSc is recognised as a field with its own core characteristics. These core elements may be envisioned as a collection of computational tools and methods related to a problem-solving mindset (Yasar et al., 2000; Yasar and Landau, 2003).
Curricula implemented through student-centred learning environments would allow for students to learn chemistry content by using CC models. The skills that students develop, such as using, creating, testing, and interpreting computational models, are skills needed by chemistry teachers for developing their TSK.
Description of the educational computational chemistry module
In this section, we describe the main components of the designed ECC module (see Fig. 3).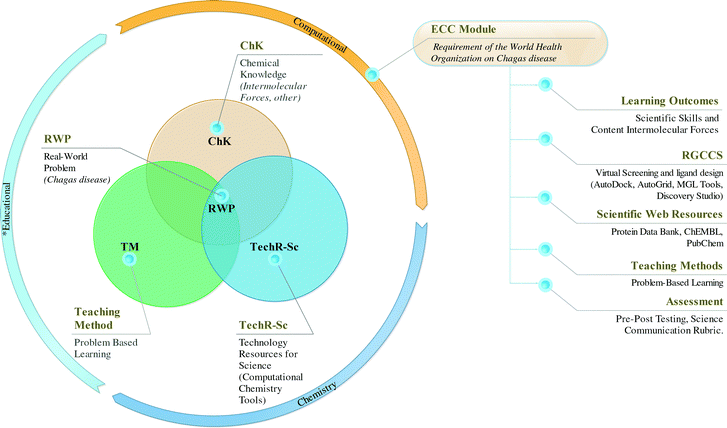 | ||
| Fig. 3 Main components that influenced the design of the ECC module: requirement of the World Health Organization on Chagas disease, for pre-service chemistry teacher training (see Appendix 2, ESI†). | ||
Chemistry topic: intermolecular forces
Intermolecular forces are present in all matter. They have been studied and classified throughout history (Burkholder et al., 2008). Moreover, intermolecular forces are considered a complicated topic for students to understand (Schurmeier et al., 2011). Interactions between neutral molecules include those between two permanent dipoles (dp–dp), between a permanent and an induced dipole (dp–di), and London's (di–di) dispersion interactions. These interactions, collectively known as the van der Waals forces, depend on molecular dipole momentum, polarizability, and ionisation energy (Bunce, 2011). In addition to these interactions, the hydrogen bond is considered a particular case of dipole interaction (dp–dp), as suggested by the IUPAC definition (Bunce and VandenPlas, 2011).Intermolecular forces are an essential concept in chemistry. They explain an expansive variety of physical and chemical properties of matter and are a driving force associated with changes in thermodynamic variables, such as internal energy and enthalpy. However, combining 3D structure and electron density distribution to determine what kind of intermolecular forces would better stabilise a molecular system, and in turn using this understanding to connect molecular structure to both the physical and chemical properties of a substance, represents a challenging task for students (Cooper et al., 2012; Cooper et al., 2015). For these reasons, we selected ‘intermolecular forces’ as the chemistry topic for the designed ECC module within the physical chemistry I course.
Computational chemistry tools: selection of RGCCS and web tools
For the ECC module designed in this case study, several types of software and scientific web resources were utilised (see Table 2). The selected tools were relevant to real-world CC-based problem-solving.| Knowledge components | Descriptive components | Software or resource (URL) |
|---|---|---|
| Computer modelling and statistical analysis | Spreadsheets | Sigma plot (https://systatsoftware.com/), Origin (http://www.originlab.com/), PsiPlot (http://www.polysoftware.com/psiplot.htm), Excel (http://office.microsoft.com/en-us/excel) |
| Chemical spreadsheets | Instant JChem (https://www.chemaxon.com/products/instant-jchem-suite/), Seurat (https://www.schrodinger.com/seurat), Bioclipse (http://www.bioclipse.net/), quattro/DS (http://www.quattro-research.com/), LICSS (https://github.com/KevinLawson/excel-cdk) | |
| Mathematical modelling and symbolic algebra software | Mathematica (https://www.wolfram.com/mathematica/), Maple (http://www.maplesoft.com/), Mathcad (http://www.ptc.com/products/mathcad/), Hydrus-1D (https://www.pc-progress.com/en/Default.aspx?hydrus-1d) | |
| Molecule editor and viewer | (Avogadro (http://avogadro.cc/), BALLView (http://www.ball-project.org/ballview/), Luscus (https://sourceforge.net/projects/luscus/), PyMol (https://sourceforge.net/projects/pymol/), J-ICE (http://j-ice.sourceforge.net/), QMForge (http://qmforge.sourceforge.net/), wxMacMolPlt. (http://brettbode.github.io/wxmacmolplt/), VIDA (https://www.eyesopen.com/vida), Chimera (https://www.cgl.ucsf.edu/chimera/), OpenAstexViewer (http://openastexviewer.net/web/), CylView (http://www.cylview.org/Home.html), Molegro Molecular Viewer (http://molegro-molecular-viewer.software.informer.com/2.5/), Qutemol (http://qutemol.sourceforge.net/), VMD (http://www.ks.uiuc.edu/Research/vmd/), Yasara (http://www.yasara.org/), ICM Browser (http://www.molsoft.com/icm_browser.html) | |
| Quantum mechanics | Gaussian 98/03/09 (http://gaussian.com/), GAMESS (http://www.msg.ameslab.gov/gamess/), Jaguar (https://www.schrodinger.com/jaguar), Spartan (https://www.wavefun.com/products/spartan.html), ABINIT (http://www.abinit.org/), ACES (http://www.qtp.ufl.edu/aces/), CP2K (https://www.cp2k.org/), JANPA (http://janpa.sourceforge.net/), NWChem (http://www.nwchem-sw.org/index.php/Main_Page), PSI4 (http://www.psicode.org/), Quantum-espresso (http://www.quantum-espresso.org/) | |
| QSAR/ADMET modelling | PaDEL-descriptor (http://www.yapcwsoft.com/dd/padeldescriptor), Chemistry aware model builder (camb, https://github.com/cambDI), Open3DGRID (http://open3dgrid.sourceforge.net), Open3DQSAR (http://open3dqsar.sourceforge.net), QSAR-tools (https://github.com/dkoes/qsar-tools), CheS-Mapper (http://ches-mapper.org), DataWarrior (http://www.openmolecules.org/datawarrior), DecoyFinder (http://urvnutrigenomica-ctns.github.io/DecoyFinder), Toxtree (http://toxtree.sourceforge.net) | |
| Virtual screening and ligand design | Pharmit (http://pharmit.sf.net), AutoDock (http://autodock.scripps.edu), AutoDock Vina (http://vina.scripps.edu), ADplugin (https://github.com/ADplugin), MGLTools (http://mgltools.scripps.edu/), PyRx (http://pyrx.sourceforge.net) | |
| Molecular dynamics | NAMD (http://www.ks.uiuc.edu/Research/namd/), GROMACS (http://www.gromacs.org/), CHARM (http://www.charmm.org/), Abalone (http://www.biomolecular-modeling.com/Abalone). | |
| Computational suites | Schrodinger (https://www.schrodinger.com/), MOE (http://www.chemcomp.com/), Sybyl-X (https://support.certara.com/software/molecular-modeling-and-simulation/sybyl-x/), Discovery Studio (http://accelrys.com/products/collaborative-science/biovia-discovery-studio/) | |
| Scientific web resources | Bioinformatics and cheminformatics database | Protein Data Bank (PDB) (https://www.rcsb.org/), ChEMBL (https://www.ebi.ac.uk/chembl/), PubChem (https://pubchem.ncbi.nlm.nih.gov/), DrugBank (https://www.drugbank.ca/), ChemSpider (www.chemspider.com/), ZINC (http://zinc.docking.org/), Hazardous Substances Data Bank (https://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB), WCSE (http://www.cheminfo.org/wikipedia), PhET (https://phet.colorado.edu/) |
(i) Avogadro was used to draw, edit and view molecules;
(ii) Autogrid 4.2 was employed to build the grid maps using a three-dimensional lattice;
(iii) AutoDock software 4.2 was used to explore the conformational states of a flexible ligand and the empirical free energy scoring function to evaluate conformations during the docking process. The parameters were taken from the default settings in AutoDock 4.2;
(iv) Discovery Studio 2016 was used for the viewing and analysis of protein–ligand complexes;
(v) Excel was used for data entry, organisation, sorting, and plotting.
In addition, scientific web resources, including Protein Data Bank (PDB), PubChem, and ChEMBL, were utilised. We also employed research-based science and mathematics simulations provided by PhET as digital educational resources: Atomic Interaction Version 1.10.00 (PhET Interactive Simulations, 2017a, 2017b) and Molecule Polarity Version 1.02.00 (PhET Interactive Simulations, 2017a, 2017b).
Teaching method: problem-based learning
The chosen pedagogical approach was PBL because this is the common way to solve problems in scientific research (Akcay, 2009). Moreover, as students were unfamiliar with PBL at the start of the module, this represented a critical opportunity from a pedagogical perspective to introduce future chemistry teachers to a strategy they will likely apply in their future careers.The topic of intermolecular forces was covered by one PBL scenario for four weeks. The PBL scenario was introduced to the students using a one-page guide consisting of a short descriptive text, presented in Appendix 2 (ESI†).
PBL scenario was carried out in small groups of 3–4 students. To promote collaboration, the classes were conducted in a classroom with big tables where students worked in team stations, rather than being seated in a traditional classroom setting. The coordinating teacher moved between the work stations, engaging with each group by:
• Asking leading and open-ended questions.
• Helping students to reflect on learning objectives in order to solve the real-world problem introduced in the PBL scenario.
• Challenging students’ thinking regarding how they could use the educational resources available.
• Raising content issues that required consideration.
• Promoting a safe learning environment in which students felt comfortable with sharing goals and ideas without fear of being ridiculed.
The PBL instruction method implemented consists of 11 stages (see examples from Appendix 3, ESI†):
(1) PBL scenario contextualisation: students read, reflect, and write about an important milestone for the contextualisation of a real-world problem.
(2) Brainstorming: students record questions, statements, facts, concepts, and constraints implicit in the problem.
(3) Systematisation: students read their notes and sort them into different categories, topics, and processes.
(4) Problem description: based on stages 2 and 3, students formulate learning goals (objectives) to solve a real-world problem.
(5) Role distribution: students designate roles based on cooperative work, where they identify and assign the learning goals (objectives) proposed in stage 4. Subsequently, the students analyse the educational resources and collect additional information to develop learning goals for the group.
(6) Aims identification: each student presents the information collected according to his/her role within the group. Afterwards and by means of cooperative work, the students identify research objectives that would allow the resolution of a real-world problem through a working methodology.
(7) Data analysis: students work on identifying and controlling variables. Then, they interpret results using grouped or tabulated data relevant to the research question.
(8) Conclusions: students formulate their conclusions considering: (i) observed regularities in the results; (ii) consistency with the hypothesis and research objectives; (iii) conclusion validity.
(9) Science communication: students present their research results to other students and the academic community via posters.
(10) Peer evaluation: students perform a peer evaluation at the end of the implementation of the ECC module, considering three categories: commitment to the assignment, accordance between role requirement and work development, and quality of work.
(11) Self-evaluation: in the end, students attend a group session and conduct a guided self-reflection on their learning. Reflection is carried out using a questionnaire that helps to identify “what” and “how” they have learned during the implementation of the ECC module.
Case study – pre-service chemistry teachers’ perceptions
Students’ perceptions towards novel educational computational chemistry learning environments have previously been assessed through a descriptive case study (Cohen et al., 2007). Similarly, we selected this approach to evaluate students’ opinions and insights about the designed learning environment.Course context
The designed ECC module was implemented in Physical chemistry I course. This advanced-level course includes many academic prerequisite requirements such as pedagogical studies (e.g. knowledge of pedagogy, learning, education policies and curriculum), chemistry studies (e.g. basics of general, inorganic chemistry, organic chemistry and analytical chemistry), teaching and learning of sciences, and pedagogical practice training (e.g. Practices I–III).Focus group procedure and participants
For the implementation of the module, the instructors divided students into groups of three students. Next, all students were asked if they would like to participate in the research. Twenty-two students gave their informed consent and participated voluntarily. These 22 students were divided into three focus groups: 6/16 students from group 1, 7/16 from group 2 and 9/16 from group 3.All participants were undergraduate level pre-service chemistry teachers in the fifth semester of an 8-semester bachelor's degree in Chemical Education at a Chilean university. The average age of participants was 22 years. Participants had no experience with the problem-based learning methodology or with the use of RGCCS. This was verified via an initial background interview.
The research sample is quite homogeneous because the sample size is small, and all respondents are studying at the same academic program and university. Also, participants’ knowledge of chemistry, pedagogy and technology are relatively uniform, and we assume that they regularly exchange experiences and ideas with each other.
As the study was conducted as a part of the regular teaching activities within the physical chemistry course, no specific ethical clearance was required. However, participating students signed an informed consent document. They were informed that the collected information would be used in a manner that would not allow for the identification of any individual, and that they always had the option to decline to participate in the focus groups.
Data gathering
Data was gathered using semi-structured interviews conducted in focus groups (Cohen et al., 2007). Interviews were carried out at the end of the semester, just after students had finished the ECC module. Interviews was recorded in video and later transcribed to preserve the fidelity and totality of the participants' discourse. The focus groups were conducted by a moderator, who had not participated in the implementation of the module.A semi-structured survey was designed with open-ended questions aimed at collecting the students' perceptions of their experiences in the ECC module implementation (interview questions are presented in Appendix 4, ESI†).
The focus group began with a descriptive open-ended question in order to encourage active participation and collect the most relevant aspects for students. Additionally, the survey included questions intended to cover aspects of three constructs of the TPASK framework: SK, TSK, and PSK. At the end of the focus group, a question was included to evaluate students’ own learning.
Data analysis
The data collected were analysed using qualitative content analysis (Hsieh and Shannon, 2005). A first segmentation of the textual corpus was carried out based on the interview questions used in the focus group.Then the transcripts of recorded focus groups were divided into discourse episodes according to turns in the conversation guided by the questions and then independently analysed and categorised into two categories: TSK and TPASK. Table 3 describes the criteria for TSK and TPASK employed for this distinction.
| Category/description | Selection criteria | Indicators employed |
|---|---|---|
|
TSK
Subject-specific knowledge in a given science domain, like molecular docking or modelling chemical kinetics, among others, that utilise emerging technologies like RGCCS. TSK represents the knowledge of how to use models to represent and apply science concepts using emergent technology. In this sense, this knowledge component is independent of its projections to pedagogical contexts, because their building is directly related to science and technology development. |
• Knowledge of computational chemistry resources (Table 1, computer modelling and statistical analysis, and scientific web resources) that can be used in real world problems of science.
• Ability to use computational chemistry resources as previously described. • Knowledge of the appropriate use of computational chemistry resources used in real world scientific problems. • Knowledge about models and protocols of computational chemistry employed by the scientific community. |
• The student identifies resources of CC that can be used to approach scientific problems.
• Students identify situations in which they have used RGCCS. • Students select appropriate software for solving a scientific problem. • Students identify molecular modelling protocols in scientific publications and chemistry models (Lennard-Jones potential, intermolecular interactions and chemical equilibrium) as part of their chemical or scientific knowledge. • The students make comparisons among different computational chemistry tools in order to model or represent a science concept. |
|
TPASK
Pre-service teacher's understanding of how to use science's emerging technology to implement learning environments that promote student science learning. However, in agreement with Cox S. and Graham C. “as the technologies used in those activities and representations become ubiquitous,” TPASK transforms into PSK (Cox and Graham, 2009).
This knowledge also refers to the understanding of models, protocols, and practices of computational science used by the scientific community and how these can be implemented into environments that promote student learning. |
• Knowledge of computational chemistry tools suited to teach chemistry topic.
• Selecting teaching approaches to facilitate the use of computational chemistry resources in order to promote science learning. • Using computational chemistry tools to improve content understanding by means of computational modelling (Table 3, e.g., molecular modelling, molecular visualisation and mathematical modelling). • Projecting the learning experience in the ECC module to pedagogical contexts (i.e. school system) as a mirror value to facilitate their future students achieving a deeper and more grounded understanding of abstract chemical concepts and processes. |
• Students name pedagogical situations in which they would use CC resources (molecular visualisation software, Maple, among others) to teach science topics.
• Students Identify teaching methods like student-centred approaches, and teaching strategies (e.g. brainstorming) used in their own learning experience within the module that they would like to implement as teachers to promote the learning of chemistry concepts using computational chemistry tools.
• Students make comparisons among teaching methods best suited for guiding students in science learning using computational chemistry tools. |
Results and discussion
Pre-service chemistry teachers’ perceptions of educational computational chemistry (RQ2)
In this section, we refer to pre-service chemistry teachers using the following symbols: Ai (i = 1–22), for each participant of the study. Currently, computational chemistry affords newer and more diverse representations, as well as greater flexibility in the use of these representations to predict, understand, or explain the structure and properties of molecules, materials and chemical reactivity. Pre-service chemistry teachers need to know not just the chemistry subject matter but also the way the subject matter can be changed by the technological advances in the field of chemistry. The ECC module was implemented using the PBL scenario: “Requirement of the World Health Organization on Chagas disease”. This approach allows students to evaluate the different molecular conformations of β-carboline derivatives into the Trypanothione Reductase active-site, considering the protein–ligand intermolecular interaction observed by mean of 3DMV. This facilitates the application of standard intermolecular interaction proofs in a real science problem. The PBL scenario was proposed to the students using a one-page descriptive text that described the challenge of identifying a potential Trypanothione Reductase inhibitor among commercially available β-carboline derivatives, using as substructure the β-CD: 1-{1-methyl-9H-pyrido[3,4-b]indol-3-yl}ethan-1-one. In this context, the students recreated the scientific work and used information about intermolecular forces to research a real-world problem. RGCCS allows students to employ 3D molecular constructions, effectively changing the nature of learning chemistry itself. These 3D molecular constructions are a form of representation in chemistry that was not available before the development of computational chemistry.The perceptions of pre-service chemistry teachers regarding this experience were analysed through descriptive categories established using TPASK framework (Table 3). The results of this analysis are synthesised below, highlighting the main findings regarding student's perceptions about both their own participation in the ECC module and how they envision applying this learning experience to their future pedagogical work.
Perceptions of pre-service chemistry teachers about their experience as students in support their TSK through the ECC module
The discourse analysis showed that pre-service chemistry teachers considered the incorporation of RGCCS to benefit their professional training. Nonetheless, across the different focus groups, two common themes emerged regarding sources of difficulty in the early stages of the module. On the one hand, student pointed to difficulties derived from their lack of previous knowledge and/or skills with ICT, not specifically related to computational chemistry tools or RGCCS, which made the process of setting up the needed software especially difficult. Acknowledging the lack of preparation in ICT as a potential flaw of the module, students recognised that the software provided access to useful computational tools distinct from those traditionally implemented in the discipline. Students viewed both this and the practice of teaching disciplinary content as advantages in the current scenario.A12: “I think that the module has several flaws, but it contributes in many things in the use of ICT because of not knowing how to use and install software, we have to improve to be able to use these tools because they are there if we do not use them we are at a disadvantage in respect to our classmates.”
On the other hand, the students frequently identified their lack of experience with computational chemistry tools and computational science in general as adding complexity to their early performance in the module
A1: “Software, simulations, in general, it's scarce that we have the opportunities we have within this programme of working with simulations, to do any type of computational experiment, so that is why I believe that for all of us the part of using the software was where we were the slowest”
A3: “to this point, the software is like the most difficult, also because we [had] never used it.”
Notwithstanding these initial difficulties, pre-service chemistry teachers expressed that, as the module progressed and through the use of RGCCS, they were able to relate chemistry concepts and understand aspects of intermolecular forces at a better level than with a direct instructional method. The highest valuations the students attributed to the use of RGCCS appear related to two specific areas of their learning process. First, they credited RGCCS in facilitating a deeper understanding of the process by which scientific conclusions are derived, without the focus on content memorisation they associate with a regular chemistry class.
A10: “since I learned things without having to study them or memorise them … I already had them (the knowledge). Accurately, and I hadn't noticed, and I could handle and explain them without studying, without reading and without memorising. That was great.”
For the students, this deeper understanding is mainly derived from the possibility RGCCS allows for experimentation, in a learning-by-doing approach guided towards the adequate resolution of the scientific problem they were presented with during the module.
A5: “It is always better to do than just copying [from a whiteboard or other written material]. When I do [something] I also had to have a process of comprehension, when I copy I am not necessarily comprehending, reading the graphics’ key does not mean that I understood the graphic, but if I do it myself, if I build it myself I do have to inform myself about I don't now like the mathematical model I am using in the graphic, what variables I am considering, and in that way to link different concepts, so doing always has more value than just copying.”
Speaking about the improvements in her own understanding of the module's subject-matter, another student expressed:
A6: “regarding content, it's good or excellent because just the fact of doing the module and you yourself searching for the information, it obligates you to study, there is no other option (…), but it obligates you to learn, to comprehend, and because you have to use what you know, at the end it stays with you in a more meaningful manner, and with the computers.”
A similar opinion emerged regarding the usefulness of RGCC to integrate and use data:
A3: “the computational tool we used, that I learned how to use, we got data that was useful for the resolution of the problem.”
Secondly, pre-service chemistry teachers pointed out that RGCCS facilitates the development of a more grounded understanding of abstract chemistry concepts, mainly through the procedures of modelling and generating visual representations.
A3: “[the] Software, it represents graphics for us, it makes it possible for us to model molecular interaction, and that it is more visible to understand chemistry from that standpoint, when you are dealing with very small molecules, with a behaviour, then it is a great tool I believe the use of this software.”
A1: “There is also the issue of abstraction, that it is difficult to suddenly imagine a molecule rotating, but in the software, it is indeed possible to see that, and that brings closer the chemistry of the smallest things, you see it as more tangible.”
An interesting finding related to the use of RGCCS in this context concerns the possibility of integrating different computational resources for the resolution of the problem at hand, as A19 points out:
A19: “For example, the visualisers of the molecules bank itself [table 2, Bioinformatics and cheminformatics database] I download molecules then I optimised them with Avogadro, and I see them in Discovery, and the Discovery I can use it very well, I see the colours, differentiate things, it is advantageous for me. I researched the [computer] programs a lot because I found them a good implementation.”
During the discussion about ways to improve the module in the future, some students identified the time constraints associated with the module implementation as a flaw, voicing that they felt the time was insufficient for thorough understanding of the interworking of the RGCCS utilised.
At times, it seemed there was an excessive focus on the concrete ways of implementing procedures with the software and not enough discussion and learning opportunities about the reasons behind the procedures implemented. Students suggested that having more time to work with the RGCCS from the beginning of the module would significantly improve their understanding of the meaning of the computational operations they learned during this experience.
One aspect that emerged as having singular relevance for the students was their exposure to a methodology that combined PBL with the use of RGCCS. Two significant trends were revealed in the focus group discussions. The first trend points to the contribution of the module to the development of students’ autonomy, specifically in connection with research work and computational science. This was the strongest trend observed across all three focus groups. The second trend relates to the pre-service chemistry teachers’ interest in real world problems of science as the main principle for organising a learning process that allowed them to experiment with computational methods in chemistry.
A3: “one is not used to working like that; we are used to the expository classes [where] we have to give the knowledge…”
A9: “as we studied a science programme, most of our activities are very guided, as I said … we always receive instructions of what we have to do. For example, this module was done as a laboratory, and we in the laboratory always have a guide that tells us what to do and practically what we have to conclude from that, so when we are given such a broad methodology, and we do not know what we have to do, that puts us in a situation of crisis (…) we don’t know where to start, so in that regard, it was difficult like they completely changed our work methodology.”
As indicated by A9, the newness of the student-centred approach of the module, in which students play an active and participatory role in their own learning process, was initially disconcerting, and even generated a sort of “crisis” in the students, as they felt disoriented regarding the steps needed for self-regulated problem-solving tasks. It was a broad consensus across the different focus groups, however, that this crisis was mainly linked to the early stages of the module implementation, and that the initial confusion progressively decreased as the module activities progressed.
A5: “later, after you got into the rhythm of the dynamic [of the module], like, to where we should go, well the concepts, there are some that you had to remember, and then when you start to link them with the equation, once you already have the information about the equation, at least to me the equation itself made more sense than if I had seen it presented in a class because if I had seen it presented in a class, I would have no idea of where it came from.”
Following this initial crisis, there was a general consensus that the model's focus on autonomous work was one of its most positive aspects. In fact, development of skills needed for an adequate level of autonomy within the frame of scientific work emerged as one of the most highly valued aspects in students’ evaluation of their own performance and learning during the module. A7 explains the way this autonomous work was carried out within an integrated student group:
A7: “… because we, knowing that learning had to be independent, we keep getting together to do the work without any manual … if we had any questions we went to the teacher, and we said teacher we have these questions, but we continue working and I think that is positive to consider, that the autonomy that was given in the beginning and that maybe at the beginning was confusing.”
The connection that the students made between PBL methodology and the achievement of higher degrees of autonomy are consistent with a previous study which showed that undergraduate students who participated in a PBL laboratory environment improved their autonomy and could take on more responsibility for their own chemistry learning (Mataka and Kowalske, 2015). These results are especially interesting within the framework of the module, in that students perceived that RGCCS and PBL methodology support each other in creating a kind of learning environment that allows for the development of self-regulated research-related skills that are fundamental for their training in the science area as future chemistry teachers. Application of RGCCS in this module facilitated exploration of different software through autonomous work in such a way that allowed the students to test different types of calculations without having the need for direct supervision and without the pressure of having negative consequences for making mistakes.
A4: “… I like that each group worked autonomously because each group could internalise itself in the use of RGCCS and do what one wanted, so even if a certain calculation did not work in practice we learned how to work with the software.”
A4: “I like that because we had had other laboratories where the teacher, for example, presented his work and you had to follow what he is doing, so if you miss a step you cannot go further, here instead you can follow your own work and is what I like the most.”
As expressed by these students, RGCCS enhanced the self-regulating skills implicit in PBL by allowing the students to make decisions regarding the validity and usefulness of their own findings and the overall process of obtaining these findings. Accordingly, the development of the skills needed for autonomy in scientific work emerged as one of the most valued aspects in students’ evaluation of their own performance and learning during the module. Students additionally credited the module in developing the following scientific skills: (a) contextualising and evaluating research questions, problems and hypothesis; (b) finding information; (c) discerning quality of information; (d) inferring and interpreting data, and (e) integrating and synthesising different kinds of information through the research process. Through improvement in these areas, pre-service chemistry teachers suggested that RGCC and PBL worked synergistically to develop their scientific thinking and fostered an atmosphere for teamwork.
(a) Contextualising and evaluating research questions, problems and hypothesis. Regarding evaluations of their learning, students highlighted improvements in their ability to manage the initial phases of research design, especially in regards to the contextualisation of the problems, objectives, and hypothesis. They linked this development with an increased capacity for understanding the coherence of the research process itself.
A13: “I think I also improved a lot in being able to pose the problem myself and see how I am progressing, the objective and I developed more scientific thinking from what I was doing myself.”
A11: “You realise that, eh, you discard, then you pose for example such a problem, then you realise which path serves me or does not serve me, that is, if I realise that the path I took, no matter how long it took me, does not serve me anymore I then take a different path and that [understanding] wasn’t there before they gave us the path, you just followed it, then that is the idea to research, to investigate what would work, what would not work, that was much more developed, to pose hypothesis also.”
(b) Finding information. Students acknowledged that participating in the module helped them identify weaknesses in their own capacity for self-regulated information seeking, explaining that the module fostered the process of discovery and experimentation.
A7: “I believe that as one has to go looking for information, it is very difficult to find information that is understandable about the potential of Lennard-jones, because to talk about intermolecular forces, it is easy because there is a lot of information about it, but in concepts that are difficult, one doesn't find information and the one we found was in English.”
A4: “I think it is to investigate because as I said before I did [not] have that ability very developed that if they do not explain it to me, I do not search further, So I think that aspect may be, because I had to search everything from the more basic, in order to be able to understand the problem from the beginning.”
(c) Discerning quality of information. The students expressed that the combination of the PBL methodology with RGCC meaningfully improved their abilities to determine what kind of information was needed for the problem-solving process. In particular, students pointed to improvements in their ability to discriminate between reliable and unreliable information. Students perceived significant self-improvement in this area:
A3: “The very fact of discriminating between reliable and unreliable information, or useful and not useful for our problems, which is already a superior cognitive ability.”
A1: “Investigating, to discriminate the information, and besides to read from the more basic stuff and at the end we all learned at our own pace, so that also made the learning much more concrete than in an expositive class. I think that was the more favourable aspect of this experience.”
(d) Inferring and interpreting data. The students evaluated the opportunity the module gave them to interpret their own data, highlighting that this competence has not been emphasised in their academic training.
A5: “The inquiry and the analysis are two things that the module strengthens a lot, because even after I had all the results, what comes next? and then we realise for example, the ability to analyse data is something that is not, not exercised much in general.”
(e) Synthetizing and reaching conclusions. Students expressed that having the opportunity to function as autonomous researchers enhanced their abilities to organise information in a way that makes meaningful connections between different sources. They perceived a noticeable increase in ability to synthetize information and derive appropriate conclusions from their own research-like process.
A11: “In scientific skills, I think that I improved them because with the module I had ideas that I would not have had with a traditional class. For example, I realised now concluding and discussing the problem, since there had to be synthesised in the head everything that one had done so that we could write something. I think I did improve, and in that aspect, I think this module is good, very good.”
A19: “I started to find out, and we got really into the role of researching about the module, after that to develop the ability to synthesise when we had to go from the 30 pages report to a poster, and that poster had to have everything and everything had to fit in it, and how to do that, what do I put in it? Furthermore, in the end, it all turned out really good because we develop a great capacity for dialoguing, for synthesising, I learned a lot with the module, it was very useful to me.”
Through the development or strengthening of these skills, students perceived a general improvement in their ability to work as scientists. They highlighted that the activities of the module elucidated the processes and methods of science more than traditional classes, thus improving the connection between theory and practice.
A4: “We are learning science and science is not only learned by listening to a textbook, but you have to be hands-on and this is hands-on in a way, so it is a benefit for us.”
A1: “For example, I see that in general there is a problem with the study of science, as a confusion, many times when doing an experiment, a practical experience is the same as conducting a research, we have a practical class in all our chemistry courses, a laboratory, but in reality, it is a recipe, they tell us ‘you have to do this and you are going to get this result, and if you don’t get that then go and find out where did you fail’. Here instead, ‘there is the problem, you decide how to solve it’ and bottom line, that is real science, to search for something coming from nothing, to search from an objective (…) that is like the current approach of science, not the misnamed experiments that are not really experiments because you already know what the final result is’”
Students linked the improvement in scientific thinking to the development of systematic collaborative work which is understood as an authoritative source for both support and knowledge acquisition:
A4: “It is true that when we were sharing with each other the knowledge that each one had, we had indeed made progress, for example with A2 we are now doing the report, and we have realised that there are things that we handle well.”
Finally, students perceived that integration of a problem-solving methodology with RGCSS, a specific tool for self-regulated experimentation, improved their overall learning as well as their ability to work collaboratively. A8 explains this idea:
A8: “For my part, regarding the content [of the curricula] good, leaning to very good, because I actually learned what I did not know anything about, that is before the module if I had been asked what is intermolecular interaction, I [would have given] a very general definition, so I evaluate it well, and regarding scientific research skills, I found it really favourable because I learned that not all software is good for something, maybe the union of software would work for the common good and can help to solve the, some problem because we didn't necessarily have to do everything with Avogadro or with Excel, and that also benefits the, the, teamwork as the “A7” says, because maybe I, I participated more in modelling molecules, “A12” more in graphics because they handled that better”
A1: “As one is focused on solving the problem one does everything possible, anything that can help to solve that problem, and in doing that one does not even realise that you learned a lot of concepts, in fact once in a laboratory with the teacher we began to talk about the concepts that we had researched, we had not even been given any kind of recipe, and many concepts came out and we had not even realised everything we had researched”
Of course, some structure and guidance are necessary: asking students to solve a problem without any specific instructions or external figure who judges the validity of the information gathered and data produced by the students would provide little benefit to student learning. In this module, finding a satisfactory answer for the problem worked as a guiding principle that provided structure to the process of self-experimentation developed by the students. Within this flexible structure, students were given the freedom to integrate information and abilities in a way that made sense to them.
A3: “it is good because you assimilate everything like very fast, at least, because in order to solve the problem you have to know this, this, that and you don't realise that you know this and this, that you know that, that you handle this, you realise everything you are handling to be able to solve the problem, then I find that in that sense it is very good because like I almost absorb the knowledge.”
Encouraging students to carry out self-regulated research in pursuit of suitable solutions to a real-world problem prompted them to integrate knowledge from different sources and positively affected their motivation on the module. In fact, students felt motivated to work beyond the scope of the direct requirements they were asked to meet, as exemplified in the following conversation between three students in the focus group:
A7: “Sometimes I feel that in studying for a class I am wasting my time, I feel that while studying I have that feeling that that is not what I want to be doing, I want to do anything but studying, and doing this [the module] there were moments where I did wanted to do it; afterwards I felt like hooked, in fact suddenly I realised it was late, and I still wanted to keep working, and that is not often the case for me.”
A13: “Something similar happened to me, I did want to finish it, but at the same time I had, I was learning to analyse data I knew I was learning, it is not the same than reading a book.”
A7: “At least for me personally, it happened that for example when I wanted to establish why that think happened, and that allowed itself for trying a lot of things, so by doing all these tests [using RGCCS] that I wanted to do because I wanted to do them, it wasn’t because [the teachers] were asking me to do them, it was a way to use the time that I felt it was pleasant, it wasn’t like a thing I felt I was obligated to do.”
Moreover, students positively viewed the change in the pedagogical–didactic approach of teaching practice. They particularly valued that the module allowed them to carry out investigative processes rather than reproduce pre-set laboratory procedures. In other words, the module allowed the pre-service chemistry teachers to develop scientific skills neglected when the focus is on merely reproducing protocols.
Educational computational chemistry as a framework for integrating novel computational chemistry into chemistry education (RQ1)
From the design process, it is clear that the knowledge and skills necessary to produce molecular visualisations and graphical representations of data, which can be applied effectively in the training of pre-service chemistry teachers, go further than chemical and computational chemistry knowledge by themselves. Pedagogical aspects such as teaching methodologies and didactic strategies should be integrated into this training. Moreover, in this work, we gave the name Educational Computational Chemistry to the construct that emerges from pedagogical consideration of computational chemistry practices in educational processes. This is based on the TPASK framework that represents the integration between PSK, TPK and TSK (see Fig. 2).The PBL learning environment was suitable to introduce CC in the ECC module, becoming one of the most positively valued aspects by the students. Accurately, they perceived that the CC tools and PBL as learning environment support each other allowing the development of self-regulated research-related skills, which are needed for autonomy in scientific work. This learning environment facilitated the autonomous exploration of different software and types of calculations. Moreover, students in focus groups mentioned they did no feel the pressure of having negative consequences for making mistakes in the exploration of different assays. This perception could be explained due to part of the work with CC tools; they were able to do it without direct supervision. In the PBL learning environment, the real-world problem acted as an organisational principle that prompted the students to integrate knowledge from different sources and positively affected their motivation on the module.
Main projections in the TPASK framework for pre-service chemistry teachers’ future pedagogical practices
The discourse analysis demonstrated that in general students do anticipate applying their learning experience in the module to their future work as teachers in the school system. They viewed that the module was relevant to the current reality of the classroom, allowing students to prepare themselves as teachers for the current demands of chemistry instruction.We observed a high level of consistency between the aspects students valued from their own experience and those they aim to implement in their own pedagogical praxis. In this sense, students valued the joining of PBL and RGCCS for both their experience as students and as a pedagogical strategy they would like to implement as teachers themselves in the future.
It is interesting to note that while students reflected extensively on the development of research-related scientific skills through participation in the module, there was little acknowledgement of the pedagogical dimension. However, when discussing their own future as teachers, the same aspects that were highlighted as contributing to the development of these skills were now pointed out as having the most potential to guide their own students learn science in a meaningful way.
As the module did not focus specifically on science pedagogy, we assume that students were drawing on their previous instruction in pedagogical and didactic courses taken during the course of their training to project their own experience as students in the module to their future pedagogical practice as chemistry teachers.
First, students imagined applying methodologies, such as combining PBL with RGCC to promote autonomy in their own students:
A3: “the children and ourselves realise that in truth the only ones responsible for our education are us and that is what is not, children do not learn because they are forced to school, In school, they want to go, they are practically kindergartens, then … this would help a little to take that conscience because one is responsible for their own education, not the mother, not the teacher, but oneself.”
Within this framework, the pre-service teachers indicated that one of the main take-aways from the module would be to integrate RGCCS into their future curricula to foster a deeper and more grounded understanding of abstract chemical concepts and processes among their students:
A4: “Well the Software also, because if you could see it like, to show maybe the molecules in a more didactical way. [in the future] when we are in the classroom and want to show them [the molecules] to our students, I think that for oneself as a student it is easier to see something like concrete rather than as something that it's being drawn for you in the whiteboard or something that is very abstract, so I think that it is also useful for that. The Avogadro and Discovery Studio would be very useful for us, as well as other software we have worked with”
Second, the idea of using real world problems that can be solved using computational science was very appealing to pre-service chemistry teachers when thinking of their own future classrooms. They highlighted that a contextualised real-world problem would provide direction and meaning to the autonomous research-like process, especially when aided by specialised software that allows for experimentation.
A7: “The kind of work that is usually given in the assignments for example, that is working in base to like almost canned content, that you just pass over and it does not have any context behind it, for example talking about thermodynamics in school is very difficult because you don’t have any context in which you can tell [to the students] hey this happens in real life, so if you propose instead that they see the content and the abilities form the standpoint of a problem that they can feel as being close or that they can work on themselves, I believe that is very positive.”
A1: “I think it is a problem of education in general, since we all study under pressure because the test is coming and after that a grade, of course (…) then oh, I have to study, and I do not know what, And in the end, it never generates a learning by interest, it is always because the test is coming up, then that is also a problem in general and that is why one also does not handle time due to lack of interest … Yes, I believe that yes, because [with the module] in the background each one builds their own way to get to solve the problem, and its note like you have to follow a prescription.”
Additionally, students declared that they hope to use the resources derived from RGCCS to develop a more autonomous teaching practice, especially in the construction of didactic materials for the classroom that facilitate a more interactive teaching method:
A5: “… I think it serves you as a teacher to do didactic material, since the PowerPoint will go with graphics that you did and did not take them from the Internet, for example, to teach “X” matter, then just know and have notions about CC the same serves you a little to go according to the new generation of teaching material, maybe a little more interactive in the classroom…”
One issue that some students identified as a challenge to implementing this kind of experience in their own classrooms is the difficulty of evaluating effort and understanding through traditional means such as tests or oral presentations. Students expressed that a single grade would not properly take into account all the work and the learning process of students in this kind of environment.
A5: “There is a big part of your work you feel is not being evaluated because the evaluation is too small for the module, sort of speaking, I mean at the end that is about how the group participated in each class, what kind of progress they show in each laboratory, that kind of things don’t fit into the report, they don’t go there, so at the end, I believe that is still important to improve the kind of evaluation that its applied for this kind of methodology. I know that it is complex, in fact, I, for example, if I think about how to apply it [the module] to my future, of course, I would like to apply it, but how do I apply it if at the end I still have to transform it into a grade.”
In summary, this analysis demonstrated that pre-service chemistry teachers value the integration of PBL and RGCCS for both for their own learning of science and as a pedagogical tool for their future careers as teachers. For the students that took part in this experience, the combination of a student-centred methodology with emerging technologies that allow for self-regulated experimentation constitutes a powerful tool for promoting the learning of science in their future classrooms.
Finally, at the centre of the ECC model, in the TPAChK framework, is a chemistry teacher's (pre-service or in-service) progressively develops different ways of thinking about how CC (TSK) can transform their future pedagogical practices.
Conclusions
This research describes the design of an ECC module in the TPASK framework. The ECC module engages pre-service chemistry teachers with a real-world problem, approached through computational chemistry. Within the ECC module, TSK refers to the knowledge of computational chemistry such as emerging technologies that integrate the knowledge of chemistry, computer science, and applied math. In this case, the 3DMV representations fully represent the bidirectional relationship of chemistry and technology, i.e. knowledge of how to represent the chemistry concepts and the technical application. Importantly, knowledge regarding computational chemistry and computational science exists independent of knowledge about their use in a pedagogical context. As this kind of knowledge becomes mainstream, that knowledge transforms into chemistry knowledge or science knowledge.The ECC module described herein provided pre-service chemistry teachers with a learning environment that employs advanced computational chemistry tools to identify a potential drug for Chagas disease. The subject-specific contents, such as intermolecular forces, are implicit in this real-world science problem used. The computational chemistry tools used in the ECC module design were selected based on their practical application in solving real-world problems. Incorporation of these technological tools into a PBL-based module for use in an undergraduate physical chemistry course yielded the following outcomes (i) students' perceived the use of computational chemistry tools as effective aids in the learning of chemistry concepts and development of scientific skills; (ii) students’ perceived that RGCCS and PBL methodology support each other in facilitating the development of self-regulated research-related skills; and (iii) the use of RGCCS in the ECC learning environments facilitated the exploration of different types of software and calculations through autonomous work.
Pre-service chemistry teachers’ reflections on their experience in the ECC module allowed them to project their acquired TSK in a potential pedagogical context. Through participating in the module, students first acquired TSK and then developed some elements of their TPASK. Concerning their perceptions about this process, pre-service chemistry teachers valued the combination of student-centred methodology with emerging technology resources for both their own learning process as students as well as their future roles as teachers. We propose that this type of technological integration could transform the educational chemistry landscape.
Future research involving the constitutive elements of TPASK and their development will have a significant impact on how pre-service and in-service chemistry teachers are trained to use CC and CSc tools in a pedagogically sound manner in the classroom. Moreover, these technology transformed learning environments need more research in Computer-Supported Collaborative Learning due to the inherently collaborative and interdisciplinary nature of CC and CSc.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This research was supported by the FGI 46-18 project (DIUMCE, Universidad Metropolitana de Ciencias de la Educación, Chile). The authors thank PIPedI20164 (Doctorate in Education Program – UMCE, Chile) and Programa Extraordinario de Becas de Postgrado – Doctorado en Educación – UMCE. We thank Alyssa Grube for assistance in language support.References
- Abraham M., Varghese V. and Tang H., (2010), Using Molecular Representations To Aid Student Understanding of Stereochemical Concepts, J. Chem. Educ., 87(12), 1425–1429.
- Agapova O. I., Jones L. L., Ushakov A. S., Ratcliffe A. E. and Varanka M. A., (2002), Encouraging independent chemistry learning through multimedia design experiences, Chem. Educ. Int., 3, AN–8, available at: http://old.iupac.org/publications/cei/vol3/0301x0300an0308.html.
- Akaygun S. and Jones L. L., (2013), Research-based design and development of a simulation of liquid-vapor equilibrium, Chem. Educ. Res. Pract., 14(3), 324–344.
- Akcay B., (2009), Problem-Based Learning in Science Education, J. Turk. Sci. Educ., 6(1), 28–39.
- Aksela M. and Lundell J., (2008), Computer-based molecular modelling: Finnish school teachers’ experiences and views, Chem. Educ. Res. Pract., 9(4), 301–308.
- Al-Balushi S. M. and Al-Hajri S. H., (2014), Associating animations with concrete models to enhance students' comprehension of different visual representations in organic chemistry, Chem. Educ. Res. Pract., 15(1), 47–58.
- Angeli C. and Valanides N., (2009), Epistemological and methodological issues for the conceptualization, development, and assessment of ICT-TPCK: advances in technological pedagogical content knowledge (TPCK), Comput. Educ., 52(1), 154–168.
- Avramiotis S. and Tsaparlis G., (2013), Using computer simulations in chemistry problem solving, Chem. Educ. Res. Pract., 14(3), 297–311.
- Baran M. and Sozbilir M., (2018), An Application of Context- and Problem-Based Learning (C-PBL) into Teaching Thermodynamics, Res. Sci. Educ., 48(4), 663–689.
- Barrows H. S., (1986), A taxonomy of problem-based learning methods, Med. Educ., 20(6), 481–486.
- Battle G. M., Allen F. H. and Ferrence G. M., (2011), Teaching Three-Dimensional Structural Chemistry Using Crystal Structure Databases. 3. The Cambridge Structural Database System: Information Content and Access Software in Educational Applications, J. Chem. Educ., 88(7), 886–890.
- Blonder R. and Mamlok-Naaman R., (2019), Teaching chemistry through contemporary research versus using a historical approach, Chem. Teach. Int., DOI:10.1515/cti-2018-0011.
- Bunce D. M., (2011), Investigating Classroom Myths through Research on Teaching and Learning, American Chemical Society, vol. 1074, ch. 1, pp. 1–4.
- Bunce D. M. and VandenPlas J. R., (2011), Investigating Classroom Myths through Research on Teaching and Learning, American Chemical Society, vol. 1074, ch. 2, pp. 5–24.
- Burkholder P. R., Purser G. H. and Cole R. S., (2008), Using Molecular Dynamics Simulation To Reinforce Student Understanding of Intermolecular Forces, J. Chem. Educ., 85(8), 1071.
- Chai C. S., Koh J. H. L. and Tsai C.-C., (2013), A Review of Technological Pedagogical Content Knowledge, Educ. Technol. Soc., 16(2), 31–51.
- Cody J. A. and Wiser D. C., (2003), Laboratory Sequence in Computational Methods for Introductory Chemistry, J. Chem. Educ., 80(7), 793.
- Cohen L., Manion L. and Morrison K., (2007), Research methods in education, 6th edn, New York, NY, US, Routledge/Taylor & Francis Group.
- Cooper M. M., Underwood S. M. and Hilley C. Z., (2012), Development and validation of the implicit information from Lewis structures instrument (IILSI): do students connect structures with properties?, Chem. Educ. Res. Pract., 13(3), 195–200.
- Cooper M. M., Williams L. C. and Underwood S. M., (2015), Student Understanding of Intermolecular Forces: A Multimodal Study, J. Chem. Educ., 92(8), 1288–1298.
- Cowden C. D. and Santiago M. F., (2016), Interdisciplinary Explorations: Promoting Critical Thinking via Problem-Based Learning in an Advanced Biochemistry Class, J. Chem. Educ., 93(3), 464–469.
- Cox S. M., (2008), A Conceptual Analysis of Technological Pedagogical Content Knowledge, pHD, Brigham Young University.
- Cox S. and Graham C. R., (2009), Diagramming TPACK in practice: using an elaborated model of the tpack framework to analyze and depict teacher knowledge, TechTrends, 53(5), 60–69.
- Dekock R. L., Madura J. D., Rioux F. and Casanova J., (2007), Reviews in Computational Chemistry, John Wiley & Sons, Inc., pp. 149–228.
- Evans M. J. and Moore J. S., (2011), A Collaborative, Wiki-Based Organic Chemistry Project Incorporating Free Chemistry Software on the Web, J. Chem. Educ., 88(6), 764–768.
- Feller S. E., Dallinger R. F. and McKinney P. C., (2004), A Program of Computational Chemistry Exercises for the First-Semester General Chemistry Course, J. Chem. Educ., 81(2), 283.
- Geldenhuys W. J., Hayes M., Van der Schyf C. J., Allen D. D. and Malan S. F., (2007), Receptor Surface Models in the Classroom: Introducing Molecular Modeling to Students in a 3-D World, J. Chem. Educ., 84(6), 979.
- Gunter T. and Alpat S. K., (2017), The effects of problem-based learning (PBL) on the academic achievement of students studying ‘Electrochemistry’, Chem. Educ. Res. Pract., 18(1), 78–98.
- Günter T., Akkuzu N. and Alpat Ş., (2017), Understanding ‘green chemistry’ and ‘sustainability’: an example of problem-based learning (PBL), Res. Sci. Technol. Educ., 35(4), 500–520.
- Hsieh H.-F. and Shannon S. E., (2005), Three Approaches to Qualitative Content Analysis, Qual. Health Res., 15(9), 1277–1288.
- Jimoyiannis A., (2010), Designing and implementing an integrated technological pedagogical science knowledge framework for science teachers professional development, Comput. Educ., 55(3), 1259–1269.
- Jones M. B., (2001), Molecular Modeling in the Undergraduate Chemistry Curriculum, J. Chem. Educ., 78(7), 867.
- Jones L. L., (2013), How Multimedia-Based Learning and Molecular Visualization Change the Landscape of Chemical Education Research, J. Chem. Educ., 90(12), 1571–1576.
- Jones L. L., Jordan K. D. and Stillings N. A., (2005), Molecular visualization in chemistry education: the role of multidisciplinary collaboration, Chem. Educ. Res. Pract., 6(3), 136–149.
- José T. J. and Williamson V. M., (2008), The Effects of a Two-Year Molecular Visualization Experience on Teachers' Attitudes, Content Knowledge, and Spatial Ability, J. Chem. Educ., 85(5), 718.
- Kang Y. and Kang F.-A., (2011), A Simple Computer-Aided Three-Dimensional Molecular Modeling for the Octant Rule, J. Chem. Educ., 88(4), 420–420.
- Kelly R. M., (2014), Using Variation Theory with Metacognitive Monitoring To Develop Insights into How Students Learn from Molecular Visualizations, J. Chem. Educ., 91(8), 1152–1161.
- Kim H., Sulaimon S., Menezes S., Son A. and Menezes W. J. C., (2011), A Comparative Study of Successful Central Nervous System Drugs Using Molecular Modeling, J. Chem. Educ., 88(10), 1389–1393.
- Krause M., Kienast S., Witteck T. and Eilks I., (2013), On the development and assessment of a computer-based learning and assessment environment for the transition from lower to upper secondary chemistry education, Chem. Educ. Res. Pract., 14(3), 345–353.
- Levy D., (2013), How Dynamic Visualization Technology can Support Molecular Reasoning, J. Sci. Educ. Technol., 22(5), 702–717.
- Linenberger K. J., Cole R. S. and Sarkar S., (2011), Looking Beyond Lewis Structures: A General Chemistry Molecular Modeling Experiment Focusing on Physical Properties and Geometry, J. Chem. Educ., 88(7), 962–965.
- Lukas S., Müller W., Huwer J., Drüke-Noe C., Koppel I., Rebholz S., Stratmann J., Theilmann F. and Weitzel H., (2019), Improving students' TPACK through learning labs: the implementation of ichemlab and steam makerspace.
- Mahaffy P., (2004), The Future Shape of Chemistry Education, Chem. Educ. Res. Pract., 5(3), 229–245.
- Mataka L. M. and Kowalske M. G., (2015), The influence of PBL on students' self-efficacy beliefs in chemistry, Chem. Educ. Res. Pract., 16(4), 929–938.
- Metz C. R. and Sendlinger S. C., (2009), CSERD—Another Important NSDL Pathway for Computational Chemistry Education, J. Chem. Educ., 86(1), 126.
- Milner-Bolotin M., (2012), Increasing Interactivity and Authenticity of Chemistry Instruction through Data Acquisition Systems and Other Technologies, J. Chem. Educ., 89(4), 477–481.
- Mishra P. and Koehler M. J., (2006), Technological pedagogical content knowledge: a framework for teacher knowledge, Teach. Coll. Rec., 108(6), 1017–1054.
- Nature.com, (2017), Computational Chemistry, http://www.nature.com/subjects/computational-chemistry, (accessed Oct. 31, 2017).
- North Carolina Science M. a. T. E. C., (n.d.), The North Carolina High School Computational Chemistry Server, http://chemistry.ncssm.edu/ accessed Apr 2017.
- Ochterski J. W., (2014), Using Computational Chemistry Activities To Promote Learning and Retention in a Secondary School General Chemistry Setting, J. Chem. Educ., 91(6), 817–822.
- Olvera B. C. and Bedolla A., (2009), La química computacional en el salón de clase, Educ. Quím., 20(2), 182–186.
- Paselk R. A. and Zoellner R. W., (2002), Molecular Modeling and Computational Chemistry at Humboldt State University, J. Chem. Educ., 79(10), 1192.
- Pfennig B. W. and Frock R. L., (1999), The Use of Molecular Modeling and VSEPR Theory in the Undergraduate Curriculum to Predict the Three-Dimensional Structure of Molecules, J. Chem. Educ., 76(7), 1018.
- PhET Interactive Simulations, (2017a), Atomic interactions, https://phet.colorado.edu/en/simulation/legacy/atomic-interactions, (accessed Oct. 31, 2017).
- PhET Interactive Simulations, (2017b), Molecule polarity, https://phet.colorado.edu/en/simulation/legacy/molecule-polarity, (accessed Oct. 31, 2017).
- Prince M., (2004), Does active learning work? A review of the research, J. Eng. Educ., 93(3), 223–231.
- Ramos M. J. and Fernandes P. A., (2005), Computer Modeling and Research in the Classroom, J. Chem. Educ., 82(7), 1021.
- Royal Society of Chemistry, (2017), Chemistry Now – Computational Chemistry, https://edu.rsc.org/resources/chemistry-now-computational-chemistry/55.article, (accessed Oct. 31, 2017).
- Ruddick K. R., Parrill A. L. and Petersen R. L., (2012), Introductory Molecular Orbital Theory: An Honors General Chemistry Computational Lab As Implemented Using Three-Dimensional Modeling Software, J. Chem. Educ., 89(11), 1358–1363.
- Schurmeier K. D., Shepler C. G., Lautenschlager G. J. and Atwood C. H., (2011), in Bunce D. M. (ed.), Investigating Classroom Myths through Reseach on Teaching and Learning, vol. 1074, pp. 137–176.
- Sendlinger S. C. and Metz C. R., (2010), Computational Chemistry for Chemistry Educators, J. Comput. Sci. Educ., 1(1), 28–32.
- Sendlinger S. C., DeCoste D. J., Dunning T. H., Dummitt D. A., Jakobsson E., Mattson D. R. and Wiziecki E. N., (2008), Transforming chemistry education through computational science, Comput. Sci. Eng., 10(5), 34–39.
- Shulman L. S., (1986), Those Who Understand: Knowledge Growth in Teaching, Educ. Res., 15(2), 4–14.
- Shulman L., (1987), Knowledge and Teaching: Foundations of the New Reform, Harvard Educ. Rev., 57(1), 1–23.
- Springer M. T., (2014), Improving Students’ Understanding of Molecular Structure through Broad-Based Use of Computer Models in the Undergraduate Organic Chemistry Lecture, J. Chem. Educ., 91(8), 1162–1168.
- Strollo C. and Davis K. L., (2017), in Kloepper K. D. and Crawford G. L. (ed.), Liberal Arts Strategies for the Chemistry Classroom, Washington: Amer Chemical Soc, vol. 1266, pp. 133–151.
- Tasker R. and Dalton R., (2006), Research into practice: visualisation of the molecular world using animations, Chem. Educ. Res. Pract., 7(2), 141–159.
- Tofan D. C., (2009), Improving Chemistry Education by Offering Salient Technology Training to Preservice Teachers. A Graduate-Level Course on Using Software To Teach Chemistry, J. Chem. Educ., 86(9), 1060.
- Toplis R., (2008), Probing student teachers' subject content knowledge in chemistry: case studies using dynamic computer models, Chem. Educ. Res. Pract., 9(1), 11–17.
- Tsai C. S., (2007), Using Computer Applications and Online Resources To Teach and Learn Pharmaceutical Chemistry, J. Chem. Educ., 84(12), 2019.
- Tuvi-Arad I. and Blonder R., (2010), Continuous symmetry and chemistry teachers: learning advanced chemistry content through novel visualization tools, Chem. Educ. Res. Pract., 11(1), 48–58.
- Venkataraman B., (2009), Visualization and interactivity in the teaching of chemistry to science and non-science students, Chem. Educ. Res. Pract., 10(1), 62–69.
- Voogt J., Fisser P., Pareja Roblin N., Tondeur J. and van Braak J., (2013), Technological pedagogical content knowledge – a review of the literature, J. Comput. Assist. Learn., 29(2), 109–121.
- Waddington D. J., Molecular Visualization in Science Education, National Science Foundation, 2001.
- Wedler H. B., Cohen S. R., Davis R. L., Harrison J. G., Siebert M. R., Willenbring D., Hamann C. S., Shaw J. T. and Tantillo D. J., (2012), Applied Computational Chemistry for the Blind and Visually Impaired, J. Chem. Educ., 89(11), 1400–1404.
- Xie Q. and Tinker R., (2006), Molecular Dynamics Simulations of Chemical Reactions for Use in Education, J. Chem. Educ., 83(1), 77.
- Yasar O. and Landau R. H., (2003), Elements of computational science and engineering education, SIAM Rev., 45(4), 787–805.
- Yasar O., Rajasethupathy K. S., Tuzun R. E., McCoy R. A. and Harkin J., (2000), A new perspective on computational science education, Comput. Sci. Eng., 2(5), 74–79.
- Ziegler B. E., (2013), Theoretical Hammett Plot for the Gas-Phase Ionization of Benzoic Acid versus Phenol: A Computational Chemistry Lab Exercise, J. Chem. Educ., 90(5), 665–668.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9rp00273a |
| This journal is © The Royal Society of Chemistry 2020 |