Essential learning outcomes for delocalization (resonance) concepts: How are they taught, practiced, and assessed in organic chemistry?
Myriam S.
Carle
 and
Alison B.
Flynn
and
Alison B.
Flynn
 *
*
Department of Chemistry & Biomolecular Sciences, University of Ottawa, Ottawa, Ontario, Canada. E-mail: Alison.flynn@uOttawa.ca
First published on 11th February 2020
Abstract
The concept of delocalization (i.e., resonance) is fundamental concept in organic chemistry but essential learning outcomes (LOs) have not previously been proposed nor has there been an analysis of how resonance is taught, despite indications in the literature that students have many non-canonical ideas about the concepts. To address this deficit, we first developed a set of ten learning outcomes believed to be essential to the concept of delocalization in organic chemistry, especially for students’ later success. Next, we analyzed how these learning outcomes are being taught, practiced and assessed in common textbooks and in a sample of exams. Five themes emerged from the analysis: (1) several of the essential intended LOs we identified are not represented in the textbooks’ teaching explanations, practice questions, or professors’ assessments; (2) the concepts related to delocalization are often taught, practiced, and assessed without associated justifications; (3) there is a large gap between when delocalization is taught and when it is used in context; (4) the link between delocalization and other concepts (e.g., reactivity) is not explicitly explained in most teaching materials; and (5) the language used around delocalization may be misleading (e.g., resonance, stability). Our analysis identified areas in which delocalization education could be improved, including with respect to teaching, practice opportunities, and assessing the concepts.
Introduction
Problem and research goals
Resonance is a critical concept that explains molecular properties and reactivity in many contexts, including materials chemistry, pharmaceutical science, solar cells, and biochemistry. Conjugated polymers have unique properties due to the delocalization of electrons and these polymers can harness energy from light and be used in solar cells (Günes et al., 2007; Jiang and McNeill, 2017). In the pharmaceutical industry, every top selling drug in 2016 contained a moiety with electron delocalization, except salts (Smith et al., 2016). The underlying concepts of resonance also help explain the reactivity of functional groups. The reactivity in many reactions can be explained using electron delocalization concepts, including electrophilic aromatic substitution, Diels–Alder, and conjugate addition reactions. In other words, resonance is a foundational aspect of organic chemistry.Educators have reported that resonance is a fundamental organic chemistry concept and one of the hardest concepts for students to understand (Duis, 2011) and ways of teaching resonance have been proposed (Gero, 1954; Delvigne, 1989; Starkey, 1995; Silverstein, 1999; Lin, 2007). However, these proposed ways of teaching delocalization did not outline what students should learn (i.e., the expected learning outcomes), what the students struggle to conceptualized, or investigated the impacts for student learning. More chemistry education research into domain-specific concepts is required, such as resonance, aromaticity and hyperconjugation (Graulich, 2015).
In the present study, we analyzed the teaching and learning of delocalization concepts by proposing essential learning outcomes and analysing how concepts and skills addressed in those learning outcomes are commonly being taught, practiced, and assessed.
The few existing studies show students’ challenges conceptualizing resonance
One previous investigation of students’ understanding of resonance concepts found that students struggled to conceptualize resonance (Taber, 2002). That study focused on how participants conceptualized molecular orbitals and electron delocalization and found that students often thought of benzene's resonance structures as alternating between two states (Fig. 1, left). The idea of “alternating” structures can be due to the everyday meaning of the word resonance, which means oscillating or alternating. In chemistry, resonance does not involve molecules alternating or oscillating between forms; their structure is a combination or hybrid of resonance contributors. To represent that hybrid, a common representation of aromatics involves a circle in the middle that represents the delocalized electrons. Taber found that the second representation type was also problematic, seen by many students as an electron reservoir inside the ring (Fig. 1, right).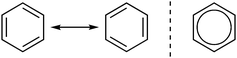 | ||
| Fig. 1 Benzene can be represented as two resonance structure (left) or with a circle to represent aromaticity (right). | ||
Betancourt-Pérez et al. (2010) analyzed students’ answers on four resonance related tasks: (1) draw the curved arrows for electron movement between resonance structures, (2) draw the alternating Lewis structures, (3) identify the most stable Lewis structure, and (4) draw the resonance hybrid. The authors identified common mistakes for each of the tasks and found a significant correlation between tasks 1 to 3 with the students’ exam scores in their first two semesters of Organic Chemistry. The authors also used a multiple-regression analysis to determine that proficiency in task 1 was the best predictor for student exam scores and that mastery of tasks 1, 2 and 3 were a good predictor for course grades.
Rather than understanding that resonance contributors represent a unified structure, student participants from a general chemistry course viewed resonance structures as dimensionalized (viewing a molecule from a different perspective) or segmented (change had occurred between the structures) (Kim et al., 2019). However, after an intervention in which students drew their own representations of resonance and determined the representations’ pros and cons, there was a significant shift in students’ view of resonance structures toward a unitization view (average of represented structures).
To more precisely communicate the idea that electrons are delocalized and not alternating between structures, we use the term “delocalization” herein except when talking about resonance structures or the resonance hybrid. While the term “mesoisomeric structures” could be used, as opposed to “resonance structures”, we found that the term was so uncommon (e.g., none of the textbooks analyzed use/teach the word) that we elected to use the more common term “resonance structures”; other contexts (e.g., in other countries) may use “mesoisomeric” more frequently, however. Similarly, “mesoisomeric hybrid” is also so uncommon that we use the term “resonance hybrid”. An expanded discussion of this idea and other instances of the use of “delocalization” instead of “resonance” are described in Appendix 1.
Theoretical framework
Learning outcomes
Learning outcomes (LOs) are the knowledge, skills, and values that students demonstrate following a learning experience such as a module, section, course, program, or degree (Collis and Biggs, 1986; Brabrand and Dahl, 2009). Clearly identified learning outcomes help focus the instructor's and learners’ attention to specific outcomes (i.e., what is demonstrably learned) instead of only the inputs (i.e., what is taught). Clearly communicated learning outcomes also allow students to track their own learning progress.Intended learning outcomes (ILOs) are the knowledge, skills and values students are expected to be able demonstrate at the end of the course (i.e., the intentions or inputs). Focused ILOs guide instruction to bring teaching strategies, learning activities (practice), and assessments into alignment (Fig. 2). The learning environment and the learners’ characteristics are also essential to consider (see the Information Processing Theory section). In this study, we use the terms LO to indicates the outcomes that students need in the future (e.g. future courses) and ILOs to indicate efforts or objectives used to help students achieved those LOs.
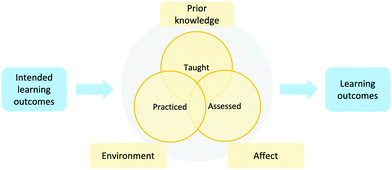 | ||
| Fig. 2 Intended learning outcomes can inform teaching strategies, learning activities, and assessment to achieve the desired learning outcomes. | ||
The taught components convey the concepts in the ILOs to the learners (e.g., videos, class notes, textbooks and supplemental resources). Pedagogical choices are also a key component of the taught piece. The practiced part of the course involves the learning opportunities available to students, including class activities and problem sets. These activities can be used by educators as formative assessments to enhance the learning activities and identify areas in which students need additional or different supports (Dixson et al., 2016) or by the students to track their own knowledge and gain experience solving problems about the concepts. The assessed components involve summative assessments (e.g., midterm and final exams) that are used to make a decision such as giving a course grade, admission into a program, or determine someone's abilities in a subject (Dixson et al., 2016). An assessment can be both summative and formative; for example, midterm exams are used to give students grades and help the educator and students track the students’ progress and guide further teaching and learning activities. For the purpose of this study, assessment refers to summative assessments, such as midterm and final exams.
To date, the only resonance-related learning outcomes that have been proposed involve drawing the set of resonance structures and identifying the major contributors to the resonance hybrid (Betancourt-Pérez et al., 2010); connections have not been made with other concepts such as molecular orbitals, relative stability/reactivity, or more advanced reactions.
Information processing theory – long-term memory
This research is guided by the information processing theory (IPT) (Ausubel et al., 1978; Sweller, 1999; Kalyuga et al., 2003; Mayer and Moreno, 2003; Schunk, 2016). The theory states that an individual has two types of memory: working memory and long-term memory (LTM). Long-term memory is where an individual stores their knowledge that can be retrieved at a later time. Information is stored as a network of concepts that are interconnected; recalling one concept can also activate the connected concepts. The information can be stored as declarative knowledge, which are facts and data obtained from events and experience, or procedural knowledge, which are the skills and procedures to perform a task (Cohen et al., 1997; Gupta and Cohen, 2002). Both declarative and procedural knowledge are stored in LTM and are activated when prompted.There are three factors that influence the storage of information in LTM: meaningfulness, elaboration, and organization. Meaningfulness means that the new knowledge must be presented in a meaningful way to the learner for the information to be integrated in LTM (Novak, 1993). Elaboration is the process adding the new pieces of information to knowledge already stored in LTM. Elaboration aids retrieval of information by providing alternate connections and adding more possible options for activation of pathways (Anderson, 1996), as well as adding more information to construct answers. Organization refers to how the information is integrated within the LTM network; a more organized LTM will facilitate the storage of information. Therefore, for a new piece of information to be stored in the LTM, it should be meaningful for the learner, elaborate on prior knowledge, and be integrated in an organized network.
Storage problems will occur when a learner has no prior knowledge to elaborate the information from, rendering it meaningless. Conceptually meaningless information can be stored in the LTM; however, it may not be part of a network and may not be retrieved. Another problem with storage is that a learner may not elaborate properly and fail to create the connection to other concepts. This is why links between concepts should be explicitly mentioned when teaching, even when seeming obvious to an instructor.
Therefore, the optimal way to introduce information or concepts for learners to integrate it in LTM is to present it meaningfully and explicitly link the new information to prior knowledge. Instruction should follow a progression in which concepts learned build upon each other.
In this study, we examined delocalization concepts through a lens of IPT, specifically what knowledge and skills are needed (LOs) for later retrieval and use for building more advanced knowledge. We investigated whether the subject of delocalization is presented in a way that is conducive for students to learn by investigating what and how the concepts are presented, how concepts are described, and what links are made between concepts.
Goals and research questions
The goals of this research were to (1) identify essential LOs related to delocalization that are needed to understand more advanced concepts in organic chemistry and related disciplines and (2) analyze how those intended learning outcomes are taught, practiced, and assessed. Having defined LOs is intended to help educators design better teaching strategies, practices, and assessments. The research questions for this study are:RQ1: What are the essential learning outcomes related to electron delocalization, by the end of a two-semester sequence in organic chemistry?
RQ2: How are the proposed delocalization ILOs being taught, practiced and assessed?
Methods
Data sources: textbooks and exams
One of the most commonly used teaching resources is textbooks; therefore, we used textbooks to gain an understanding of how delocalization concepts are being taught and practiced in organic chemistry courses. We analyzed seven introductory to Organic Chemistry, as they give a representation of what students are taught and intended to learn, providing insight into potential essential learning outcomes (Solomons and Fryhle, 2000, 2011; Wade, 2010; Klein, 2012; Smith, 2014; Ogilvie et al., 2018; Vollhardt and Schore, 2018). Six of the textbooks organize the information in the traditional functional group approach, while one textbook (Ogilvie et al., 2018) uses a mechanistic approach.We also analyzed summative assessments from two main sources: (i) 51 different organic chemistry midterm and final exams at research-intensive universities in Canada, provided by the professors of those courses and (ii) information provided to us about the American Chemical Society's Organic Chemistry Practice exam, given by the ACS Exams Institute. The Practice Exam is designed to cover all topic areas equally; thus, the distribution of items on the Practice Exam does not necessarily reflect the distribution of items on a released exam.
Data analysis
Validation of the LOs
Seven experts were consulted, including two post-doctoral researchers and five professors with doctorates in organic chemistry. They were sent a survey in which they were asked to rank each of the 33 ILOs for Organic Chemistry II as essential, useful but not essential, not essential, or to be left for later courses. They were also given the opportunity to add any ILOs they felt were missing. For each ILO, a content validity ratio was calculated to determine the importance of that ILO (Zamanzadeh et al., 2015). A content validity ratio over 0.8 indicates that the content of the item was considered essential by the majority of the experts. The survey also asked whether they used the terms resonance, delocalization, or both when teaching the concept to students and why. From these results and our coding of the textbooks, ten ILOs were determined as essential for the students to achieve.Taught. With all 33 ILOs in hand, we revisited the textbooks to determine how those ILOs were being taught (Solomons and Fryhle, 2000, 2011; Wade, 2010; Klein, 2012; Smith, 2014; Ogilvie et al., 2018; Vollhardt and Schore, 2018). One, or multiple, of the 33 ILOs was assigned to each section that taught a delocalization concept (Fig. 3). We also coded any inconsistencies or non-specific language using a deductive coding approach (Creswell, 2012). When a paragraph of the textbook addressed delocalization, we coded how the subject was being taught and the language used. From this analysis we also identified common themes in how delocalization was being explained in the textbooks. An example of the coding can be found in Appendix 3.
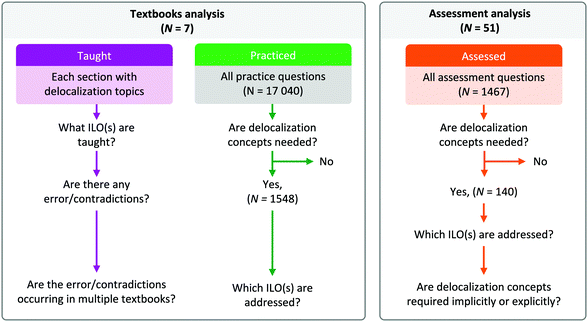 | ||
| Fig. 3 Method to analyze the taught, practiced, and assessed aspects of delocalization concepts in organic chemistry. | ||
Practiced. We coded 17
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 end-of-chapter questions from the seven textbooks. Each question was coded as either requiring or not requiring delocalization concepts to answer. Questions that did not fully address any ILOs were coded accordingly. For the questions involving delocalization concepts, we identified the associated ILOs.
040 end-of-chapter questions from the seven textbooks. Each question was coded as either requiring or not requiring delocalization concepts to answer. Questions that did not fully address any ILOs were coded accordingly. For the questions involving delocalization concepts, we identified the associated ILOs.
Assessed. Summative assessments (N = 51) were provided by seven professors, representing 25 different courses from 2011 to 2018, from research-intensive Canadian universities. All professors consented to have their assessments used in this research. The assessments included final examination and mid-term assessments and were analyzed for the presence of delocalization concepts. The majority (N = 47) only contained open ended questions, two had a mix of open-ended questions and multiple choices, and two assessments contained multiple-choice questions only.
Within the assessments, we coded each item (i.e., question) as requiring delocalization concepts or not; if the item did require delocalization concepts, we identified which ILOs were being addressed. We also analyzed the items requiring delocalization concepts to determine whether the use of delocalization knowledge was implicitly or explicitly required. To be coded as explicitly requiring the use of delocalization knowledge, the question needed to state that resonance/delocalization or a related concept had to be part of the answer. For example, “Draw the mechanism of an electrophilic aromatic substitution, including all resonance structures” would be considered explicit, while “Which proton is more acidic? (in which one of the conjugate bases can have delocalized electrons) would be considered implicit.
Reliability of the coding
To establish reliability of the coding, 15% of the textbook questions were randomly selected to be coded by a second rater. Four chapters from each textbook were also randomly selected for the second rater to code, which amounted to 909 of the textbook questions. The raters obtained a 91% agreement and a Krippendorff α of 0.7 before discussions, which is within the acceptable limit for reliability of coding (Krippendorff, 2004). The two coders also met and discussed the questions they had coded differently until agreement was reached.Results and discussion
RQ1: Proposed essential learning outcomes for delocalization
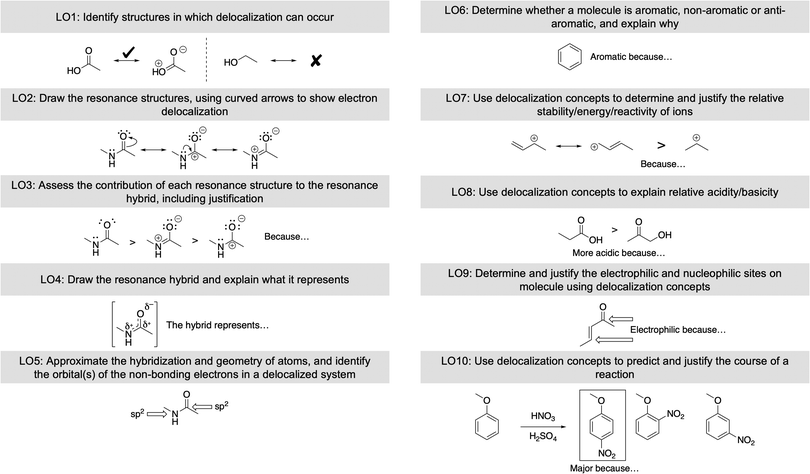
The ten LOs that arose from the analysis described in the methods section represent a combination of the authors’ perspectives, analysis of more advanced contexts (e.g., advanced organic chemistry, biochemistry), textbook analysis, and expert validation (Fig. 4). The essential LOs are therefore meant to be representative of the skills and knowledge a student needs to have upon completing two semesters of organic chemistry. The LOs are outlined in such a way that they build upon each other.The first LO identified is to Identify structures in which delocalization can occur (“Identify”), which is a fundamental skill that is nested within many of the other learning outcomes. The ability for students to quickly recognize whether delocalization is present within a molecule is necessary in many areas such as acid–base, stability, and properties of molecules. This ability also proves useful beyond organic chemistry, in disciplines such as biochemistry and physical chemistry.
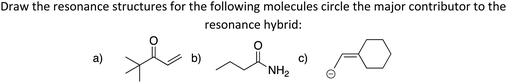
The second learning outcome is to Draw the resonance structures, using curved arrows to show electron delocalization (“Draw”); this LO requires skills with the electron-pushing formalism (EPF) and drawing Lewis structures. Previous research about resonance has shown a significant correlation between this LO and the students’ overall success in organic chemistry courses (Betancourt-Pérez et al., 2010).
The third LO is to Assess the contribution of each resonance structure to the resonance hybrid, including justification (“Contribution”). The goal is for students to rank the relative contribution of each resonance structure toward the resonance hybrid, leading eventually to an estimate of the reactivity of various sites.
Learning outcome four is to Draw the resonance hybrid and explain what is represents (“Hybrid”). We decided to include this LO although only five of the seven of the experts surveyed deemed it essential and it was only explained in four of the textbooks. We included this LO with the intent that competency with the hybrid representation could help dispel the notion that structures are alternating and give an alternate visualization of the concept of delocalization. This intent is supported by research findings that students better viewed resonance structures as an average representation of the structure after building their own representations and having a discussion about the pros and cons of representations (Kim et al., 2019). Accordingly, teaching students how to draw the resonance hybrid and explaining what it represents could help students build a more scientifically accurate mental model of delocalization.
The fifth LO is to Approximate the hybridization and geometry of atoms, and identify the orbital(s) of the non-bonding electrons in a delocalized system (“Hybridization”). This learning outcome builds on the previous four outcomes since the learner must first identify whether electrons are delocalized through the π system, then approximate which structures are significant enough to affect the hybridization of an atom. The wording of LO5 was changed to its current form to be as thorough as possible; however; data in this study were analyzed in LO5's original version: “Approximate the hybridization and geometry of atoms in delocalized system.”
The sixth learning outcome is to Determine whether a molecule is aromatic, non-aromatic, or anti-aromatic, and explain why (“Aromatic”). Aromatic, non-aromatic, and anti-aromatic molecules have vastly different properties due to orbital and electronic effects. Being able to conceptualize why a molecule is aromatic could allow students to better predict how the molecules will react.
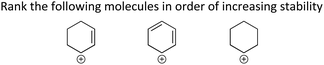
The seventh LO, Use delocalization concepts to determine and justify the relative stability/energy/reactivity of ions (“Stability/Reactivity”), and the eighth LO, Use delocalization concepts to determine the relative acidity/basicity of an atom (“Acid/Base”) are similar. Delocalization is often the basis for a molecule's relative stability or acidity/basicity and using delocalization concepts has been previous identified as part of key learning outcomes for acid–base chemistry (Stoyanovich et al., 2015).
Learning outcome nine is to Determine the electrophilic and nucleophilic sites on a molecule using delocalization concepts as the justification (“Electrophilic/Nucleophilic”). Identifying nucleophilic and electrophilic sites is a key component to determining the reactivity of molecules; delocalization can affect the location of those sites.
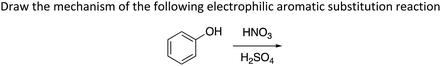
The tenth learning outcome is to Use delocalization concepts to predict and justify the course of a reaction (“Reaction”). This LO encompasses many of the previous LOs and applies the concepts in context. Delocalization can affect the regioselectivity of a reaction or whether a reaction occurs at all (e.g., electrophilic aromatic substitution regioselectivity is dictated primarily by the extent of delocalization in the transition state leading to the arenium). Being able to identify how delocalization affects reactions stands to equip students with better skills to solve organic chemistry problems.
Of the ten learning outcomes established, seven (LO3 – Contribution, LO4 – Hybrid, LO6 – Aromatic, LO7 – Stability, LO8 – Acid/Base, LO9 – Electrophilic/Nucleophilic, LO10 – Reaction) require a justification or explanation. Causal explanations address the reasons why a phenomenon occurs and allows learners, citizens, and scientists to go beyond a set of rules to understand, predict, and explain the cause and effect relationships that underpin phenomena (Talanquer and Pollard, 2010; Sevian and Talanquer, 2014; Cooper, 2015; Weinrich and Talanquer, 2016; Bodé et al., 2019). Moreover, the US’ National Research Council's Framework has emphasized the importance for students to develop causal reasoning skills when providing scientific explanations or arguments (National Research Council, 2012).
RQ2: How are the proposed delocalization intended LOs being taught, practiced, and assessed?
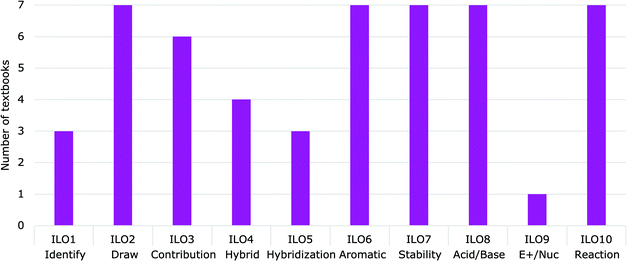
TaughtOf the ten proposed essential LOs, five were demonstrated in all of the textbooks analyzed (Fig. 5) and four were underrepresented. ILO9 (Electrophilic/Nucleophilic) was taught in only one of the textbooks (Ogilvie et al., 2018), leaving it to students to make the connection between delocalization concepts and electrophilic/nucleophilic sites on a molecule. Similarly, ILO1 (Identify) was only explicitly demonstrated in three of the textbooks (Klein, 2012; Smith, 2014; Ogilvie et al., 2018). Similarly, only a few textbooks explained explicitly how to Approximate the hybridization and geometry of the atoms involved in delocalization (ILO5) (Wade, 2010; Klein, 2012; Smith, 2014). Draw the resonance hybrid and explain what it represents (ILO4) was also under-represented. The resonance hybrid was shown in all of the textbooks, with four of them explaining how to draw the hybrid and what the dashed bonds represent (Solomons and Fryhle, 2000, 2011; Smith, 2014; Ogilvie et al., 2018). Two of the textbooks explained that the hybrid is a representation of all resonance structures but did not explain how to draw the hybrid or what the dashed bonds represent (Wade, 2010; Vollhardt and Schore, 2018). The remaining textbook mentioned the hybrid is the “true” representation but did not show an image of the hybrid (Klein, 2012). Explaining the hybrid structure and its components could help enhance students’ representational competency and mental models of delocalization concepts.
Within the textbooks analyzed, the ILOs were addressed in two main areas: the beginning and the middle of the textbooks (Fig. 6). ILOs 2 through 4 (Draw, Contribution, and Hybrid) were shown within the first two chapters of each book. ILO7 (Stability) and ILO8 (Acid/Base) were shown in the following few chapters, when introducing the acid–base and substitution/elimination reactions, respectively. ILO6 (Aromatic) and ILO10 (Reaction) were taught in the middle of the textbooks when conjugation and aromaticity were introduced. ILO5 (Hybridization) appeared in different places, in that two textbooks taught the concept in the first two chapters, while the remainder addressed it when introducing aromaticity. This placement could be occurring because the orbitals, hybridization and geometry is taught after delocalization in four of the textbooks (Solomons and Fryhle, 2000, 2011; Wade, 2010; Vollhardt and Schore, 2018) while the other three teach delocalization after having gone over hybridization. The order in which those concepts are taught makes a difference of over ten chapters between the textbooks analyzed in when the concept of hybridization in terms of delocalization is taught.
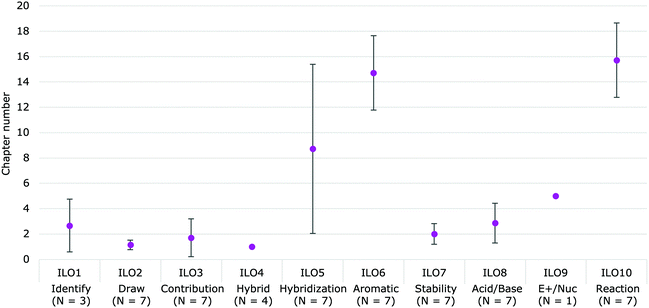 | ||
| Fig. 6 The average location in the textbooks where the ILOs are taught. Error bar denote the standard deviation. | ||
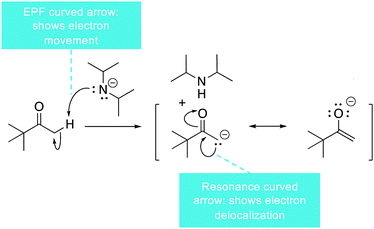
Another potential issue involves the slightly differing meaning of the electron pushing formalism symbolism for reactions and resonance structures (Fig. 7). For example, in the reaction shown in Fig. 7, there are curved arrows that show electron movement for a reaction and curved arrows that show electron delocalization. This symbolism could add to the idea that student have that resonance structures are alternating and the electrons are moving. This potential confusing could be mitigated by clearly explaining the different ways the curved arrows are used and by using and explaining the hybrid (ILO4).
Another misalignment with the concept of delocalization is that resonance structures are often labelled as “stable” and describing relative stability of resonance structures could be misleading because resonance structures do not exist as discreet entities. Instead, referring to the individual structures as contributors (i.e., major contributor, minor contributor) avoids giving a real property (stability) to a non-real entity, as do some textbooks (Klein, 2012; Smith, 2014; Ogilvie et al., 2018).
All the textbooks analyzed used the term resonance more often than the word delocalization. When asked, experts reported they use the terms resonance and delocalization interchangeably. One expert stated: “Resonance is a term that is too prevalent, so we need to teach it. Delocalization is also useful and arguably a better term pedagogically.” This quote shows that resonance is an integrated part of the organic chemistry culture, despite being the less precise term for the phenomenon. We analyzed two more advanced textbooks (Fleming, 2009; Clayden et al., 2012) and found that they refer to the concept as delocalization; they also mentioned that chemists will often use the word resonance. Clayden et al. state in the textbook that they avoid the use of the term “resonance” and only uses “delocalization” to avoid confusion:
“How to describe delocalization? What word should be used to describe delocalization is a vexed question. Terms such as resonance, mesomerism, conjugation, and delocalization are only a few of the ones you will find in books. You will already have noticed that we’re avoiding resonance because it carries the suggestion that the structure is somehow oscillating between localized structures.” (Clayden et al., 2012, p. 145). Explicitly teaching these limitations and using precise terminology could help avoid confusion and help learners integrate the concept.
In the textbooks analyzed, causal reasoning was not usually included when teaching delocalization; rule-based reasoning was used instead (Kraft et al., 2010). As one example, the relative contributions to a resonance hybrid are taught as a set of rules: the highest maximum number of bonds to an atom or maximum number of atoms with a full octet, minimum number of charges, charge separation, etc. Four textbooks used chemical stability as the reasoning for the relative contributions to the hybrid (Solomons and Fryhle, 2000, 2011; Wade, 2010; Vollhardt and Schore, 2018). Only one of the textbooks (Klein, 2012) gave reasons as to why a contributor is major (i.e., the causal links).
Another example of a common organic chemistry rule related to delocalization is Hückel's rule. Hückel's rule states: “monocyclic planar (or almost planar) systems of trigonally (or sometimes diagonally) hybridized atoms that contain (4n + 2) π-electrons (where n is a non-negative integer) will exhibit aromatic character.” (McNaught and Wilkinson, 1997). This rule does not explain why these factors are required for aromaticity, such as planarity and π-orbital alignment. By incorporating causal reasoning into the learning outcomes and educational design, students stand to be better positioned to move from rote memorization toward more meaningful learning.
For example, ILO9 (Electrophilic/Nucleophilic) was only explicitly taught in one of the textbooks (Ogilvie et al., 2018). Analysing structures for electron delocalization can reveal areas of high and low electron density, which can be used to identify nucleophilic and electrophilic sites. Delocalization is taught before the concepts of nucleophiles and electrophiles, and when nucleophiles and electrophiles are taught, the effect of delocalization is not explained. The link between delocalization and electron density may be obvious to experts but this connection is often only made implicitly in textbooks and so may never become apparent to learners.
Similarly, ILO5 (Hybridization) is not explicitly explained in four of the textbooks. One of the three that did explain this learning outcome mentioned it in a solved practice question, not as part of the main text (Wade, 2010). Ogilvie et al. (2018) gave a brief explanation in Chapter 5 (Organic reaction mechanisms: using curved arrows to analyze reaction mechanisms) (p. 220) although delocalization was first introduced in Chapter 1 (Carbon and its compounds). Klein (2012) gave a detailed explanation of how to identify the hybridization and geometry of the atoms in an amide functional group. Greater explanations are likely needed around the concepts of hybridization and how some non-bonding electrons form part of the π system.
ILO8 (Acid/Base) and ILO7 (Stability) were explained only very briefly in most of the textbooks. The effect of delocalization on a molecule's stability was briefly explained in the first chapter (second chapter in Klein (2012)); however, the concept was not revisited when describing ions. Two of the textbooks explained the reasons why delocalization affects acidity/basicity (Smith, 2014; Ogilvie et al., 2018). The others make broad statement such as: “In this case, the charge is delocalized over both oxygen atoms. Such a negative charge will be more stable than a negative charge localized on one oxygen” (Klein, 2012 p. 110). This explanation will promote rule-based reasoning and does not explain why delocalization stabilizes ions.
Our analysis shows that the concept of delocalization is sometimes overlooked in key areas, such as electron density and reactivity, hybridization, acid–base chemistry, and stability. The concepts are taught in silos without an explicit connection being made, which could lead to difficulty storing the information in LTM due to the lack of the explicit links.
Practiced
Practice problems and feedback help students build organic chemistry skills can help build metacognitive skills, both ideally promoting meaningful learning (Grove and Lowery Bretz, 2012). Practice questions help students store information in LTM by repeatably elaborating pieces of information and organizing the information into a network (Gupta and Cohen, 2002).We analyzed the 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 end-of-chapter questions from the seven textbooks; of those, 9% (N = 1548) related to delocalization (Fig. 8). Many questions included sub questions (i.e., a, b, c, etc.). The questions varied from multiple choice (e.g., select the most stable molecule) to constructed response questions (e.g., draw the mechanism of this reaction).
040 end-of-chapter questions from the seven textbooks; of those, 9% (N = 1548) related to delocalization (Fig. 8). Many questions included sub questions (i.e., a, b, c, etc.). The questions varied from multiple choice (e.g., select the most stable molecule) to constructed response questions (e.g., draw the mechanism of this reaction).
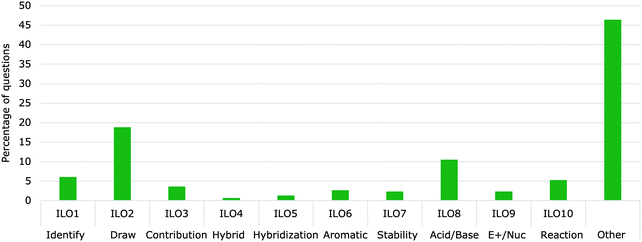 | ||
| Fig. 8 Percentage of questions related to the LOs from all delocalization questions (N = 1548). “Other” represents any delocalization questions that do not fully address one of the 10 essential LOs. | ||
Of the ten essential intended LOs, ILO2 (Draw) was the most commonly practiced with 18% of all delocalization questions. The remaining intended LOs were not present as often with ILO5 (Hybridization), ILO7 (Stability) and ILO9 (Electrophilic/Nucleophilic) each representing less than 2% of the questions about delocalization.
The least practiced essential ILO was ILO4 (Hybrid), with only 0.7% of all the delocalization questions being related to it. Only three of the seven textbooks had practice questions related to the resonance hybrid (Solomons and Fryhle, 2011; Smith, 2014; Vollhardt and Schore, 2018).
The “Other” category occurred more often than all the other intended ILOs since most questions did not fully address the essential ILOs and instead related to the ones considered non-essential (Fig. 8). Many of the questions did address parts of the essential ILOs but did not require justifications or explanations. These were coded as partially assessing the ILOs and were added to the “Other” category along with questions related to delocalization by not within the ten essential LOs. For example, the “Other” category includes Predict the product of an aromatic electrophilic substitution reaction. Solving electrophilic aromatic substitutions reactions could involve the knowledge of delocalization but can usually be readily answered by memorizing the directing groups’ effects without knowing the reasons for those effects. Therefore, simply predicting the product does not fully address LO10 (reaction) since it lacks the justification component. Predict the product of an electrophilic substitution reaction represented 20% of all delocalization questions while Draw the electrophilic aromatic substitution mechanism with all structures representing delocalization occurred only in 2.7% of the questions. Similarly, the LO Use Hückel's rule to determine if a molecule is aromatic, anti-aromatic or non-aromatic was found in 2.5% of all delocalization questions, while justifying aromaticity using other parameters was found in 1.1% of questions. Appendix 2 shows the distribution of questions to all of the 33 ILOs.
While the “non-essential” LOs could be important, having over 50% of the practice questions addressing them identifies a concern about how questions are designed and chosen. Shifting the questions' proportions to align with the more the essential LOs could promote better understanding of delocalization.
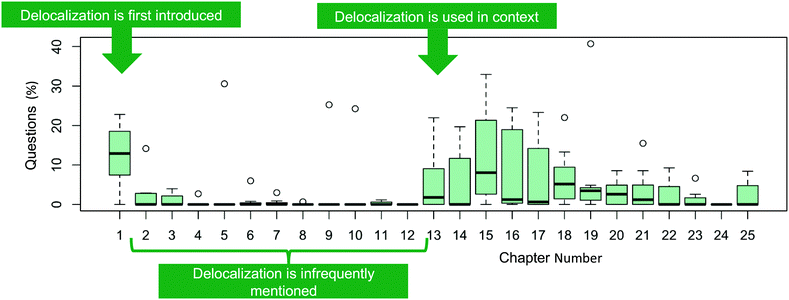 | ||
| Fig. 9 Distribution of delocalization question (%) in each chapter from the seven textbooks. N = 1548. | ||
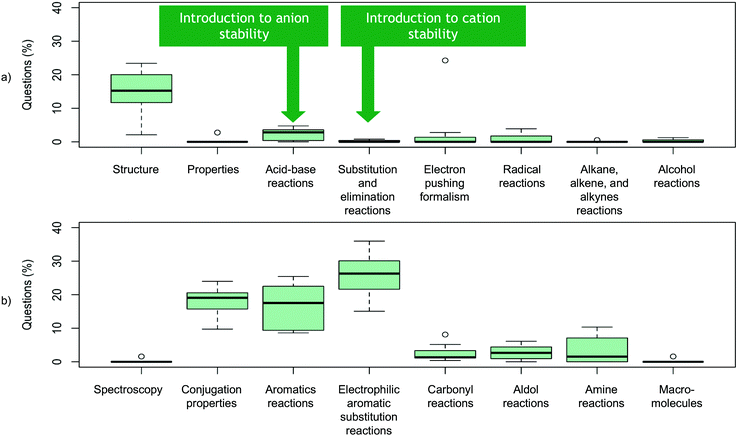
The first-time delocalization is used in context is in the acid–base chapter to talk about acidity/basicity, which appears six chapters after delocalization has been taught, on average. We propose introducing delocalization when it is needed, specifically at this point and as has been done in previous work (Stoyanovich et al., 2015). This placement would mean introducing delocalization when introducing acid–base chemistry instead of when introducing Lewis structures. This placement would ideally help the students understand why delocalization concepts are important. Teaching delocalization later would also allow the students to have gained the prior knowledge required (e.g., drawing and interpreting Lewis structures, electron-pushing arrows, and hybridization) to integrate the new knowledge of delocalization.
Delocalization affects reactivity, stability and properties of a molecule, therefore we anticipated seeing questions related to delocalization in chapters about structure (including ions and radicals), conjugation, aromaticity, and reactions (e.g., acid–base, elimination, substitution, and electrophilic aromatic substitution). Our analysis found that sections on structure, conjugated systems, aromatic chemistry, and electrophilic aromatic substitution reactions did have a higher percentage of delocalization questions, while acid–base chemistry, elimination and substitution reactions, and radicals had a low number of delocalization questions (Fig. 10). Only 1.4% of all delocalization questions were related to ion stability with delocalization, a subject that is highly important for substitution and elimination reactions.
Assessed
Assessments are used to assess the progress toward the intended LOs and should be aligned with how a concept is being taught and practiced, as well as the intended learning outcomes themselves.In terms of the breakdown by intended learning outcome, LO4 (Hybrid) was addressed only in one of the questions analyzed (Fig. 11). This could be due to the convention that practicing organic chemists use the resonance structures representations as opposed to the resonance hybrid.
LO9 (Electrophilic/Nucleophilic) was completely absent from the assessments analyzed, but 6% of the questions partially addressed this LO. For example, a question asking students to classify substituents as ortho, meta or para directing does not fully address the LO, because it does not require the delocalization justification and could be simply memorized. Similarly, questions related to the ILO: Predict the electrophilic aromatic substitution product requires an understanding of the regioselectivity but may be answered without having the skill and knowledge of LO9 (Electrophilic/Nucleophilic).
LO 10: Use delocalization concepts to predict and explain the course of a reaction represented 10% of the assessment questions; however, “Predict the product” questions, which do not require justification or drawing of the resonance structures, that involved delocalization represented 16% of the questions. While students may have the skills to use delocalization to predict a reaction, without the justification or drawing of the structures, LO 10 cannot be fully assessed.
There was a similar number of questions in both Organic Chemistry I and Organic Chemistry II exams (67 and 76 respectively) but there was a difference in the nature of LOs that were assessed (Fig. 11). Assessments in Organic Chemistry I focussed more on drawing resonance structures and assessing the structures (LO2 – Draw, LO3 – Contribution, LO5 – Hybridization, LO7 – Stability and LO8 – Acid/Base) while Organic Chemistry II assessments focused more on reactions using delocalization (LO6 – Aromatic, LO7 – Stability, LO8 – Acid/Base and LO10 – Reaction).
We also reached out to ACS Exams to determine the weight of delocalization on the ACS Organic Chemistry Practice Exam. One item (out of 50) on the Organic Chemistry Practice Exam explicitly assessed delocalization. Other questions may indirectly assess delocalization; however, no in-depth analysis was done.
Implicit vs. explicit delocalization questions
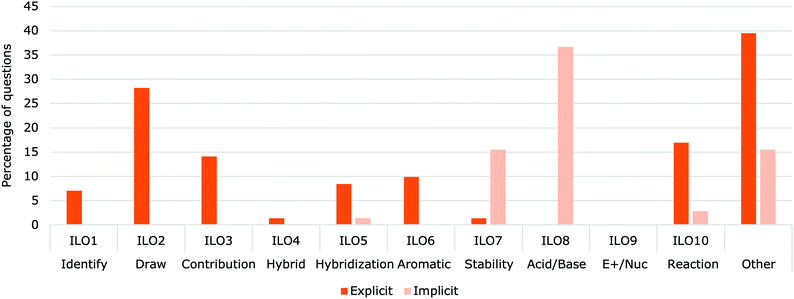
Questions that implicitly involved delocalization concepts had significantly greater points value (M = 6.82, SD = 9.47) than explicit delocalization questions (M = 3.14, SD = 3.81) on the summative assessments (t(49) = 6.91, p < 0.001). Questions that implicitly involve delocalization concepts do not state that the use of delocalization is required, but answering the question requires the student to use knowledge of delocalization. Explicit delocalization questions clearly state the requirement for using delocalization/resonance concepts. Both implicit and explicit questions are useful and assess different aspect of the concept.Some LOs were almost always explicit (LO1 – Identify, LO2 – Draw, LO3 – Contribution, LO5 – Hybridization and LO6 – Aromatic). These LOs deal with the structures and property of delocalization, while the remaining LOs are typically used in a context. For example, all of the delocalization questions about LO8 (Acid/Base) were implicit. The most common acid–base question required that students compare two protons in different molecular environments with one of them having delocalized electrons in their conjugate base (Fig. 12). Similarly, questions aligned with LO7 (Stability) were often implicit. Questions aligned with LO10 (Reaction) were both implicit and explicit; the majority of the question about LO10 explicitly stated that resonance structures should be drawn for full marks. However, there were also several questions in which a mechanism was all that was required, and that delocalization was implicit.
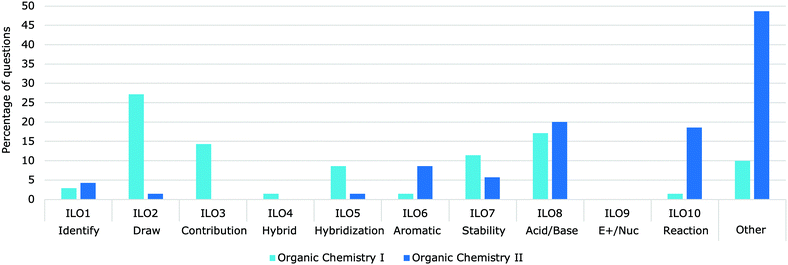 | ||
| Fig. 12 Percent of delocalization question (N = 143) related to each ILO on professors' assessments (N = 51) in Organic Chemistry I (dark blue) and Organic Chemistry II (pale blue). | ||
Conclusion
Through this study, we proposed ten essential learning outcomes (LOs) for the subject of delocalization (Fig. 4), designed based on key concepts related to delocalization and considering ways in which delocalization is used in more advanced contexts (e.g., identifying sites of high/low electron density). Identifying explicit essential LOs can help align curricular and instructional practices, including assessment.We conducted a textbook analysis using those ten intended LOs and found that they were not always the focus of how delocalization was taught, practiced, and assessed. Some of the intended LOs were underrepresented in the textbook explanations or the practice questions (e.g., end-of-chapter questions). In particular, underrepresented learning outcomes in explanations were LO1 (Identify), LO4 (Hybrid), LO5 (Hybridization), and LO9 (Electrophilic/Nucleophilic). In textbook practice questions and summative assessments, only LO2 (Draw) and LO8 (Acid/base) were well-represented; questions related to the other essential ILOs were infrequently asked. There were many delocalization questions related to non-essential ILOs (Fig. 8). Moreover, there was a large gap in most textbooks between the chapter when delocalization concepts were first explained and the chapter in which the concepts were used in context (10 chapters, on average), posing likely learning issues for students in seeing the relevance of delocalization initially and connecting concepts later (Fig. 9).
We found potential areas of confusion in the way delocalization concepts were explained, which could be mitigated by more detailed and explicit explanations, including: (i) curved arrows can be used to describe electron movement in reactions (EPF) and to explain the differences between resonance structures (not electron movement per se since the electrons are delocalized and structures are not alternating), (ii) resonance structures were frequently described in terms of relative stability; however, since these structures do not exist discretely, it would be more appropriate to describe them in terms of their relative contribution to the resonance hybrid, and (iii) the term “resonance” has been interpreted by students as structures that alternate; instead, the term “delocalization” could be more precise and help students develop more accurate mental model of delocalization.
The focus in most questions (in the textbooks and on the exams) and explanations (in the textbook) was on rule-based explanations as opposed to causal explanations. Summative assessments questions (e.g., final exams) followed a similar pattern as the practice questions. Teaching and assessing by including causal reasoning can help students consolidate ideas and provides opportunities to practice building explanations and arguments from evidence.
Limitations
Textbooks are a common component of chemistry courses and were used in this study to capture how delocalization concepts are taught; however, they do not represent the entire educational picture. Instructors may not follow exactly the layout of the textbook, use supplementary material, or may not use a textbook at all. Nevertheless, textbooks reflect how authors (and textbook adopters) believe chemistry should be taught and therefore provide one lens into how a concept is being taught. Another limitation of this study is that no student data was analyzed; therefore, we cannot make claims about students’ achievement of the essential LOs.Implications for teaching and learning
The ten evidence-based essential LOs can be used by educators in their courses to guide instruction and assessments. The essential LOs will ideally also be shared with students to communicate the expectations and skills they should master and help them focus their studying. The learning outcomes could also be used in the design of new curricula in a backward design method (Richards, 2013). The method involves first identifying the knowledge and skills that need to be gained by the end of the curriculum then selecting the appropriate methods for instruction and assessment. These learning outcomes can act as the goals of the instruction and help guide curriculum design and learning opportunities for the university organic chemistry sequence.Educators could review their own formative and summative assessment questions with the essential learning outcomes in mind to identify areas where they may wish to adjust their own instruction and assessments.
Implications for research
New research could be guided by the ten essential LOs established and how delocalization is taught, practiced, and assessed.The LOs proposed also build on each other; therefore, investigating how students progress through the LOs could be investigated. The LOs could be used as the basis for a learning progression about the subject of delocalization.
We have introduced an approach to look at how a concept is being taught and found evidence-based essential LOs that can guide instruction. This approach could be used on other chemistry concept to identify essential LOs.
Conflicts of interest
There are no conflicts to declare.Appendix 1
Which term is appropriate: resonance or delocalization?
Resonance is often introduced as a tool to represent a molecule that cannot be represented by a single Lewis structure (Ogilvie et al., 2018). Several Lewis structures with differences in π and non-bonding electrons are used to portray a single molecule, but the discrete resonance structures do not exist. A molecule is actually a combination all of the resonance structures, which can be represented using the resonance hybrid; however, the hybrid structure does not show the number of electrons that are delocalized.The International Union of Pure and Applied Chemistry (IUPAC) defines resonance as: “the representation of the electronic structure of a molecular entity in terms of contributing structures…” (McNaught and Wilkinson, 2006).
Kerber sparked a debate when he stated that resonance was a misleading term and should be replaced by the word “delocalization” (Kerber, 2006). He advocated using the word “delocalization” and “delocalization forms” instead of “resonance”. Other chemists have opposed Kerber's views and stated that “resonance” is both conceptually and historically more accurate (Jensen, 2006). This type of argument can also be demonstrated in the fact that several advanced organic chemistry textbooks (Fleming, 2009; Clayden et al., 2012) have decided to not use the word “resonance” but instead use the word “delocalization”. Those authors also note that delocalization is often referred to as resonance.
According to the IUPAC definition, “resonance” refers to any Lewis structure that contributes to the wavefunctions, however unlikely that structure is (McNaught and Wilkinson, 2006). This definition would mean that delocalization across σ bonds are resonance structures and that hyperconjugation would also fall under the definition of resonance (Mullins, 2012). Therefore, resonance structures do not solely differ on placements of π electrons and non-bonding electrons, but include any structure that contributes to the wavefunction. Electrons being shared over multiple atoms in π orbitals is actually delocalization (McNaught and Wilkinson, 2014a), while the Lewis structures associated with the π system represent mesomerism (McNaught and Wilkinson, 2014b).
Appendix 2
LOs and the percentage of questions related to each ILO
Thirty-three LOs were derived from the first coding, which were then validated by experts (Table 1). Table 1 also shows the percentage of end-of-chapter questions that were related to each of the individual ILOs. The three of the ten essential LOs do not appear in their entirety in the table; due to an aggregation of several of the 33 ILOs. Some of the ILOs are too fine-grained and as such were added together to form one essential LO, such as LO10 (Reaction) which includes “Draw the nucleophilic aromatic substitution mechanism including all structures representing delocalization” and “Use delocalization to explain regioselectivity” among others. Therefore, LO10 includes all ILOs that require the concept of delocalization in the context of a reaction. Similarly, LO7 (Stability) is all encompassing of all ions, therefore the ILOs related to anion stability and cation stability were binned together. On the other hand, LO2 (Draw) requires two of the 33 ILOs to be present to be addressed: “Draw the resonance structures” and “Use curved arrows to show electron delocalization”. For most of the coding the electron-pushing formalism was implicit in the questions, therefore questions that require to draw resonance structures (without explicitly stating to use the EPF), were coded for the essential learning outcomes.| Intended learning outcomes | Questions, % |
|---|---|
| 29. Predict the product of an electrophilic aromatic substitution (EAS) reaction | 19.9 |
| 1. Draw the resonance structures (ILO2) | 15.4 |
| 27. Describe the effect of resonance on a molecule acidity/basicity (ILO8) | 10.1 |
| 11. Predict whether a molecule is aromatic, anti-aromatic or non-aromatic | 6.3 |
| 4. Identify structures where electron delocalization can occur (ILO1) | 5.8 |
| 24. Classify aromatic substituents as activating or deactivating | 4.1 |
| 31. Predict the product of a nucleophilic aromatic substitution reaction (SNAr) | 3.4 |
| 5. Assess contribution of each resonance structures to the hybrid, including justification (ILO3) | 3.1 |
| 32. Determine if the nucleophilic reaction of a conjugated system gives the kinetic or thermodynamic product | 3.0 |
| 12. Justify why a molecule is aromatic (or anti-aromatic or non-aromatic) using concept of conjugation, planarity, cyclic structures and Hückel's rule (ILO6) | 2.8 |
| 28. Draw the electrophilic aromatic substitution (EAS) mechanism with all structures representing delocalization (ILO10) | 2.7 |
| 14. Use Hückel's rule to determine if a molecule is aromatic, anti-aromatic or non-aromatic | 2.5 |
| 18. Correlate delocalized structures to spectroscopic data | 2.1 |
| 33. Draw the mechanism of a 1,2 or 1,4 conjugate addition | 2.1 |
| 25. Use delocalization to explain regioselectivity (ILO10) | 1.7 |
| 26. Predict electrophilic and nucleophilic sites on molecules (ILO9) | 1.7 |
| 16. Use delocalization to explain bond length | 1.3 |
| 20. Use delocalization to explain cation energy/stability (ILO7) | 1.3 |
| 2. Draw the electron pushing formalism arrows to represent electron delocalization (ILO2) | 1.2 |
| 15. Correlate structure with macroscopic properties, such as light absorption. | 1.2 |
| 17. Explain how delocalization affects bond rotation | 1.2 |
| 8. Identify hybridization of atoms involved in delocalization (ILO5) | 1.1 |
| 13. Use Frost circles to justify aromaticity or anti-aromaticity | 1.0 |
| 23. Use delocalization to explain radical energy/stability | 1.0 |
| 3. Draw the resonance hybrid including the partial charges (ILO4) | 0.7 |
| 19. Explain the effect of delocalization on heat of hydrogenation | 0.7 |
| 30. Draw the S N Ar (nucleophilic aromatic substitution) mechanism with all structures representing delocalization (ILO10) | 0.7 |
| 6. Draw the orbitals involved in electron delocalization | 0.6 |
| 22. Use delocalization to explain arenium energy/stability | 0.6 |
| 7. Draw the MO diagram of a molecules with resonance | 0.3 |
| 21. Use delocalization to explain anion energy/stability (ILO7) | 0.3 |
| 9. Explain the effect of delocalization on the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) | 0.0 |
| 10. Explain π orbitals alignment and the molecule planarity required for delocalization to occur | 0.0 |
Appendix 3
Example of taught-practiced-assessed (TPA) coding
To code for how a paragraph or section is teaching resonance, each paragraph in sections related to delocalization was assigned an ILO (or multiple) that was being demonstrated in the paragraph. Fig. 13 shows an example of a section in a textbook. The callouts on the right show the ILOs that were coded for each paragraph. The callout within the text show the types of recurring themes that were highlighted throughout the coding.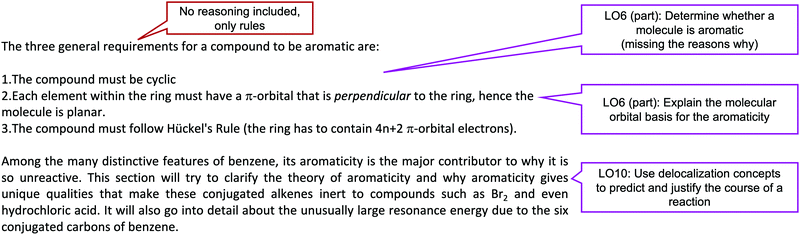 | ||
| Fig. 13 Example of how textbook paragraphs were coded. Excerpt taken from Chemistry Libretexts (2019). | ||
The series of practice questions would be coded using all 33 ILOs that have been proposed, not just the ten essential ILOs (Table 2). Some ILOs were present in the questions, but not completely; in those cases the ILOs were coded as present but with a note stating why it was not fully addressed.
Acknowledgements
We thank the following people who provided us with past assessments and/or expert advice: Dr André Beauchemin, Dr Amanda Bongers, Dr Kathy-Sarah Focsaneanu, Dr Stephen MacNeil, Dr Katherine McGilvray, Dr Barb Morra, Dr Leanne Racicot, Dr Effiette Sauer, Dr Jaclyn Stewart, and Dr Alison Thompson. We also thank Keith Lapierre for his work on the inter-rater reliability. MSC thanks the Social Sciences and Humanities Research Council of Canada for funding through a Canadian Graduate Scholarship – Doctoral (CGS-D).References
- Anderson J. R., (1996), ACT: a simple theory of complex cognition, Am. Psychol., 51(4), 355–365.
- Arterhan C. S. C. and Schwarz B. B., (2007), The effects of monological and dialogical argumentation on concept learning in evolutionary theory, J. Educ. Psychol., 99, 626–639.
- Ausubel D. P., Novak J. D., and Hanesian H., (1978), Educational psychology: A cognitive view, Holt: Rinehart and Winston.
- Berland L. K. and Reiser B. J., (2009), Making sense of argumentation and explanation, Sci. Educ., 93(1), 26–55.
- Betancourt-Pérez R., Olivera L. J., and Rodríguez J. E., (2010), Assessment of organic chemistry students’ knowledge of resonance-related structures, J. Chem. Educ., 87(5), 547–551.
- Bodé N. E., Deng J. M., and Flynn A. B., (2019), Getting Past the Rules and to the WHY: Causal Mechanistic Arguments When Judging the Plausibility of Organic Reaction Mechanisms, J. Chem. Educ., 96(6), 1068–1082.
- Brabrand C. and Dahl B., (2009), Using the SOLO taxonomy to analyze competence progression of university science curricula, High. Educ., 58(4), 531–549.
- Chemistry LibreTexts, (2019), Aromaticity. [online] Available at: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Arenes/Properties_of_Arenes/Aromaticity [Accessed 7 Feb., 2020].
- Clayden J., Greeves N. and Warren S., (2012), Organic Chemistry, 2nd edn, Oxford University Press Inc.
- Cohen N. J., Poldrack R. A., and Eichenbaum H., (1997), Memory for Items and Memory for Relations in the Procedural/Declarative Memory Framework, Memory, 5(1–2), 131–178.
- Collis K. F. and Biggs J. B., (1986), Using The SOLO Taxonomy, Set Res. Inf. Teach., 2(4), 4.
- Cooper M. M., (2015), Why Ask Why? J. Chem. Educ., 92(8), 1273–1279.
- Creswell J. W., (2012), Educational Research: Planning, Conducting, and Evaluating Quantitative and Qualitative Research, Pearson.
- Delvigne F., (1989), A visual aid for teaching the resonance concept, J. Chem. Educ., 66(6), 461.
- Dixson D. D., Worrell F. C., Dixson D. D., and Worrell F. C., (2016), Formative and Summative Assessment in the Classroom, p. 5841.
- Duis J. M., (2011), Organic Chemistry Educators‚ Perspectives on Fundamental Concepts and Misconceptions: An Exploratory Study, J. Chem. Educ., 88(3), 346–350.
- Fleming I., (2009), Molecular Orbitals and Organic Chemical Reactions, Student edn, Wiley.
- Gero A., (1954), Predicting Reaction of a Resonance Hybrid from Minor Canonical Structures, J. Chem. Educ., 31(3), 136.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16(1), 9–21.
- Grove N. P. and Lowery Bretz S., (2012), A continuum of learning: from rote memorization to meaningful learning in organic chemistry, Chem. Educ. Res. Pract., 13(3), 201–208.
- Günes S., Neugebauer H., and Sariciftci N. S., (2007), Conjugated polymer-based organic solar cells, Chem. Rev., 107(4), 1324–1338.
- Gupta P. and Cohen N. J., (2002), Theoretical and computational analysis of skill learning, repetition priming, and procedural memory, Psychol. Rev., 109(2), 401–448.
- Jensen W. B., (2006), More on the Nature of Resonance, J. Chem. Educ., 83(65), 223–227.
- Jiang Y. and McNeill J., (2017), Light-harvesting and amplified energy transfer in conjugated polymer nanoparticles, Chem. Rev., 117(2), 838–859.
- Kalyuga S., Ayres P., Chandler P., and Sweller J., (2003), Cognitive Load Measurement as a Means to Advance Cognitive Load Theory, Educ. Psychol., 38, 23–31.
- Kerber R. C., (2006), If It's Resonance, What Is Resonating? J. Chem. Educ., 83(2), 223.
- Kim T. D., Wright L. K., and Miller K., (2019), An examination of students’ perceptions of the Kekulé resonance representation using a perceptual learning theory lens, Chem. Educ. Res. Pract., 20(4), 659–666.
- Klein D., (2012), Organic Chemistry, John Wiley & Sons, Inc.
- Kraft A., Strickland A. M., and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11(4), 281–292.
- Krippendorff K., (2004), Reliability in Content Analysis, Hum. Commun. Res., 30(3), 411–433.
- Lin S., (2007), Aromatic Bagels: An Edible Resonance Analogy, J. Chem. Educ., 84(5), 779.
- Mayer R. E. and Moreno R., (2003), Cognitive Load Measurement as a Means to Advance Cognitive Load Theory, Educ. Psychol., 1520(38), 43–52.
- McNaught A. D. and Wilkinson A., (1997), IUPAC. Compendium Of Chemical Terminology, 2nd ed. (the “Gold Book”), Blackwell Scientific Publications.
- McNaught A. D. and Wilkinson A, (2014a), IUPAC Gold Book - Delocalization, IUPAC, Compendium of Chemical Terminology (the “gold book”).
- McNaught A. D. and Wilkinson A., (2014b), IUPAC Gold Book - Mesomerism, IUPAC, Compendium of Chemical Terminology (the “gold book”).
- McNaught A. D. and Wilkinson A., (2006), IUPAC Gold Book - Resonance, 2nd edn, Blackwell Scientific Publications.
- Mullins J. J., (2012), Hyperconjugation: a more coherent approach, J. Chem. Educ., 89(7), 834–836.
- National Research Council, (2012), A Framework for K-12 Science Education.
- Novak J. D., (1993), Human constructivism: a unification of psychological and epistemological phenomena in meaning making, Int. J. Pers. Constr. Psychol., 6(2), 167–193.
- Ogilvie W., Ackyord N., Browning C. S., Deslongchamps G., Lee F., and Sauer E., (2018), Organic Chemistry Mechanistic Patterns, 1st edn, Nelson.
- Richards J. C., (2013), Curriculum approaches in language teaching: forward, central, and backward design, RELC J., 44(1), 5–33.
- Schunk D., (2016), Learning Theories: An Educational Perspective, 6th edn, Pearson.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: a learning progression on chemical thinking, Chem. Educ. Res. Pract., 15(1), 10–23.
- Silverstein T. P., (1999), The “Big Dog-Puppy Dog” Analogy for Resonance, J. Chem. Educ., 76(2), 206.
- Smith D. T., Delost M. D., Qureshi H., and Njaroarson J. T., (2016), Top 200 Pharmaceutical Products by Retail Sales.
- Smith J. G., (2014), Organic Chemistry, 4th edn, McGraw Hill.
- Solomons T. W. G. and Fryhle C. B., (2000), Chimie organique, Traduction, Modulo.
- Solomons T. W. G. and Fryhle C. B., (2011), Organic Chemistry, 10th edn, John Wiley & Sons, Inc.
- Starkey R., (1995), Resonance Analogy Using Cartoon Characters, J. Chem. Educ., 72(6), 542.
- Stoyanovich C., Gandhi A., and Flynn A. B., (2015), Acid–Base Learning Outcomes for Students in an Introductory Organic Chemistry Course, J. Chem. Educ., 92(2), 220–229.
- Sweller J., (1999), Instructional design in technical areas, ACER Press.
- Taber K. S., (2002), Compounding Quanta: Probing the Frontiers of Student Understanding of Molecular Orbitals, Chem. Educ. Res. Pract., 3(2), 159–173.
- Talanquer V. and Pollard J., (2010), Let's teach how we think instead of what we know, Chem. Educ. Res. Pract., 11(2), 74–83.
- Vollhardt P. and Schore N., (2018), Organic Chemistry: Structure and Function, 6th edn, W.H. Freeman and Company.
- Wade L. G., (2010), Organic Chemistry, 7th edn, Pearson Prentice Hall.
- Weinrich M. L. and Talanquer V., (2016), Mapping Students’ Modes of Reasoning When Thinking About Chemical Reactions Used to Make a Desired Product, Chem. Educ. Res. Pract., 17(2), 394–406.
- Zamanzadeh V., Ghahramanian A., Rassouli M., Abbaszadeh A., and H. Alavi-Majd, (2015), Design and Implementation Content Validity Study: Development of an instrument for measuring Patient-Centered Communication, J. Caring Sci., 4(2), 165–178.
| This journal is © The Royal Society of Chemistry 2020 |