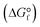Pyrolysis of mixtures of methane and ethane: activation of methane with the aid of radicals generated from ethane
Hitoshi
Ogihara
 *,
Hiroki
Tajima
and
Hideki
Kurokawa
*,
Hiroki
Tajima
and
Hideki
Kurokawa

Graduate School of Science and Engineering, Saitama University, 255 Shimo-Okubo, Sakura-ku, Saitama 338-8570, Japan. E-mail: ogihara@mail.saitama-u.ac.jp
First published on 14th November 2019
Abstract
Direct chemical conversion of methane (CH4) has been actively researched in order to use natural gas as a chemical resource. However, the high stability of CH4 molecules hinders the chemical conversion of CH4. In this study, we investigated pyrolysis of mixtures of CH4 and ethane (C2H6) at 973–1073 K. Even though CH4 alone did not react in the temperature range, mixtures of CH4/C2H6 and of Ar/C2H6 showed different pyrolysis behaviours; the co-existence of CH4 significantly increased yields of propylene (C3H6), propane (C3H8) and toluene. Mass spectrometry analysis using 13C-labeled CH4 revealed that carbon contained in CH4 was incorporated into the pyrolysis products. The results suggested that CH4 was activated with the aid of C2H6. We assumed that CH4 was attacked by radical species generated from pyrolysis of C2H6 and was converted into methyl radicals. The CH4-derived methyl radicals were incorporated into pyrolysis products via radical reactions. This study clarified that CH4 can be activated by radicals generated from co-existing molecules without the help of catalysts or extremely high temperature.
Introduction
Innovations in shale gas extraction technology are boosting the use of natural gas in various fields. Natural gas is expected to play an active role as a raw material for the chemical industry. This is because petroleum, which is a fossil resource that plays a major role in the current chemical industry, is depleting rapidly. Methane (CH4), a main component of natural gas, is industrially used to produce synthesis gas via steam reforming. In addition, ethane (C2H6), a secondary component of natural gas, can be converted to ethylene (C2H4) via thermal cracking. Steam reforming and thermal cracking are typical examples of methods of chemical conversion of natural gas, however, although industrial interest in natural gas conversion has intensified, most natural gas is presently used as fuel for thermal power generation etc. In other words, natural gas plays a more limited role in the current chemical industry compared with petroleum.The reason is attributed to the high stability of the CH4 molecule; the high stability of CH4 hampers the chemical conversion processes for natural gas.1 Thus, the development of technology to convert natural gas into essential chemicals is being intensively researched. In particular, direct conversion processes of CH4 such as coupling to produce lower olefins2–7 and aromatization8,9 are being rigorously explored.
In contrast, Periana et al. developed a catalyst for the selective oxidation of mixtures of CH4, C2H6 and C3H8 to alcohol esters.10 Such direct conversion of CH4-based hydrocarbon mixtures is interesting from the viewpoint of direct utilization of natural gas because natural gas is a CH4-based hydrocarbon mixture (C2H6 and C3H8 are present as secondary and tertiary components). In related works, dehydrogenative conversion of lower olefins containing CH4 using zeolite-supported catalysts has also been investigated.10–15
However, the chemical conversion of CH4-based hydrocarbon mixtures is not easy because the reactivities of CH4 and other hydrocarbons are quite different; in brief, CH4 is much more stable than other hydrocarbons. Pyrolysis of CH4 has been intensively investigated,16–20 and among various hydrocarbons, CH4 requires extreme high temperatures (>approx. 1473 K) for pyrolysis reactions.1,18 However, such high temperatures are too severe for other hydrocarbons. For most hydrocarbons, coke should be formed by deep dehydrogenation under the conditions appropriate for pyrolysis of CH4. Conversely, reaction conditions suitable for pyrolysis of other hydrocarbons such as C2H6 and C3H8 are too mild to activate CH4 molecules, indicating CH4 does not react.
So far, most studies have focused on catalysts that enable the direct activation of CH4. However, indirect activation routes would be feasible; for example, OH radicals generated from H2O on a molten salt catalyst activate CH4 molecules and promote the coupling of CH4.21,22 In addition, CH4 activation by gas phase atomic clusters has been investigated.23 Thus, if active radical species can be generated, CH4 would be activated with the aid of the radicals. In this study, pyrolysis of mixtures of CH4 and C2H6 was examined. We clarified that CH4 was activated by C2H6-derived radicals; as a result, even under mild conditions where CH4 alone did not react, CH4 molecules played a role in the pyrolysis reaction and were incorporated into pyrolysis products.
Experimental
Pyrolysis reactions
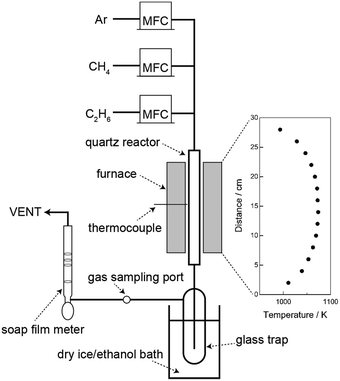
Schematic diagram of reactor system is shown in Fig. 1. Pyrolysis reactions were carried out with a quartz reactor (i.d. = 10 mm, o.d. = 12 mm) that was heated with an electric furnace. The temperature profile for the furnace is shown in Fig. 1. Flow rates of CH4 (99.999%), C2H6 (99.7%), and Ar (99.99%) were controlled by using mass flow controllers. The reactor was purged by flowing Ar for 10 min, and then heated to reaction temperature (973–1073 K). By introducing mixtures of CH4 and C2H6 or of Ar and C2H6, pyrolysis was carried out for 1 h. Hereafter, mixtures of CH4 and C2H6 and of Ar and C2H6 will be denoted as CH4/C2H6 and Ar/C2H6, respectively. Volume fractions of C2H6 in the gas mixtures were adjusted to 0.17, 0.25, and 0.50, and flow rates of the gas mixtures were controlled at 10, 30, and 50 mL min−1. The flow rates of the outlet gas were measured by a soap film flowmeter.During the pyrolysis reaction, outlet gas of 0.5 mL was collected with a gas-tight syringe and injected into gas chromatographs every 15 min. For H2, CH4, and C2H6, a gas chromatograph (Shimadzu GC-8A, TCD) equipped with a packed column (Active carbon) was used at 473 K (injection/detector) and 443 K (column) under flowing Ar as a carrier gas. For C2H4, C2H2, and C3 hydrocarbons, a gas chromatograph (Shimadzu GC-8A, FID) equipped with a packed column (Unibeads 1S) was used at 453 K (injection/detector) and 383 K (column) under flowing N2 as a carrier gas. To quantify gaseous products, calibration curves for all products were prepared by injecting different volume of the gases. The gases were collected from a gas cylinder (99.999% H2, 99.999% CH4, and 99.7% C2H6) or a gas can (GL Science Inc.; 99.5% C2H4, 0.100% C2H2, 99.5% C3H6, and 99.5% C3H8). Formation rates of the products were calculated based on the flow rates and the GC analysis.
Aromatics (benzene, toluene, styrene, and naphthalene) formed by the pyrolysis were collected in a glass trap cooled with a dry ice/ethanol bath. After the reaction, the trapped aromatics were dissolved in acetonitrile and then 30 mM butyl acetate in acetonitrile (1 or 2 mL) was added as an internal standard. The obtained solution was injected into a gas chromatograph (Shimazu, GC-18A) equipped with a capillary column (Shinwa chemical industries ltd., ULBON HR-1, 0.25 mm i.d., 30 m) under flowing N2 as a carrier gas. Temperature for injection/detector was settled at 523 K and temperature for column was raised from 313 K to 473 K at 10 K min−1. To quantify the aromatics, solutions containing different amounts of benzene (99.5%, Kanto Chemical Co., Inc.), toluene (99.5%, Kanto Chemical Co., Inc.), styrene (99.0%, Kanto Chemical Co., Inc.), naphthalene (98.0%, Kanto Chemical Co., Inc.), and butyl acetate (99.0%, FUJIFILM Wako Pure Chemical Corporation) in acetonitrile (99.5%, Kanto Chemical Co., Inc.) were prepared and calibration curves were prepared by injecting the solutions into the GC.
Conversions of CH4 and C2H6 were calculated based on gas composition analysed by GC and flow rates of mixture gas. Mass balance was calculated from ratios of carbon atoms for components of feed and outlet gases and trapped solutions. Equilibrium conversion and standard free energy of formation for hydrocarbons 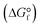 was calculated using HSC Chemistry (Outotec).
was calculated using HSC Chemistry (Outotec).
Pyrolysis reactions using 13C-labeled CH4
Pyrolysis reactions using 13C-labeled CH4 (13CH4; Watari Co. Ltd.) were carried out on a closed gas-circulation system (224 mL) equipped with an electric furnace. The closed gas-circulation system was mainly made of Pyrex glass and a reactor that was heated with an electric furnace was made of quartz glass. After evacuating the closed gas-circulation system, 13CH4/C2H6 (20 kPa/20 kPa) or He/C2H6 (20 kPa/20 kPa) were introduced. The reactor was heated to 1073 K under circulating the gas mixtures and maintained for 2 h. During the reaction, the aromatics were collected in a glass trap cooled with a dry ice/ethanol bath. After the reaction, the trapped aromatics were dissolved in acetonitrile and mass spectra of the aromatics were obtained with GC-MS (Bruker, SCION SQ) equipped with a capillary column (Bruker, BR-5 ms, 0.25 mm i.d., 30 m).Results and discussion
In this study, pyrolysis reactions of two different gas mixtures (i.e., CH4/C2H6 and Ar/C2H6) were carried out by controlling three reaction parameters: (1) reaction temperature, (2) gas flow rate, and (3) gas composition. Consequently, 55 experimental results were obtained and are summarized in Table 1. In these reactions, the pyrolysis products were hydrogen (H2), CH4, C2H4, acetylene (C2H2), propylene (C3H6), C3H8, benzene (C6H6), toluene (C7H8), styrene (C8H8), and naphthalene (C10H8). Coke was also produced depending on the reaction conditions. Note that CH4 conversions under CH4/C2H6 conditions were calculated based on the amount of CH4 in the inlet and the outlet gases, therefore, when CH4 was formed from C2H6 (eqn (1) and (2)), the amount of CH4 in the outlet gas increased so that the CH4 conversion became negative.| C2H6 → 2CH3˙ | (1) |
| CH3˙ + C2H6 → CH4 + C2H5˙ | (2) |
| Entry | Reaction temp./K | Reactant gas | Volume fraction of C2H6 | Flow rate/mL min−1 | Yield/μmol h−1 | Conv.b/% | Mass balance/% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CH4a | C2H6 | C2H4 | C2H2 | C3H8 | C3H6 | C6H6 | C7H8 | C8H8 | C10H8 | CH4 | C2H6 | ||||||
| a CH4 yields for CH4/C2H6 conditions contained not only formed CH4 but also feed CH4. b Average value in pyrolysis for 1 h. c Although C2H6 and C2H4 was formed by dehydrogenation reactions, H2 could not be detected because hydrocarbon was analysed by using GC-FID at the highest sensitivity but H2 was analysed by using GC-TCD. d The CH4 conversion was calculated on the basis of the yields of C2H6 and C2H4. | ||||||||||||||||||
| 1 | 973 | Ar/C2H6 | 0.5 | 10 | 5011 | 731 | 6978 | 4258 | 11 | 4 | 76 | 20 | 2 | 1 | 0.3 | n/a | 43.9 | 94.2 |
| 2 | 973 | CH4/C2H6 | 0.5 | 10 | 5138 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356 356 |
7444 | 4496 | 13 | 12 | 109 | 16 | 2 | 1 | 0.4 | −1.7 | 42.3 | 96.7 |
| 3 | 1023 | Ar/C2H6 | 0.5 | 10 | 7457 | 2010 | 3360 | 5418 | 28 | 7 | 121 | 182 | 14 | 14 | 12 | n/a | 73.1 | 85.1 |
| 4 | 1023 | CH4/C2H6 | 0.5 | 10 | 7900 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 746 746 |
3607 | 5668 | 29 | 16 | 181 | 202 | 15 | 12 | 12 | −20.1 | 72 | 93.2 |
| 5 | 1073 | Ar/C2H6 | 0.5 | 10 | 8764 | 5831 | 1238 | 3882 | 57 | 3 | 60 | 508 | 20 | 31 | 62 | n/a | 90.1 | 80.5 |
| 6 | 1073 | CH4/C2H6 | 0.5 | 10 | 9648 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610 610 |
1524 | 4204 | 54 | 7 | 109 | 522 | 30 | 34 | 58 | −44.7 | 88 | 89.8 |
| 7 | 973 | Ar/C2H6 | 0.25 | 10 | 2898 | 319 | 3346 | 2678 | 11 | 1 | 32 | 6 | 0.4 | n.d. | n.d. | n/a | 47.5 | 97.9 |
| 8 | 973 | CH4/C2H6 | 0.25 | 10 | 2710 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 680 |
3393 | 2481 | 12 | 8 | 63 | 5 | 0.4 | n.d. | n.d. | 2.1 | 46.8 | 96.0 |
| 9 | 1023 | Ar/C2H6 | 0.25 | 10 | 4494 | 1559 | 1157 | 3364 | 26 | 2 | 52 | 88 | 5 | 6 | 7 | n/a | 79.6 | 89.5 |
| 10 | 1023 | CH4/C2H6 | 0.25 | 10 | 4590 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 406 406 |
1485 | 3331 | 25 | 9 | 123 | 86 | 8 | 5 | 5 | −1.2 | 76.8 | 94.2 |
| 11 | 1073 | Ar/C2H6 | 0.25 | 10 | 5280 | 1819 | 311 | 2463 | 50 | 1 | 28 | 239 | 8 | 14 | 30 | n/a | 94.3 | 73.0 |
| 12 | 1073 | CH4/C2H6 | 0.25 | 10 | 6029 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 788 788 |
612 | 2672 | 47 | 4 | 90 | 251 | 17 | 15 | 24 | −8.4 | 90.4 | 92.7 |
| 13 | 973 | Ar/C2H6 | 0.17 | 10 | 1916 | 165 | 1953 | 1771 | 10 | 0.4 | 15 | 4 | 0.3 | n.d. | n.d. | n/a | 53.7 | 91.1 |
| 14 | 973 | CH4/C2H6 | 0.17 | 10 | 1873 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490 490 |
2259 | 1619 | 9 | 6 | 47 | 2 | 0.3 | n.d. | n.d. | 1.2 | 46.1 | 97.2 |
| 15 | 1023 | Ar/C2H6 | 0.17 | 10 | 3071 | 811 | 693 | 2238 | 24 | 1 | 25 | 60 | 2 | 2 | 4 | n/a | 83.5 | 85.3 |
| 16 | 1023 | CH4/C2H6 | 0.17 | 10 | 3404 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 104 104 |
947 | 2382 | 26 | 6 | 103 | 47 | 6 | 4 | 3 | −0.9 | 77.4 | 97.3 |
| 17 | 1073 | Ar/C2H6 | 0.17 | 10 | 3599 | 1571 | 209 | 1667 | 47 | 0.2 | 14 | 129 | 3 | 6 | 14 | n/a | 95 | 75.7 |
| 18 | 1073 | CH4/C2H6 | 0.17 | 10 | 4568 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 411 411 |
334 | 1984 | 48 | 3 | 81 | 154 | 12 | 9 | 16 | −2.2 | 92 | 94.4 |
| 19 | 973 | Ar/C2H6 | 0.5 | 30 | 9882 | 473 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 007 007 |
8797 | 25 | 2 | 68 | 1 | n.d. | n.d. | n.d. | n/a | 26.2 | 98.7 |
| 20 | 973 | CH4/C2H6 | 0.5 | 30 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149 149 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 915 915 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 705 705 |
8386 | 25 | 16 | 103 | 1 | n.d. | n.d. | n.d. | −1.5 | 27.3 | 97.9 |
| 21 | 1023 | Ar/C2H6 | 0.5 | 30 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 085 085 |
3170 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 316 316 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 459 459 |
80 | 17 | 332 | 132 | 5 | 3 | 3 | n/a | 61.7 | 96.0 |
| 22 | 1023 | CH4/C2H6 | 0.5 | 30 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 066 066 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 303 303 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 881 881 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 799 799 |
82 | 43 | 450 | 123 | 12 | 7 | 3 | −5.9 | 61.3 | 94.8 |
| 23 | 1073 | Ar/C2H6 | 0.5 | 30 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 284 284 |
7769 | 5463 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 287 287 |
209 | 13 | 314 | 756 | 17 | 24 | 34 | n/a | 84.9 | 81.9 |
| 24 | 1073 | CH4/C2H6 | 0.5 | 30 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 551 551 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 205 205 |
5678 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353 353 |
206 | 33 | 537 | 736 | 24 | 27 | 28 | −20.6 | 84.1 | 92.1 |
| 25 | 973 | Ar/C2H6 | 0.25 | 30 | 5687 | 227 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 796 796 |
5395 | 24 | 1 | 28 | n.d. | n.d. | n.d. | n.d. | n/a | 31 | 98.8 |
| 26 | 973 | CH4/C2H6 | 0.25 | 30 | 5152 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 286 286 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 357 357 |
4871 | 24 | 16 | 68 | n.d. | n.d. | n.d. | n.d. | 1.9 | 30.1 | 97.7 |
| 27 | 1023 | Ar/C2H6 | 0.25 | 30 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 473 473 |
1368 | 6223 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 941 941 |
74 | 5 | 133 | 68 | 7 | 5 | 2 | n/a | 65.7 | 101.4 |
| 28 | 1023 | CH4/C2H6 | 0.25 | 30 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 930 |
59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 433 433 |
6637 | 9604 | 64 | 29 | 299 | 46 | 6 | 3 | 0.8 | 1.3 | 65.1 | 95.0 |
| 29 | 1073 | Ar/C2H6 | 0.25 | 30 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 412 412 |
4165 | 2047 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 793 793 |
199 | 3 | 130 | 292 | 3 | 6 | 12 | n/a | 88.6 | 89.8 |
| 30 | 1073 | CH4/C2H6 | 0.25 | 30 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353 353 |
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 212 212 |
2355 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
185 | 18 | 385 | 252 | 7 | 7 | 19 | −3.3 | 87.5 | 93.2 |
| 31 | 973 | Ar/C2H6 | 0.17 | 30 | 3817 | 137 | 8570 | 3838 | 23 | 0.4 | 16 | n.d. | n.d. | n.d. | n.d. | n/a | 32.7 | 98.4 |
| 32 | 973 | CH4/C2H6 | 0.17 | 30 | 3214 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 877 877 |
9227 | 3024 | 22 | 11 | 47 | n.d. | n.d. | n.d. | n.d. | −0.4 | 27.8 | 99.4 |
| 33 | 1023 | Ar/C2H6 | 0.17 | 30 | 8998 | 1045 | 3574 | 7723 | 68 | 3 | 23 | 46 | 3 | 3 | 1 | n/a | 76.8 | 88.8 |
| 34 | 1023 | CH4/C2H6 | 0.17 | 30 | 9164 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 408 408 |
4536 | 7323 | 62 | 22 | 263 | 33 | 4 | 2 | 0.5 | −0.8 | 68.9 | 98.3 |
| 35 | 1073 | Ar/C2H6 | 0.17 | 30 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 468 468 |
2966 | 987 | 7602 | 185 | 1 | 19 | 202 | 2 | 4 | 15 | n/a | 99.1 | 77.5 |
| 36 | 1073 | CH4/C2H6 | 0.17 | 30 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015 015 |
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 175 175 |
876 | 8141 | 167 | 13 | 353 | 180 | 6 | 5 | 10 | −0.7 | 94.3 | 94.0 |
| 37 | 973 | Ar/C2H6 | 0.5 | 50 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 156 156 |
415 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 438 438 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 555 555 |
41 | 1 | 53 | n.d. | n.d. | n.d. | n.d. | n/a | 20.5 | 98.4 |
| 38 | 973 | CH4/C2H6 | 0.5 | 50 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 481 481 |
67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 289 289 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 763 763 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319 319 |
38 | 20 | 84 | n.d. | n.d. | n.d. | n.d. | −0.3 | 19.3 | 99.4 |
| 39 | 1023 | Ar/C2H6 | 0.5 | 50 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 276 276 |
2931 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434 434 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 272 272 |
112 | 20 | 412 | 49 | 3 | 1 | 0.6 | n/a | 53.3 | 94.9 |
| 40 | 1023 | CH4/C2H6 | 0.5 | 50 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106 106 |
69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 008 008 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123 123 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
112 | 70 | 596 | 49 | 2 | 1 | 0.6 | −2.8 | 53.4 | 96.0 |
| 41 | 1073 | Ar/C2H6 | 0.5 | 50 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 302 302 |
8525 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 065 065 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 253 253 |
391 | 25 | 599 | 611 | 10 | 12 | 20 | n/a | 82.3 | 83.6 |
| 42 | 1073 | CH4/C2H6 | 0.5 | 50 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 454 454 |
76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 148 148 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 998 998 |
390 | 64 | 972 | 594 | 14 | 14 | 20 | −13.6 | 80.7 | 92.4 |
| 43 | 973 | Ar/C2H6 | 0.25 | 50 | 7165 | 215 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 122 122 |
7180 | 37 | 0.5 | 25 | n.d. | n.d. | n.d. | n.d. | n/a | 23.7 | 98.5 |
| 44 | 973 | CH4/C2H6 | 0.25 | 50 | 6892 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 540 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 831 831 |
5894 | 37 | 20 | 61 | n.d. | n.d. | n.d. | n.d. | 1.1 | 24.1 | 97.1 |
| 45 | 1023 | Ar/C2H6 | 0.25 | 50 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 571 571 |
1796 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 678 678 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 951 951 |
101 | 7 | 171 | 37 | 2 | 2 | 0.5 | n/a | 61.5 | 94.0 |
| 46 | 1023 | CH4/C2H6 | 0.25 | 50 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 142 142 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 294 294 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487 487 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
85 | 46 | 333 | 20 | 2 | 1 | n.d. | −0.6 | 55.8 | 94.9 |
| 47 | 1073 | Ar/C2H6 | 0.25 | 50 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 145 145 |
5836 | 4560 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 518 518 |
315 | 7 | 239 | 278 | 2 | 4 | 11 | n/a | 86.1 | 86.6 |
| 48 | 1073 | CH4/C2H6 | 0.25 | 50 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 294 294 |
103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 283 283 |
5319 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925 925 |
312 | 39 | 722 | 231 | 5 | 4 | 7 | −5.4 | 83.3 | 98.0 |
| 49 | 973 | Ar/C2H6 | 0.17 | 50 | 4429 | 148 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111 111 |
4620 | 36 | 0.2 | 12 | n.d. | n.d. | n.d. | n.d. | n/a | 32.2 | 90.4 |
| 50 | 973 | CH4/C2H6 | 0.17 | 50 | 3599 | 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133 133 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 386 386 |
3948 | 34 | 15 | 95 | n.d. | n.d. | n.d. | n.d. | 2.7 | 23.7 | 96.4 |
| 51 | 1023 | Ar/C2H6 | 0.17 | 50 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 528 528 |
936 | 7243 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 989 989 |
87 | 3 | 81 | 23 | 0.7 | 1 | 0.3 | n/a | 65.2 | 91.0 |
| 52 | 1023 | CH4/C2H6 | 0.17 | 50 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 820 |
109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 066 066 |
9741 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 206 206 |
73 | 37 | 305 | 8 | 0.3 | n.d. | n.d. | −0.5 | 54.9 | 99.0 |
| 53 | 1073 | Ar/C2H6 | 0.17 | 50 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 047 047 |
3318 | 1941 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027 027 |
303 | 2 | 113 | 192 | 0.9 | 2 | 10 | n/a | 90.6 | 85.0 |
| 54 | 1073 | CH4/C2H6 | 0.17 | 50 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 563 563 |
109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 098 098 |
3235 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 611 611 |
266 | 24 | 595 | 125 | 3 | 2 | 4 | −0.7 | 85 | 96.2 |
| 55 | 1073 | CH4 | 0 | 10 | n.d.c | n/a | 3 | 1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.002d | n/a | 102.0 |
Before considering the main subject (i.e., activation of CH4 with the aid of C2H6), the basic effects of the reaction parameters on the pyrolysis behaviour will be considered.
Effect of reaction temperature
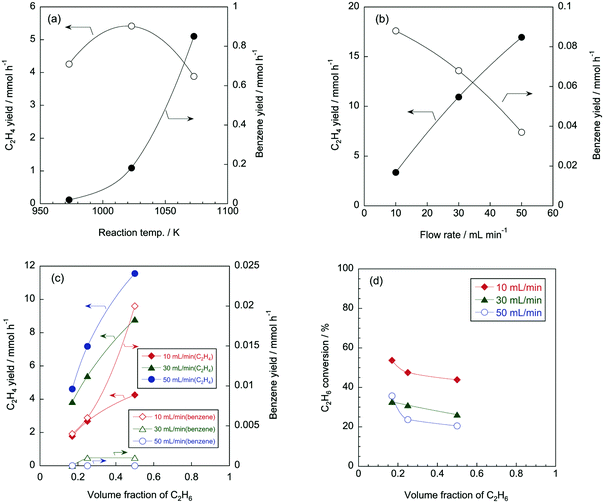
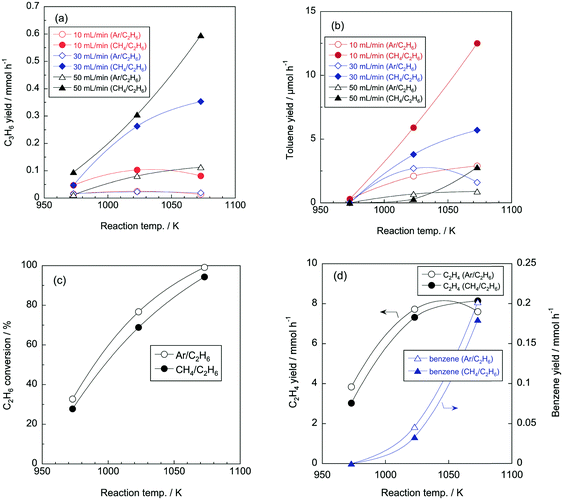
To discuss the effect of reaction temperature, we used several results of Ar/C2H6 conditions as examples (entries 1, 3, 5 in Table 1). C2H6 conversions increased to 44, 73, and 90% as reaction temperature increased to 973, 1023, and 1073 K, which is because the pyrolysis reactions are endothermic. Fig. 2a shows C2H4 and benzene yields at different reaction temperatures. Because C2H4 and benzene were the main products in the reaction, their yields may become higher with increasing C2H6 conversion with the reaction temperatures. While the benzene yield increased with the reaction temperatures, the C2H4 yield dropped at 1073 K. As for the formation of H2, dehydrogenation of C2H6 to C2H4 mainly contributed at 973 K. For example, in entry 1, the yields of C2H4 and H2 was 4258 and 5011 μmol h−1; the amounts C2H4 and H2 were similar. At higher temperature, the formation of benzene and coke also contributed to the formation of H2.According to previous studies on CH4 pyrolysis,24,25 benzene is formed by the recombination of propargyl radicals (C3H3˙), which are generated via H abstraction reactions of C2H6.
| C2H6 + H˙ → C2H5˙ + H2 | (3) |
| C2H5˙ → C2H4 + H˙ | (4) |
| C2H4 + H˙ → C2H3˙ + H2 | (5) |
| C2H3˙ → C2H2 + H˙ | (6) |
| C2H2 + CH3˙ → C3H4 + H˙ | (7) |
| C3H4 + H˙ → C3H3˙ + H2 | (8) |
| C3H3˙ + C3H3˙ → C6H6 | (9) |
In addition, there is another model that cyclopentadienyl radical is an important intermediate.26,27
Considering that C2H4 is the primary product in the pyrolysis of C2H6,28 C2H4 concentration should increase at high reaction temperatures because C2H6 conversion increased with the reaction temperature. Furthermore, as shown in eqn (3)–(9), benzene is formed via C2H4; therefore, it is likely that benzene formation is enhanced with increasing C2H4 concentration at high temperature. Consequently, the C2H4 yield was suppressed apparently by consuming C2H4 to form benzene. This is the reason why the C2H4 yield dropped at 1073 K.
Mass balance for carbon atoms was shown in Table 1. At 973 K, the mass balance was approx. 100%, while the mass valance decreased as increasing reaction temperature. This is because the formation of coke, indeed, the wall of the quartz reactor became black after the pyrolysis reaction at high temperature.
Effect of gas flow rate
The effect of gas flow rate on the pyrolysis of Ar/C2H6 was considered by using entries 9, 27, 45 in Table 1 as typical examples. As the flow rate of the reactant gas increased to 10, 30, and 50 mL min−1, C2H6 conversions decreased to 80, 66, and 62%, respectively. The high flow rate means a short residence time of reactant gases in the reactor, which should result in the decrease in C2H6 conversion. Yields of lower hydrocarbons such as C2 and C3 increased when the flow rate was high, and conversely, yields of aromatics decreased. As typical examples, the effect of the flow rate on yields of C2H4 and benzene is shown in Fig. 2b. With increasing gas flow rates, the C2H4 yields increased and the benzene yields decreased. As described above, benzene is formed via the recombination of propargyl radicals that are successively formed by H abstraction reactions of C2H6. Such a successive reaction is likely to be affected by the residence time of reactant gases; long residence time is favourable for the formation of benzene. Therefore, high flow rate, that is, short residence time, resulted in increasing yield of the intermediate product, namely C2H4.Effect of volume fraction of C2H6
The volume fraction of C2H6, that is the concentration of C2H6, is a reaction parameter in this study. As shown in Table 1, product yields became higher with higher C2H6 concentrations, and product distributions were almost the same regardless of the C2H6 concentration. Fig. 2c shows C2H4 and benzene yields at different volume fraction of C2H6 and flow rates. C2H4 and benzene yields tended to increase as a function of the volume fraction of C2H6. As described above, H abstraction from C2H6 provides C2H4 and benzene. Thus, it is reasonable that increase in volume fraction of C2H6 contributed to the formation of C2H4 and benzene. In addition, similar to Fig. 2b, as increasing flow rates, C2H4 yield increased and benzene yield decreased regardless of volume fraction of C2H6.Fig. 2d shows C2H6 conversion at different volume fractions of C2H6 in Ar. C2H6 conversion was in inverse proportion to the volume fraction of C2H6. The dominant reaction in the pyrolysis of C2H6 is the dehydrogenation of C2H6 into C2H4 (C2H6 → C2H4 + H2). The equilibrium conversions for the reaction are as follows: 71, 67, and 62% for the volume fractions of 0.17, 0.25, and 0.5, respectively. This order is in accordance with the tendency of C2H6 conversion shown in Fig. 2d.
Pyrolysis of CH4/C2H6 mixtures
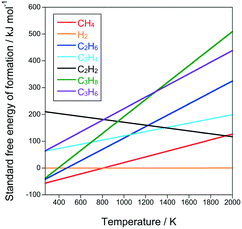
A CH4 molecule is so stable that CH4 alone did not react under the reaction conditions in this study. Even under the most severe condition (i.e., the highest temperature and the lowest flow rate), the CH4 conversion was approximately 0% (entry 55 in Table 1). Thermodynamic data also suggest that CH4 is much more stable than other hydrocarbons (Fig. 3). Thus, in this study, CH4 is expected to behave as an inert molecule, such as Ar and He. In other words, results in pyrolysis of CH4/C2H6 and Ar/C2H6 are assumed to be the same, however, their pyrolysis results were quite different. Fig. 4a shows C3H6 yields under CH4/C2H6 and Ar/C2H6 conditions at different temperatures and flow rates. Interestingly, the C3H6 yields were greatly increased in the presence of CH4. For example, under the condition at 1073 K and 50 mL min−1, C3H6 yields for Ar/C2H6 and CH4/C2H6 were 0.11 and 0.60 mmol h−1, respectively, indicating the co-existing CH4 promoted the C3H6 formation by more than five times. The same trend was observed for C3H8 formation.Fig. 4b shows the effect of co-existing CH4 on toluene yields. Similar to the C3 hydrocarbons, co-existing CH4 significantly enhanced toluene formation. For example, under the condition at 1073 K and 10 mL min−1, toluene yield increased by more than four times (from 2.9 to 12.5 μmol h−1).
From the perspective of the transition from petrochemical to natural gas chemistry, it is necessary to reconsider the production process of C3H6, which is an essential molecule in the chemical industry. In petrochemicals, C3H6 (and benzene, toluene, xylene) are obtained as by-products of naphtha cracking to produce C2H4. In contrast, C2H4 synthesis from C2H6 in natural gas produces poor by-products. In this regard, the enhancement of C3H6 formation by co-feed of CH4 to C2H6 would be worthwhile from the viewpoint of natural gas utilization.
Fig. 4c shows C2H6 conversion for CH4/C2H6 and Ar/C2H6 conditions. As described earlier, C2H6 conversion increases with reaction temperatures, and the co-existence of CH4 slightly suppressed the C2H6 conversion. Probably, thermal cracking of C2H6 to form CH4 would be inhibited by the presence of CH4, which can be a reason why C2H6 conversion slightly decreased under CH4/C2H6 conditions. In addition, the slight decrease in C2H6 conversion in the presence of CH4 indicates that the increase in C3 hydrocarbons and toluene is irrelevant to C2H6 conversion. Fig. 4d shows C2H4 and benzene yields for CH4/C2H6 and Ar/C2H6 conditions. As expected from Fig. 4c, C2H4 and benzene were less formed under CH4/C2H6 conditions because C2H6 conversion was suppressed in the presence of CH4. As described later, we considered that methyl radicals were generated from CH4 with the aid of radicals formed from C2H6. Fig. 4 shows that while C2H4 yield was hardly affected by CH4, the formation of C3 hydrocarbon was enhanced in the presence of CH4. From the results, we presumed that methyl radicals derived from CH4 promoted the recombination reaction of C2H3˙ (or C2H5˙) and CH3˙ to form C3 hydrocarbons.
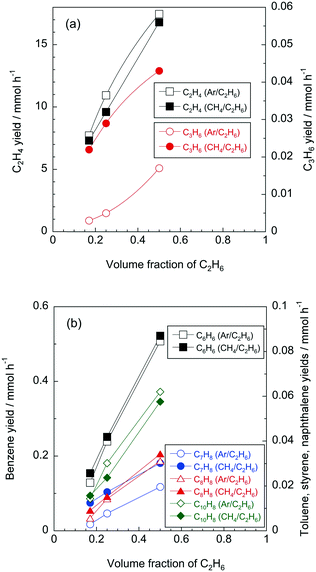
Fig. 5 shows the effect of volume fractions of C2H6 on yields of lower olefins. Regardless of reaction conditions, all the product yields became higher with increasing C2H6 concentration. Considering that products were mainly formed from the pyrolysis of C2H6, it is likely that the product yields were proportional to the concentration of C2H6. As shown in Fig. 5a, it appeared that the C2H4 yields slightly decreased with the presence of CH4. The co-existence of CH4 contributed to the decrease in C2H6 conversion (Fig. 4c), thus, it is possible that the yield of the main product (i.e., C2H4) decreased with the presence of CH4. On the other hand, the C3H6 yield was significantly increased by the co-existing CH4 regardless of the C2H6 concentration. This tendency is the same as shown in Fig. 4a. Fig. 5b shows yields of aromatics at various volume fractions of C2H6. Like the lower olefins, aromatics yields also increased with the concentration of C2H6. Comparing CH4/C2H6 and Ar/C2H6 conditions, we can see that there were no significant effects of CH4 on the benzene, styrene and naphthalene yields, while only the toluene yield increased with the CH4/C2H6 conditions, which is similar to the results shown in Fig. 4b.
Based on the above results, the effects of co-existing CH4 on the pyrolysis reactions are summarized as follows:
• C3H6, C3H8 and toluene yields increased significantly in the presence of CH4.
• C2H6 conversion was slightly lowered in the presence of CH4.
• The influence of co-existing CH4 on main products (i.e., C2H4 and benzene) yields was small.
Mass spectrometry analysis using 13C-labeled CH4
It is well-known that CH4 molecules are significantly stable because of both their structural symmetry and strong C–H bond.1 Indeed, pyrolysis of CH4 hardly took place when only CH4 was heated to 1073 K (entry 55 in Table 1). Thus, it is not surprising that CH4 molecules in CH4/C2H6 behaved the same as an inert gas, however, CH4/C2H6 showed different pyrolysis behaviour from Ar/C2H6. This result strongly suggests that CH4 molecules in C2H6 played a role in the pyrolysis reaction. In order to understand the role of CH4, pyrolysis using 13CH4 was carried out. If CH4 molecules were activated and converted into pyrolysis products, mass spectra of the pyrolysis products should be changed by incorporating 13C into them.Fig. 6 shows mass spectra of the aromatic compounds formed by pyrolysis of 13CH4/C2H6 or He/C2H6 at 1073 K (mass spectra of C2 and C3 hydrocarbons could not be obtained due to the limitation of separation in the GC-MS system). It was no wonder that typical mass spectra of benzene and toluene were observed under the He/C2H6 condition (Fig. 6a and b). On the other hand, it is interesting that mass spectra of benzene and toluene formed from 13CH4/C2H6 (Fig. 6c and d) were different from those of He/C2H6; benzene and toluene from 13CH4/C2H6 had higher m/z values. The m/z values at the highest peaks were 78 (benzene from He/C2H6), 80 (benzene from 13CH4/C2H6), 91 (toluene from He/C2H6) and 94 (toluene from 13CH4/C2H6). The increase in m/z of benzene and toluene in the presence of 13CH4 strongly suggested that 13C derived from CH4 was incorporated into benzene and toluene during the pyrolysis reactions.
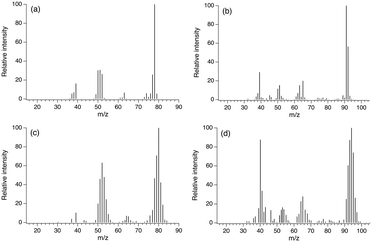 | ||
| Fig. 6 Mass spectra of (a and c) benzene and (b and d) toluene formed by dehydrogenation of (a and b) He/C2H6 and (c and d) 13CH4/C2H6 at 1073 K. | ||
Reaction mechanism
The mass spectra shown in Fig. 6 indicated that CH4 was incorporated into the products in the presence of C2H6 even though CH4 alone did not react, which implies CH4 molecules were activated with the aid of C2H6. Considering that no catalysts were used in this study, we assumed that radical species generated from C2H6 would play a role to activate the stable CH4 molecules.It is known that various radical species are formed from C2H6 at above 773–873 K.29 If the radicals abstract H˙ from CH4 molecules, CH4 molecules generate radical species (mainly methyl radicals):
| CH4 + H˙ → CH3˙ + H2 | (10) |
| CH4 + C2H5˙ → CH3˙ + C2H6 | (11) |
Consequently, the methyl radicals derived from CH4 molecules can convert into C3H6 and C3H8 by the following radical reactions:
| C2H3˙ + CH3˙ → C3H6 | (12) |
| C2H5˙ + CH3˙ → C3H8 | (13) |
In the above recombination reactions, CH4 molecules are incorporated into C3 hydrocarbons. For example, by combining eqn (5), (10), and (12), we know that a CH4 molecule is consumed to form a C3H6 molecule (CH4 + C2H4 + 2H˙ → C3H6 + 2H2).
Also, when the methyl radical reacts with a phenyl radical, toluene is formed:
| C6H5˙ + CH3˙ → C7H8 | (14) |
The significant increases in C3 hydrocarbons and toluene yields under CH4/C2H6 conditions can be explained by the above mechanism; the increase in the concentration of methyl radicals promoted the formation of C3 hydrocarbons and toluene. As shown in the mass spectra, CH4-derived carbon was also incorporated into benzene, which can be explained by the recombination of propargyl radicals that is formed from 13CH3˙ and C2H2 (eqn (7)–(9)).
Current strategies for CH4 activation can roughly be categorized into (1) the development of catalysts that efficiently activate C–H bonds in CH4 and (2) pyrolysis of CH4, where coupling of methyl radicals proceeds with the aid of extreme high temperature (>approx. 1473 K). In this study, an alternative route for the chemical conversion of CH4 was proposed: radicals generated from co-existing molecules (in this study, C2H6) activate CH4 molecules.
Conclusions
Pyrolysis reactions of mixtures of CH4/C2H6 or Ar/C2H6 were carried out at 973–1073 K. Even though CH4 alone did not react in the temperature range, the pyrolysis behaviour was quite different in the presence of CH4; the formation of C3 hydrocarbons and toluene was enhanced. In contrast, C2H4 and benzene yields were not affected by the co-existing CH4. Mass spectrometry analysis using 13C-labeled CH4 revealed that CH4-derived carbon was incorporated into the pyrolysis products, indicating CH4 molecules were activated and played a role in the pyrolysis reactions. Probably, radicals generated from C2H6 abstracted H˙ from CH4 so that CH4-derived methyl radicals would be formed. By reacting the methyl radicals with C2H3˙ and C6H5˙, C3H6 and toluene were formed. In other words, the increase in the concentration of methyl radicals enhanced the formation of C3 hydrocarbons and toluene. This study clarified that CH4 can be activated by radicals generated from co-existing molecules without the help of catalysts or extreme high temperature.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the technology development project carried out in Japan Petroleum Energy Center (JPEC) under the commission of the Ministry of Economy, Trade and Industry (METI) and also by JST CREST, Grant Number JPMJCR15P4. We appreciate technical support of Mr. Ohshima (Technical Support Center, Saitama University) for pyrolysis reactions on the closed gas-circulation system and of Mr. Niimi (Comprehensive Analysis Center for Science, Saitama University) for GC-MS analyses.Notes and references
- U. P. M. Ashik, W. M. A. W. Daud and H. F. Abbas, Renewable Sustainable Energy Rev., 2015, 44, 221–256 CrossRef CAS.
- K. Otsuka, K. Jinno and A. Morikawa, Chem. Lett., 1985, 499–500 CrossRef CAS.
- T. Ito and J. H. Lunsford, Nature, 1985, 314, 721–722 CrossRef CAS.
- G. E. Keller and M. M. Bhasin, J. Catal., 1982, 73, 9–19 CrossRef CAS.
- X. G. Guo, G. Z. Fang, G. Li, H. Ma, H. J. Fan, L. Yu, C. Ma, X. Wu, D. H. Deng, M. M. Wei, D. L. Tan, R. Si, S. Zhang, J. Q. Li, L. T. Sun, Z. C. Tang, X. L. Pan and X. H. Bao, Science, 2014, 344, 616–619 CrossRef CAS PubMed.
- Y. Nishikawa, H. Ogihara and I. Yamanaka, ChemistrySelect, 2017, 2, 4572–4576 CrossRef CAS.
- A. Galadima and O. Muraza, J. Ind. Eng. Chem., 2016, 37, 1–13 CrossRef CAS.
- J. J. Spivey and G. Hutchings, Chem. Soc. Rev., 2014, 43, 792–803 RSC.
- L. S. Wang, L. X. Tao, M. S. Xie, G. F. Xu, J. S. Huang and Y. D. Xu, Catal. Lett., 1993, 21, 35–41 CrossRef CAS.
- B. G. Hashiguchi, M. M. Konnick, S. M. Bischof, S. J. Gustafson, D. Devarajan, N. Gunsalus, D. H. Ess and R. A. Periana, Science, 2014, 343, 1232–1237 CrossRef CAS PubMed.
- P. M. Bijani, M. Sohrabi and S. Sahebdelfar, Ind. Eng. Chem. Res., 2014, 53, 572–581 CrossRef CAS.
- M. C. J. Bradford, M. Te, M. Konduru and D. X. Fuentes, Appl. Catal., A, 2004, 266, 55–66 CrossRef CAS.
- V. R. Choudhary, A. K. Kinage and T. V. Choudhary, Science, 1997, 275, 1286–1288 CrossRef CAS PubMed.
- J. J. Guo, H. Lou and X. M. Zheng, J. Nat. Gas Chem., 2009, 18, 260–272 CrossRef CAS.
- J. H. Lunsford, P. Qiu, M. P. Rosynek and Z. Q. Yu, J. Phys. Chem. B, 1998, 102, 167–173 CrossRef CAS.
- F. Billaud, F. Baronnet, E. Freund, C. Busson and J. Weill, Rev. Inst. Fr. Pet., 1989, 44, 813–823 CrossRef CAS.
- O. Olsvik and F. Billaud, Thermochim. Acta, 1994, 232, 155–169 CrossRef CAS.
- A. Holmen, O. Olsvik and O. A. Rokstad, Fuel Process. Technol., 1995, 42, 249–267 CrossRef CAS.
- O. Olsvik, O. A. Rokstad and A. Holmen, Chem. Eng. Technol., 1995, 18, 349–358 CrossRef CAS.
- G. Fau, N. Gascoin, P. Gillard and J. Steelant, J. Anal. Appl. Pyrolysis, 2013, 104, 1–9 CrossRef CAS.
- K. Takanabe and E. Iglesia, Angew. Chem., Int. Ed., 2008, 47, 7689–7693 CrossRef CAS PubMed.
- K. Takanabe, A. M. Khan, Y. Tang, L. Nguyen, A. Ziani, B. W. Jacobs, A. M. Elbaz, S. M. Sarathy and F. Tao, Angew. Chem., Int. Ed., 2017, 56, 10403–10407 CrossRef CAS PubMed.
- Y. X. Zhao, Z. Y. Li, Y. Yang and S. G. He, Acc. Chem. Res., 2018, 51, 2603–2610 CrossRef CAS PubMed.
- C. Keramiotis, G. Vourliotakis, G. Skevis, M. A. Founti, C. Esarte, N. E. Sanchez, A. Millera, R. Bilbao and M. U. Alzueta, Energy, 2012, 43, 103–110 CrossRef CAS.
- J. A. Miller and S. J. Klippenstein, J. Phys. Chem. A, 2003, 107, 7783–7799 CrossRef CAS.
- A. M. Dean, J. Phys. Chem., 1990, 94, 1432–1439 CrossRef CAS.
- D. M. Matheu, A. M. Dean, J. M. Grenda and W. H. Green, J. Phys. Chem. A, 2003, 107, 8552–8565 CrossRef CAS.
- G. F. Glasier and P. D. Pacey, Carbon, 2001, 39, 15–23 CrossRef CAS.
- K. M. Sundaram and G. F. Froment, Ind. Eng. Chem. Fundam., 1978, 17, 174–182 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |