Biomass-derived carbon-supported Ni catalyst: an effective heterogeneous non-noble metal catalyst for the hydrogenation of nitro compounds
Qingjie
Tang
,
Ziliang
Yuan
,
Shiwei
Jin
*,
Kaiyue
Yao
,
Hanmin
Yang
 *,
Quan
Chi
and
Bing
Liu
*,
Quan
Chi
and
Bing
Liu
 *
*
Key Laboratory of Catalysis and Materials Sciences of the Ministry of Education, South-Central University for Nationalities, Wuhan, 430074, People's Republic of China. E-mail: jinsw@mail.scuec.edu.cn; yhm20181011@163.com; liubing@mail.scuec.edu.cn; Fax: +86 27 67842572; Tel: +86 27 67842572
First published on 7th November 2019
Abstract
There has been a great deal of attention to the development of heterogeneous non-noble metal catalysts for the selective and mild hydrogenation of nitro compounds into primary amines. Herein, a biomass-derived carbon material supported Ni catalyst (Ni/C) was facilely prepared by a one-pot pyrolysis process, and the as-prepared Ni/C catalyst demonstrated a high catalytic activity for the hydrogenation of nitro compounds into primary amines at room temperature. The Ni/C catalyst not only demonstrated a high catalytic activity but also showed a good tolerance to other functional groups. Structurally diverse primary amines were achieved in yields from 92% to 99% within a few hours at room temperature under 5 bar H2. Furthermore, the Ni/C catalyst showed good reusability without the loss of its activity.
Introduction
Amines are very important industrial intermediates which have been widely used as the building blocks for pharmaceuticals, agrochemicals, pigments, dyes and fine chemicals.1 Therefore, there has been a growing interest in the synthesis of amines in recent years.2,3 Nitro compounds are readily available with good stability, which serve as the starting materials for the synthesis of primary amines.4 Traditionally, the synthesis of primary amines via the reduction of nitro compounds was conducted by the use of stoichiometric reducing agents, such as sulfides, Fe, and Zn. The use of stoichiometric reducing agents resulted in a high cost towards the reduction of nitro compounds into primary amines. In addition, these methods also released lots of waste acids and residues. Therefore, catalytic hydrogenation of nitro compounds represents a green and sustainable method for the synthesis of primary amines.5–8In recent years, both H2 and other hydrogen donors such as isopropanol and formic acid have been extensively studied for the hydrogenation of nitro compounds into primary amines over different kinds of catalysts.9,10 H2 has been considered as an economic and sustainable reducing agent with only the release of water. Catalytic hydrogenation of nitro compounds into primary amines with H2 has been performed by either homogeneous or heterogeneous catalytic systems. The use of homogenous catalytic systems generally demonstrated high catalytic activity for the hydrogenation of nitro compounds because of the flexible contact of the active sites with the substrates.11 However, it is difficult to recycle the homogeneous catalysts. Heterogeneous catalytic systems can overcome the drawbacks of homogeneous catalytic systems and thus have attracted much more interest for the development of effective heterogeneous catalytic systems for the hydrogenation of nitro compounds into primary amines.
Heterogeneous noble metal catalysts such as supported Pd, Au, and Ru have been early used in the hydrogenation of nitroarenes to synthesize functionalized anilines due to their extraordinary activities.12,13 However, the high cost of noble metal catalysts greatly limits their practical applications. In addition, it is a great challenge in the chemoselective reduction of nitroarenes bearing reducible groups (C–Br, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and so on).14
C, and so on).14
In recent years, many non-noble metal catalysts, mainly including Co, Ni and Fe, have been studied for the hydrogenation of nitro compounds into primary amines.15–18 Of various inexpensive catalysts, supported Ni catalysts have attracted substantial interest for catalytic hydrogenation reactions due to the excellent catalytic performance of Ni nanoparticles.19,20 Among various kinds of supported Ni catalysts, carbon material supported Ni catalysts have been widely studied because carbon materials demonstrated some excellent merits such as high surface area, unique electronic properties, and high chemical, thermal, and mechanical stability.21 However, the synthesis of most carbon-based catalysts typically forgoes environmentally benign approaches in favor of complex and expensive procedures that include acid oxidation, washing, impregnation, and reduction. The high cost of many novel carbon nanomaterials (such as graphene and carbon nanotubes) makes their large-scale use less practical. From the viewpoints of green and sustainable chemistry, it is highly desirable to prepare Ni/C catalysts with high catalytic activity for the hydrogenation of nitro compounds into primary amines via facile and green strategies from renewable resources.
Biomass is an abundant renewable resource which has attracted great interest for the preparation of various kinds of carbon-based materials.22,23 Among various kinds of biomass, cotton is mainly composed of cellulose fibers with abundance in nature and a low cost, which can serve as a good candidate for the preparation of carbon materials.24 Herein, one kind of Ni/C catalyst was successfully prepared via the one-pot pyrolysis of a cotton–Ni(NO3)2 composite and used for the hydrogenation of nitro compounds towards the chemoselective synthesis of structurally diverse amines.
Experimental
Materials
All of the chemicals were purchased from Aladdin Chemicals Co. Ltd. (Beijing, China). All of the solvents were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).Catalyst preparation
Medical absorbent cotton was adopted as the raw material and used without further purification. Briefly, 0.5 g of cotton was ultrasonically dispersed in 0.1 M Ni(NO3)2 solution (10 mL) for 10 minutes to remove adhered air bubbles, and then the mixture was kept still at room temperature for 24 h. Then the cotton–Ni(NO3)2 composite was removed and dried at 80 °C for another 24 h. The dried sample was loaded into a tube furnace and heated at 400 °C in a H2 atmosphere for 3 h at a heating rate of 2 °C min−1. The final black product was cooled to room temperature under a hydrogen atmosphere. The as-prepared catalyst was denoted as Ni/C catalyst.Catalyst characterization
Transmission electron microscopy (TEM) was performed on an FEI Tecnai G2-20 instrument. X-ray powder diffraction (XRD) measurements were conducted on a Bruker Advanced D8 powder diffractometer (Cu Kα) operating in the 2θ range of 10–80° at a scanning rate of 0.016° s−1. X-ray photoelectron spectroscopy (XPS) experiments were carried out on a Thermo VG Scientific ESCA MultiLab-2000 spectrometer with a monochromatized Al Kα source (1486.6 eV) at a constant analyzer pass energy of 25 eV. The nickel content was determined by inductively coupled plasma atomic emission spectrometry (ICP-AES) on an IRIS Intrepid II XSP instrument (Thermo Electron Corporation). Nitrogen physisorption measurements were conducted at 77 K on a Quantachrome Autosorb-1-C-MS instrument. The surface area was determined by the standard BET method based on the relative pressure between 0.05 and 0.20. The pore size distribution was calculated using the non-local density functional theory method.General procedure of the hydrogenation of nitro compounds
The catalytic hydrogenation was carried out in a 40 mL stainless steel reactor. Typically, nitrobenzene (1 mmol), water (10 mL), the Ni/C catalyst (20 mg) and a magnetic bar were charged into the reactor. The air in the reactor was removed by flushing with H2 five times; then the reactor was charged with 5 bar H2, and finally the reactor was sealed. Then the reaction was started at room temperature with magnetic stirring at 800 RPM for 4 h. After the reaction was complete, the Ni/C catalyst was collected using an external magnet, and the clear liquid solution was extracted with dichloromethane. Ethyl benzene was used as the internal standard, and the liquid mixture was analyzed by gas chromatography.Analytical methods
Product analysis was carried out on an Agilent 7890A gas chromatography (GC) instrument, which was equipped with a crosslinked capillary HP-5 column (30 m × 0.32 mm × 0.4 mm) and a flame ionization detector. The analytical conditions were as follows: the flow rate of the N2 carrier gas was 40 mL min−1, and the injection port temperature was 300 °C. The GC oven temperature program was conducted as follows: the temperature program ranges from 40 °C to 280 °C at a heating rate of 10 °C min−1 and then held at 280 °C for 30 min. The detector temperature was set to 300 °C. The content of each compound was determined based on the internal standard.Catalyst recycling experiments
After the reaction, the Ni/C catalyst was collected via centrifugation, and the spent catalyst was washed with water and ethanol successively five times. Then it was dried in a vacuum oven overnight at 40 °C, and the spent catalyst was used for the next run under the same reaction conditions. Other cycles were repeated using the same procedures.Results and discussion
Catalyst preparation and characterization
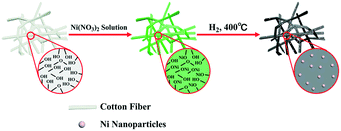
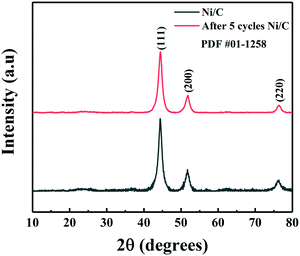
Due to the presence of abundant oxygen-containing groups in cotton, it has a good ability to absorb Ni(NO3)2. The procedure of the preparation of the Ni/C catalyst is shown in Scheme 1. The preparative process is a simple, green, and low-cost strategy, which involves two steps. Firstly, Ni(NO3)2 was absorbed onto the surface of cotton at room temperature to generate the cotton–Ni(NO3)2 composite. Then the cotton–Ni(NO3)2 composite was calcined at 400 °C under a H2 atmosphere to generate the Ni/C catalyst. The Ni content in the Ni/C catalyst was determined to be 42 wt% by ICP-AES. In order to further investigate the stability of the catalyst in the reaction system, we measured the solution after the reaction by ICP-AES and found no Ni element, indicating that our catalyst has good stability.The XRD patterns of the as-obtained Ni/C catalyst are shown in Fig. 1. Three peaks at 44.4°, 51.9° and 76.5° were clearly observed in the XRD patterns of the Ni/C catalyst, which corresponded to crystalline facets of Ni (111), Ni (200), and Ni (220), respectively.20 From Fig. 1, we can see that the XRD pattern of the catalyst after 5 reaction cycles is almost the same, indicating that our catalyst has good stability. Therefore, we can conclude that the Ni2+ absorbed on cotton has been successfully reduced to metallic Ni by H2 at 400 °C. Generally, the XRD peak at 26.0° was assigned to graphitic carbon. The disappearance of this peak in the XRD pattern of our prepared Ni/C catalyst suggested that the carbon in the Ni/C catalyst might be amorphous.
The TEM image of the as-prepared Ni/C catalyst is shown in Fig. 2. It clearly shows that Ni nanoparticles were homogeneously distributed on the surface of the carbon materials, and no significant aggregation of Ni nanoparticles was observed before and after the reaction. These results revealed that Ni nanoparticles would have a strong interaction with the carbon materials, which was unlike the traditional impregnation method. The particle size distribution of Ni nanoparticles in Fig. 2 clearly indicated that nickel nanoparticles have a narrow size distribution, and the average size of nickel nanoparticles was calculated to be 9.5 nm. Moreover, the high-resolution TEM (HRTEM, Fig. 2c) image revealed that the lattice fringe of the Ni nanoparticles had an interplanar spacing of 0.205 nm, which is ascribed to the (111) plane of metallic Ni nanoparticles.
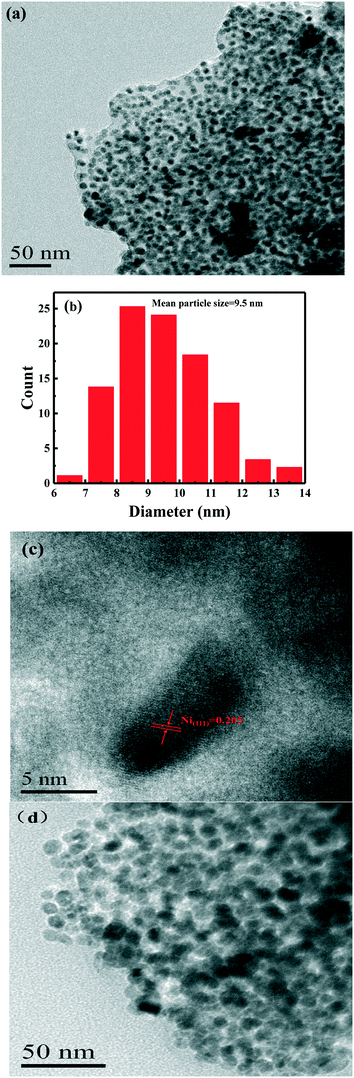 | ||
| Fig. 2 TEM image (a), particle size distribution image (b), (c) HRTEM image of the Ni/C catalyst and (d) TEM image after reaction. | ||
XPS was used to examine the oxidation states of elements on the surface of the Ni/C catalyst. The XPS survey spectrum confirms the presence of mainly Ni, C, N, and O in the catalyst (Fig. 3). As shown in Fig. 3, the C 1s XPS spectra of the Ni/C catalyst show one main peak at 284.6 eV, corresponding to sp2-hybridized graphitic carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). In addition, two other weak peaks at the binding energies of 286.1 eV and 288.9 eV should be attributed to C–O and O–C
C). In addition, two other weak peaks at the binding energies of 286.1 eV and 288.9 eV should be attributed to C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species, respectively.25 As shown in Fig. 3, the two fitted peaks at ∼852.7 and ∼869.6 eV were assigned to the binding energies of metallic Ni 2p3/2 and Ni 2p1/2, respectively. The fitted Ni 2p3/2 peak at 855.6 and the Ni 2p1/2 peak at 873.3 eV can be assigned to the divalent valence state Ni2+,20 which was due to the surface oxidation of metallic Ni nanoparticles during storage in the air. Obviously, most of the nickel nanoparticles were present in their oxidized state as detected by XPS, which revealed that the metallic nickel on the surface of nickel nanoparticles was majorly oxidized into its oxidized state either by the treatment with 1% O2 in the nitrogen atmosphere or by the air during the storage.
O species, respectively.25 As shown in Fig. 3, the two fitted peaks at ∼852.7 and ∼869.6 eV were assigned to the binding energies of metallic Ni 2p3/2 and Ni 2p1/2, respectively. The fitted Ni 2p3/2 peak at 855.6 and the Ni 2p1/2 peak at 873.3 eV can be assigned to the divalent valence state Ni2+,20 which was due to the surface oxidation of metallic Ni nanoparticles during storage in the air. Obviously, most of the nickel nanoparticles were present in their oxidized state as detected by XPS, which revealed that the metallic nickel on the surface of nickel nanoparticles was majorly oxidized into its oxidized state either by the treatment with 1% O2 in the nitrogen atmosphere or by the air during the storage.
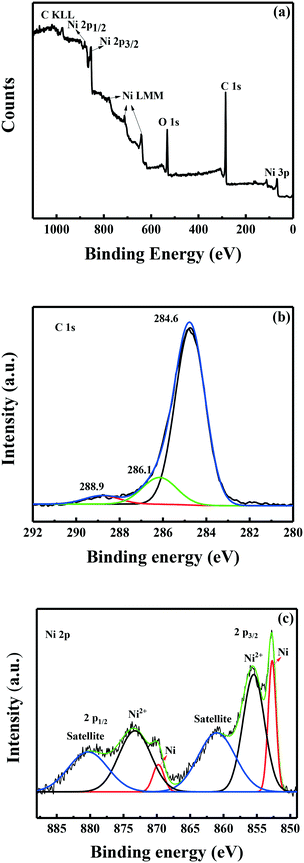 | ||
| Fig. 3 XPS spectra of the Ni/C catalyst. (a) The survey spectrum; (b) the C 1s XPS spectrum; (c) the Ni 2p XPS spectrum. | ||
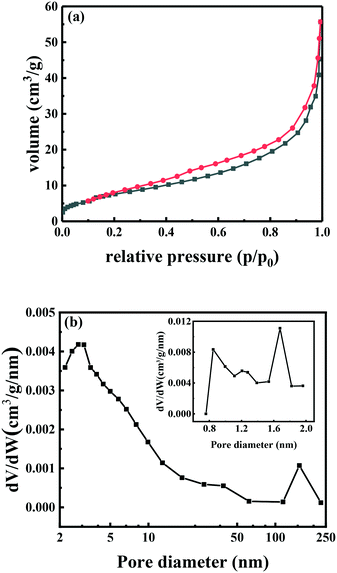
N2 adsorption–desorption of the Ni/C catalyst shows an H1 type hysteresis loop and a type IV isotherm (Fig. 4a), which is a characteristic isotherm for mesoporous materials. However, the sorption isotherm does not level out in a plateau at relative pressures p/p0 > 0.9, indicating the existence of macropores. The pore size distribution in Fig. 4b also shows that three types of macropores, mesopores and micropores were present in the Ni/C catalyst. In addition, all of the macropores, mesopores and micropores had a large size distribution. The total surface area of the Ni/C catalyst was determined to be 41.8 m2 g−1, and the total pore volume was calculated to be 0.09 cm3 g−1.
Catalytic hydrogenation of nitrobenzene
The catalytic activity of the as-prepared Ni/C catalyst was evaluated by the hydrogenation of nitrobenzene. Firstly, the effect of the reaction solvents on the hydrogenation of nitrobenzene was studied, which was performed at 40 °C and 10 bar H2. As shown in Table 1, the reaction solvents showed a great influence on nitrobenzene conversion as well as the product distribution. Generally, the hydrogenation activity of nitrobenzene exhibits a positive relation with the solvent polarity. The hydrogenation of nitrobenzene in non-polar solvents such as hexane and dichloromethane produced low nitrobenzene conversions (Table 1, entries 1 and 2). The poor catalytic performance of the Ni/C catalyst in the non-polar solvents should be caused by its poor dispersion in these non-polar solvents, and thus contact between the substrates and the active sites of nickel nanoparticles became much more difficult. As expected, moderate to quantitative conversions of nitrobenzene were attained in the polar solvents (Table 1, entries 3–8), and the nitrobenzene conversion seems to increase with an increase of the solvent polarity. The conversion of nitrobenzene in ethanol was 70.0% after 4 h and it further increased to 90.2% and 100% in methanol and water, respectively (Table 1, entries 5–8). Interestingly, the product distribution was also found to be affected by the reaction solvents. Phenylhydroxylamine was found to be the intermediate of the hydrogenation of nitrobenzene into aniline. Interestingly, it was found to be present in the oxygen-containing polar solvents, and the possible reason should be that these oxygen-containing polar solvents have the ability to stabilize the intermediate phenylhydroxylamine via the hydrogen bonds.| Entry | Solvent | Con. (%) | Selectivity (%) | |
|---|---|---|---|---|
| 1 | 2 | |||
| a Reaction conditions: nitrobenzene (1 mmol), Ni/C catalyst (20 mg), solvent (10 mL), H2 (10 bar), 40 °C and 4 h. | ||||
| 1 | Hexane | 29.2 | 71.2 | 28.8 |
| 2 | Dichloromethane | 26.5 | 100 | 0 |
| 3 | THF | 50.0 | 70.7 | 29.3 |
| 4 | iso-PrOH | 53.9 | 74.2 | 25.8 |
| 5 | Acetonitrile | 64.5 | 100 | 0 |
| 6 | EtOH | 70.0 | 88.7 | 11.3 |
| 7 | MeOH | 90.2 | 82.4 | 17.6 |
| 8 | H2O | 100 | 100 | 0 |
The best results were achieved in water, which produced the quantitative conversion of nitrobenzene into aniline at 40 °C after 4 h (Table 1, entry 8). The use of water as the reaction solvent is much more preferable because water is a green solvent at a low cost without any toxicity. To the best of our knowledge, there has been no report on the use of supported Ni catalysts for the hydrogenation of nitro compounds under such mild conditions. In recent years, single metal atom catalysts have aroused great interest for chemical reactions because of their high activity, but the recently reported single Ni atom catalyst for the hydrogenation of nitro compounds was carried out at 120 °C and 3 MPa H2 pressure.6
The effect of the reaction temperature on the hydrogenation of nitrobenzene was studied over the Ni/C catalyst, and the results are shown in Fig. 5. It was found that the Ni/C catalyst demonstrated excellent catalytic activity even at room temperature, and a moderate nitrobenzene conversion of 54.4% was achieved at room temperature after 2 h. The hydrogenation of nitrobenzene was sensitive to the reaction temperature. Nitrobenzene conversion greatly increased to 78.6% by the increase of the reaction temperature to 40 °C, and a full nitrobenzene conversion was achieved at 60 °C after 2 h. The selectivity of the intermediate phenylhydroxylamine decreased from 55.6% at 25 °C to 12.2% at 40 °C, and it was not observed at 60 °C. Generally, the intermediates of the hydrogenation of nitro compounds were not stable and could not be detected by gas chromatography at high reaction temperatures. Therefore, few literature studies have reported on the intermediates of the hydrogenation of nitro compounds over supported metal catalysts at high reaction temperatures.
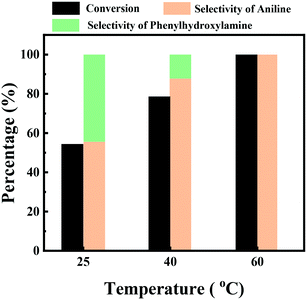 | ||
| Fig. 5 The effect of the reaction temperature on the hydrogenation of nitrobenzene. Reaction conditions: nitrobenzene (1 mmol), Ni/C catalyst (20 mg), water (10 mL), H2 (10 bar), 25–60 °C and 2 h. | ||
Then the effect of the hydrogen pressure on the hydrogenation of nitrobenzene over the Ni/C catalyst was studied at room temperature, and the results are shown in Fig. 6. The conversion of nitrobenzene was observed to increase with an increase of hydrogen pressure. For example, the conversion of nitrobenzene was 41.5% under 2.5 bar H2 pressure, and then it increased to 76.3% under 5 bar H2 pressure. On further increasing the hydrogen pressure to 10 bar, the nitrobenzene conversion continuously increased to 90.0%. The increase of nitrobenzene conversion with an increase of hydrogen pressure should be caused by the increase of hydrogen concentration in the reaction solution with an increase of hydrogen pressure. Aniline and the intermediate phenylhydroxylamine were produced at 25 °C under different hydrogen pressure, and these results again suggested that the stability of the phenylhydroxylamine intermediate was sensitive to the reaction temperature, not hydrogen pressure.
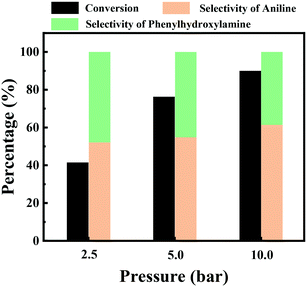 | ||
| Fig. 6 The effect of hydrogen pressure on the hydrogenation of nitrobenzene. Reaction conditions: nitrobenzene (1 mmol), Ni/C catalyst (20 mg), water (10 mL), H2 (2.5–10 bar), 25 °C and 4 h. | ||
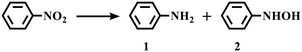
The time course of the product distribution was recorded for the hydrogenation of nitrobenzene at 25 °C and 5 bar H2 over the Ni/C catalyst (Fig. 7). The conversion of nitrobenzene gradually increased during the reaction process, and its content greatly decreased at an early reaction stage, which was due to the high concentration of nitrobenzene at the early reaction stage. The yield of aniline also gradually increased during the reaction process, and it reached 100% after 7 h at 5 bar H2 pressure and 25 °C. The content of phenylhydroxylamine first increased to 40.8% from the beginning to 5 h and then it decreased from 40.8% at 5 h to zero after 7 h. These results suggested that the hydrogenation of nitrobenzene into phenylhydroxylamine was a slow step. Generally, two reaction pathways are accepted for the hydrogenation of nitrobenzene into aniline.26 One way involves phenylhydroxylamine as the intermediate, which is called the direct pathway (Scheme 2). The other way proceeds via the azoxybenzene intermediate. Clearly, the hydrogenation of nitrobenzene into aniline proceeds via the direct way over the Ni/C catalyst (Fig. 8).
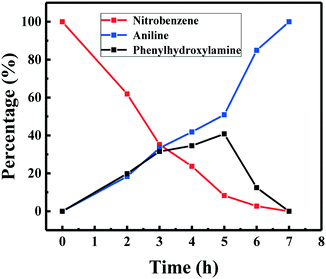 | ||
| Fig. 7 Time course of the product distribution on the hydrogenation of nitrobenzene. Reaction conditions: nitrobenzene (1 mmol), Ni/C catalyst (20 mg), water (10 mL), H2 (5 bar), 25 °C. | ||
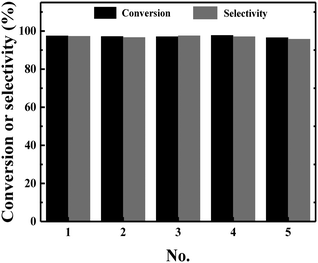 | ||
| Fig. 8 Catalyst recycling experiments of the Ni/C catalyst. Reaction conditions: nitrobenzene (1 mmol), Ni/C catalyst (20 mg), water (10 mL), H2 (5 bar), 25 °C, 6 h. | ||
Substrate scope
The scope of the developed method was studied for the hydrogenation of various nitroarenes over the Ni/C catalyst at room temperature and 5 bar H2 pressure. As shown in Table 2, different kinds of nitro compounds including aromatic and aliphatic nitro compounds were successfully converted into the primary amines with excellent yields. Substrates with electron-donating groups demonstrated a slightly higher catalytic activity than substrates with electron-withdrawing groups (Table 2, entries 4–6 vs. 7–11). Halogen-substituted nitroarenes were smoothly converted into the corresponding amines without dehalogenation. In addition, the substrates bearing other functional groups such as hydroxyl, nitrile and carbonyl groups were also successfully converted into the corresponding amines without the reduction of these reducible groups (Table 2, entries 6, 10 and 11). Furthermore, the Ni/C catalyst was also active for the hydrogenation of aliphatic nitro compounds to aliphatic amines under mild conditions. All of these results revealed that the Ni/C catalyst exhibits high activity for the selective hydrogenation of nitro compounds. In order to further study its application, we increased the substrate to 10 mmol. Under the conditions of 100 mg of catalyst, 20 bar H2, 24 h, and water as solvent, the conversion was 83.3% and the yield of aniline was 80.6%.| Entry | Structure | Time (h) | Con. (%) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: nitrobenzene (1 mmol), Ni/C catalyst (20 mg), water (10 mL), H2 (5 bar), 25 °C. b Reaction conditions: solvent (water 7 ml, methanol 3 ml). | ||||
| 1a |
|
7 | 100 | 100 |
| 2a |
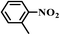
|
8.5 | 100 | 99 |
| 3a |
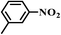
|
7 | 100 | 97 |
| 4a |
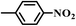
|
6.5 | 100 | 94 |
| 5b |
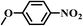
|
5.5 | 100 | 96 |
| 6b |
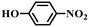
|
6.5 | 100 | 99 |
| 7a |
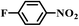
|
7.5 | 100 | 99 |
| 8b |
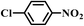
|
7.5 | 100 | 98 |
| 9b |

|
8 | 100 | 92 |
| 10b |
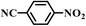
|
7.5 | 98 | 96 |
| 11b |
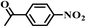
|
7.5 | 98.6 | 97 |
| 12a |
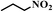
|
6 | 100 | 99 |
Catalyst recycling experiment
The distinct advantage of heterogeneous catalysts over homogeneous catalysts is that the heterogeneous catalysts can be facilely recycled and reused. Therefore, the recycling experiments of the Ni/C catalyst were investigated by the hydrogenation of nitrobenzene at room temperature under 5 bar H2 pressure. After the first run, the Ni/C catalyst was collected using an external magnet and washed with water and ethanol, respectively. The spent catalyst was dried in a vacuum oven and then used for the next run under the same conditions. Other cycles were repeated using the same procedures. It was noted that the conversion of nitrobenzene as well as the selectivity of aniline remained stable during the five runs. These results suggested that the Ni/C catalyst was stable during the reaction process, and the excellent activity of the Ni/C catalyst should be due to its high catalytic activity at room temperature as well as the strong interaction of nickel nanoparticles with the carbon support.Conclusions
In summary, the Ni/C catalyst was facilely prepared by a one-step pyrolysis strategy from naturally abundant cotton as the resource and Ni(NO3)2 as the metal precursor. The Ni/C catalyst demonstrated excellent catalytic activity towards the hydrogenation of nitro compounds at room temperature under 5 bar H2. To the best of our knowledge, no other heterogeneous non-noble metal catalysts have been reported to be so active for the hydrogenation of nitro compounds at room temperature. Our results revealed that the hydrogenation of nitro compounds proceeds via the direct pathway with hydroxylamine as the intermediate. In addition, the Ni/C catalyst was highly stable without loss of its catalytic activity during the recycling experiments. This study may offer a new strategy for the rational design and application of biomass-derived carbon material supported non-noble metal catalysts for organic transformation reactions under mild conditions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project was supported by the Special Fund for Basic Scientific Research of Central Colleges, South-Central University for Nationalities (CZZ 19005), and the National Natural Science Foundation of China (No. 2182175).Notes and references
- (a) P. N. Rylander, Catalytic Hydrogenation in Organic Syntheses, Academic Press, 1979 Search PubMed; (b) S. Nishimura, Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis, John Wiley & Sons, 2001 Search PubMed; (c) R. M. Bullock, Science, 2013, 342, 1054–1055 CrossRef CAS PubMed; (d) A. M. Smith and R. Whyman, Chem. Rev., 2014, 114, 5477–5510 CrossRef CAS PubMed.
- X. F. Tan, S. Gao, W. J. Zeng, S. Xin, Q. Yin and X. M. Zhang, J. Am. Chem. Soc., 2018, 140, 2024–2027 CrossRef CAS PubMed.
- H. T. Guo, R. X. Gao, M. M. Sun, H. Guo, B. W. Wang and L. G. Chen, ChemSusChem, 2019, 12, 487–494 CrossRef CAS PubMed.
- N. Ono, The Nitro Group in Organic Synthesis, Wiley, New York, NY, 2001 Search PubMed.
- S. Sadjadi, M. Akbari, B. Leger, E. Monflier and M. M. Heravi, ACS Sustainable Chem. Eng., 2019, 7, 6720–6731 CrossRef CAS.
- F. Yang, M. J. Wang, W. Liu, B. Yang, Y. Wang, J. Luo, Y. S. Tang, L. Q. Hou, Y. Li, Z. H. Li, B. Zhang, W. Yang and Y. F. Li, Green Chem., 2019, 21, 704–711 RSC.
- Q. W. Liu, Y. Xu, X. Q. Qiu, C. J. Huang and M. Liu, J. Catal., 2019, 370, 55–60 CrossRef CAS.
- X. D. Chen, K. Shen, D. Ding, J. Y. Chen, T. Fan, R. F. Wu and Y. W. Li, ACS Catal., 2018, 8, 10641–10648 CrossRef CAS.
- X. L. Xu, J. J. Luo, L. P. Li, D. Zhang, Y. Wang and G. S. Li, Green Chem., 2018, 20, 2038–2046 RSC.
- C. J. Liao, B. Liu, Q. Chi and Z. Z. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 44421–44429 CrossRef CAS.
- E. Pedrajas, I. Sorribes, A. L. Gushchin, Y. A. Laricheva, K. Junge, M. Beller and R. Llusar, ChemCatChem, 2017, 9, 1128–1134 CrossRef CAS.
- S. Sadjadi, M. Akbari, E. Monflier, M. M. Heravi and B. Leger, New J. Chem., 2018, 42, 15733–15742 RSC.
- A. Corma and P. Serna, Science, 2006, 313, 332–334 CrossRef CAS.
- H. S. Wei, X. Y. Liu, A. Q. Wang, L. L. Zhang, B. T. Qiao, X. F. Yang, Y. Q. Huang, S. Miao, J. Y. Liu and T. Zhang, Nat. Commun., 2014, 5, 5634 CrossRef CAS.
- R. Milian, L. C. Liu, M. Boronat and A. Corma, J. Catal., 2018, 364, 19–30 CrossRef.
- F. W. Zhang, C. Zhao, S. Chen, H. Li, H. Q. Yang and X.-M. Zhang, J. Catal., 2017, 348, 212–222 CrossRef CAS.
- P. Zhou, L. Jiang, F. Wang, K. J. Deng, K. L. Lv and Z. Z. Zhang, Sci. Adv., 2017, 3, e1601945 CrossRef PubMed.
- D. Formenti, F. Ferretti, F. K. Scharnagl and M. Beller, Chem. Rev., 2018, 119, 2611–2680 CrossRef PubMed.
- J. Park, A. Riaz, D. Verma, H. J. Lee, H. M. Woo and J. Kim, ChemSusChem, 2019, 12, 1743–1762 CrossRef CAS PubMed.
- Y. M. Zhang, H. M. Yang, Q. Chi and Z. Z. Zhang, ChemSusChem, 2019, 12, 1246–1255 CrossRef CAS PubMed.
- D. W. Liu, L. N. Zhang, W. P. Han, M. X. Tang, L. G. Zhou, Y. Zhang, X. K. Li, Z. F. Qin and H. Q. Yang, Chem. Eng. J., 2019, 369, 386–393 CrossRef CAS.
- Z. H. Wang, D. K. Shen, C. F. Wu and S. Gu, Green Chem., 2018, 20, 5031–5057 RSC.
- O. Fromm, A. Heckmann, U. C. Rodehorst, J. Frerichs, D. Becker, M. Winter and T. Placke, Carbon, 2018, 128, 147–163 CrossRef CAS.
- Z. W. Sun, X. H. Duan, C. Srinivasakannan and X. Wang, Sci. Adv. Mater., 2018, 10, 724–733 CrossRef CAS.
- C. K. Poh, Z. Q. Tian, J. J. Gao, Z. L. Liu, J. Y. Lin, Y. P. Feng and F. B. Su, J. Mater. Chem., 2012, 22, 13643–13652 RSC.
- A. Mahata, R. K. Rai, I. Choudhuri, S. K. Singh and B. Pathak, Phys. Chem. Chem. Phys., 2014, 16, 26365–26374 RSC.
| This journal is © The Royal Society of Chemistry 2020 |