Open Access Article
Open Access ArticleSelective leaching of lead from lead smelter residues using EDTA†
Thupten Palden ab,
Lieven Machiels
ab,
Lieven Machiels ab,
Bieke Onghena
ab,
Bieke Onghena ab,
Mercedes Regadío
ab,
Mercedes Regadío a and
Koen Binnemans
a and
Koen Binnemans *a
*a
aKU Leuven, Department of Chemistry, Celestijnenlaan 200F, P.O. box 2404, B-3001 Leuven, Belgium. E-mail: Koen.Binnemans@kuleuven.be
bSIM vzw, Technologiepark 935, B-9052 Zwijnaarde, Belgium
First published on 18th November 2020
Abstract
Ethylenediaminetetraacetic acid (EDTA) has been widely used as an effective reagent for removal of lead from soil because of its high lead extraction efficiency caused by the high thermodynamic stability of the Pb(II)–EDTA complex. In this study, EDTA was used as a lixiviant for recovery of lead from residues (matte and slag) of secondary lead smelter plants. The residues were composed mainly of iron (34–66 wt%) and lead (7–11 wt%). Leaching parameters (EDTA concentration, pH, temperature, liquid-to-solid ratio and leaching time) were optimized. The optimum leaching efficiency was achieved when leached for 1 h at room temperature using 0.05 mol L−1 EDTA at a liquid-to-solid ratio of 5 mL g−1. At such conditions, 72 to 80% of lead and less than 1% of iron were leached from both matte and slag. The high selectivity towards lead with minimal co-dissolution of iron is a major advantage since it reduces the chemical consumption and simplifies the downstream processes. Although the stability constants of the complexes Fe(III)–EDTA, Fe(II)–EDTA and Pb–EDTA are all large (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KS 25.1, 14.33 and 18.04, respectively), the leaching of iron was most likely limited by its presence in insoluble phases such as iron oxides, sulfides and silicates in the residues. 100% leaching of lead was achieved by a multi-step leaching process where the leaching residues were contacted three times by a fresh EDTA solution. To recover EDTA, first iron was precipitated as iron hydroxide by raising the pH of pregnant leach solution (PLS) above 12.6 using sodium hydroxide, followed by precipitation of lead as lead sulfide by adding ammonium sulfide. The recovered EDTA was successfully reused two times for leaching without significant changes in leaching yields.
KS 25.1, 14.33 and 18.04, respectively), the leaching of iron was most likely limited by its presence in insoluble phases such as iron oxides, sulfides and silicates in the residues. 100% leaching of lead was achieved by a multi-step leaching process where the leaching residues were contacted three times by a fresh EDTA solution. To recover EDTA, first iron was precipitated as iron hydroxide by raising the pH of pregnant leach solution (PLS) above 12.6 using sodium hydroxide, followed by precipitation of lead as lead sulfide by adding ammonium sulfide. The recovered EDTA was successfully reused two times for leaching without significant changes in leaching yields.
Introduction
A total of 11.9 million tons of lead metal was produced globally in 2019, and 61% of that amount was produced by recycling of lead-containing scrap by secondary lead smelters.1 The secondary lead smelters produce metallic lead as their main product but also a large amount of by-products. During the smelting process, molten lead sinks to the bottom of the furnace and is tapped separately for further refining. The less dense mineral phases (i.e., the smelting residue) float on the top of the molten lead and these are tapped into a separate pot to settle. There, the denser matte consisting mostly of molten sulfide sinks to the bottom and the slag consisting of molten silicate floats on the top.2,3 After cooling, the matte is physically separated from the slag. About 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of lead-rich residues (matte and slag) are being produced yearly in Europe alone during this smelting process.4 These residues are composed mostly of iron (30–70%) and lead (6–10%), but some amounts of tin, antimony, nickel and zinc are present as well. Therefore, they can be reused as secondary resources for many valuable metals. In addition, matte and slag landfills are known to release lead, a toxic but economically important metal, to the environment, and recovery of the valuable metals such as lead will generate a new residue that is safer to landfill.5
000 tonnes of lead-rich residues (matte and slag) are being produced yearly in Europe alone during this smelting process.4 These residues are composed mostly of iron (30–70%) and lead (6–10%), but some amounts of tin, antimony, nickel and zinc are present as well. Therefore, they can be reused as secondary resources for many valuable metals. In addition, matte and slag landfills are known to release lead, a toxic but economically important metal, to the environment, and recovery of the valuable metals such as lead will generate a new residue that is safer to landfill.5
Some research has focused on the valorization of these residues as a construction material.6,7 However, the recovery of the valuable metals prior to their application as construction material was not considered. Hence these approaches result in a great loss of valuable metals in addition to the potential risk of leaching the toxic metals to the environment. Few studies have investigated the recovery of valuable metals from these lead-containing residues. Kim et al. studied the selective leaching of lead and other minor metals from lead smelter residues using nitric acid.2,8 They investigated in detail the effect of roasting, pressure leaching, and addition of the ferric ion as an oxidant to enhance lead leaching. With their optimized system, about 90% of lead was leached with minimal co-dissolution of iron. However, the leaching system employs nitric acid which is highly corrosive and powerful oxidant and, requires roasting which is energy intensive. Moreover, it did not leach any lead from slag making it only applicable for matte. Forte et al. employed a solvometallurgical leaching process using concentrated acetic acid to recover the valuable metals from the lead smelter residues.9 The process could leach 90% of lead with 6% co-leaching of iron from matte and lower lead leaching of 70% from the slag. Although this process is novel and promising, its main challenge is to convince the stakeholders to use a pure acetic acid at industrial scale since it has a strong stringent odor and it has not been applied on a commercial scale by a metallurgical industry yet. Moreover, liquid-to-solid ratio of 20 L kg−1 required for the acetic acid leaching is too high, making the process unattractive for commercialization.
Ethylenediaminetetraacetic acid (EDTA) is a chelating agent that can form stable Pb(II)–EDTA complexes.9 In fact, EDTA has been widely used as an effective reagent for decontamination of lead from soil because of its high lead extraction efficiency enabled by high thermodynamic stability of lead–EDTA complexes.10–23 Moreover, EDTA can be recuperated and recycled which is an economical and environmental importance since EDTA is relatively expensive and only slow biodegradable.24–26 In a recent study, Smaniotto et al. investigated the recovery of lead from recycled lead–acid battery slag using EDTA as a lixiviant.5
In this paper, we presents the development of a process to selectively recover lead from the residues (matte and slag) of a secondary lead smelter using EDTA as lixiviant. Firstly, the operative parameters (concentration, pH, liquid-to-solid ratio, temperature) were firstly optimized. Secondly, the recovery of EDTA and its subsequent reusability with fresh residues, was studied in detail. Finally, the scaling up of the leaching system was tested in a 1 L reactor.
Experimental
Chemicals
The slag and matte were kindly provided by a European secondary lead producer. Disodium ethylenediaminetetraacetic acid (0.1 mol L−1, Na2EDTA) and sodium hydroxide (NaOH, pearl) was purchased from Fisher Scientific (Loughborough, United Kingdom). Disodium ethylenediaminetetraacetic acid (0.2 mol L−1, Na2EDTA) was purchased from Honeywell Fluka (Seelze, Germany). Hydrochloric acid (37 wt%, HCl in water) and boric acid (99.5%, H3BO4 in water) was supplied by VWR Chemicals (Leuven, Belgium). Nitric acid (65 wt% HNO3 in water) and iron, lead, zinc and rhodium standard solutions (1000 mg L−1) were purchased from Chem-Lab NV (Zedelgem, Belgium). Hydrofluoric acid (48 wt%, HF in water) and ammonium sulfide (20 wt%, (NH4)2S in water) were purchased from Sigma-Aldrich (Diegem, Belgium). All chemicals were used as received without any further purification.Instrumentation
The matte and slag were ground and sieved using a mortar grinder (Pulverisette 2, Fritsch, Germany) and a vibratory sieve shaker (Analysette 3, Fritsch, Germany). The materials were digested using a microwave digester (Mars 6, CEM, USA). The metal content in solution was measured by inductively coupled plasma optical emission spectroscopy (ICP-OES, Optima 8300, Perkin Elmer, USA) from PerkinElmer. The mineral phases in the solid materials were identified by powder X-ray diffraction (XRD) analysis using a Bruker D2 Phaser diffractometer. The leaching was carried out on a RCT classic heating plate (IKA). The phase disengagement between the solid and liquid after leaching was carried out by centrifugation using Heraeus Labofuge 200. The scalability of the leaching at optimized conditions was studied using a customized 1 L jacketed laboratory reactor, linked to an automatic filtration unit (LabKit 36167) constructed by HiTec Zang GmbH (Herzogenrath, Germany).Methodology
The matte and slag (as received) were dried in an oven at 100 °C for 24 h to remove any trace of moisture. The materials were ground using a mortar grinder and sieved below 250 μm in particle size using a vibratory sieve shaker. The procedure for quantitative X-ray diffraction analysis (QXRD) was adapted from the work of Snelling et al. and Machiels et al.27,28 Samples were spiked with 20 wt% of Al2O3 internal standard and ground in ethanol for 5 min using a McCrone Micronizing Mill. X-ray powder diffraction (XRD) was used for the phase identification of the crystalline fraction. Experimental parameters for XRD analysis were: 2θ: 10–80°, CuKα, acceleration voltage: 45 kV, acceleration current: 30 mA, a step size of 0.020° and a counting time of 1 s per step, spin mode. Phase identification was done using the Bruker Diffrac+ software while Rietveld quantitative phase analysis (QPA) was performed using the Topas Academic software. A fundamental parameter approach was used, meaning that instrumental contributions to the peak shapes were directly calculated. The following parameters were refined: background, sample displacement, scale factors of all phases, lattice parameters, crystallite size and lattice strain. The background was refined using a cosine Chebyshev polynomial function of 15 parameters. The metal content of the materials was determined after fully dissolving 100 mg of the milled sample in an acid mixture of 4 mL of 37 wt% HCl, 5 mL of 65 wt% HNO3 and 1 mL of 48 wt% HF using microwave-assisted acid digestion. The samples were digested using a one stage program where the samples heated from room temperature to 180 °C in 5.5 min and held for 9.5 min at 1000 W. After the digestion, the excess HF was rendered safe by complexation with 10 mL of 4 wt% of H3BO3 and enhanced by microwave digestion where the samples were heated up to 170 °C in 15 min and held for 10 min. After the neutralization, the digestion vessels were cooled and the digested solutions were transferred to volumetric flasks and filled up to 100 mL with ultrapure water for analysis. The sample dissolution via microwave digestion was done in triplicates to check the reproducibility of the composition. The metal concentrations in each of the digested acid solution was measured by ICP-OES with ±5% of error associated with the measurements.The initial leaching experiments were carried out by adding 200 mg of matte or slag and 2 mL of EDTA solution in a 4 mL glass vial and magnetically stirred on a heating plate. The following leaching conditions were applied: a liquid-to-solid ratio (L/S) of 10 mL g−1, a temperature of 60 °C, a contact time of 2 h and a stirring speed of 600 rpm. The upscaling of the leaching system was tested for 1 L of EDTA in a 1 L batch reactor. Attention had to be paid when choosing the type of vial for leaching experiments. The iron- and lead-rich residues have a high mass density and were difficult to stir by a magnetic stirring bar. As a result, the solids residues were not homogenously distributed in the EDTA solution, especially in the longitudinal leaching vials giving a low leaching efficiency of lead. An appropriate vial must be chosen for each volume of the lixiviant. Otherwise, maximum leaching of lead could not be realised using inappropriate vial. Therefore, 4 mL vials were used for lixiviant volume of less than 2 mL and 10 mL vial for lixiviant volume between 2–5 mL.
The pregnant leach solution (PLS) was separated from the solid residue by centrifugation (5300 rpm, 10 min). The finer particles suspended in the PLS were further separated by a syringe filter made of a polyester membrane (Chromafil PET, 0.45 μm pore size). The metal concentration in the PLS was measured using ICP-OES and the leaching efficiency EL (%), was calculated according to eqn (1):
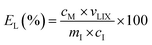 | (1) |
The EDTA in the PLS was recovered by precipitation of the dissolved iron by adding 12 mol L−1 NaOH; followed by precipitation of lead by adding 2.93 mol L−1 (NH4)2S. The precipitation efficiency EP (%) was calculated by mass balance according to the following equation:
 | (2) |
Results and discussion
Characterization of matte and slag
The matte and slag were composed mainly of iron (34–66 wt%) and lead (7–11 wt%) (Table 1). The iron and lead were present in several mineral phases (Table 2, Fig. S1 and S2†). In the matte, iron was mainly present in the form of sulfide (FeS) with small amounts of oxides (FeO, Fe3O4). In the slag, iron was mainly present as silicates (Fe2SiO4, CaFeSiO4) with a significant amount of sulfide (FeS) and small amounts of oxides (Fe3O4, FeO). It must be noted that 91% of the iron in matte and slag were in divalent state. Lead was present in elemental state (Pb), oxide (PbO) and carbonate hydroxide (Pb3(CO3)2(OH)2) form in both matte and slag, and additionally, in sulfide (PbS) form in the slag. Amorphous phases and the minor phases that were in low concentration were not detected by XRD. These unidentified phases contributed to less than 5% of the mass of the residues. To avoid complications in presenting a very large array of data, only data for iron and lead are compared and further discussed. The other metals were in low concentrations and their impact on iron and lead leaching would be minimal.| Metal | Matte (wt%) | Slag (wt%) |
|---|---|---|
| Fe | 66.3 | 34.5 |
| Pb | 10.9 | 6.8 |
| Zn | 0.35 | 0.61 |
| Cu | 0.82 | 0.27 |
| Si | 0.26 | 13.49 |
| Sn | 0.21 | 0.34 |
| Ni | 0.11 | 0.03 |
| Ca | 0.06 | 4.28 |
| Cr | 0.05 | 0.33 |
| Matte | Slag | ||
|---|---|---|---|
| Mineral phases | wt% | Mineral phases | wt% |
| FeS (troilite) | 71 | Fe2SiO4 (fayalite) | 34 |
| FeO (wüstite) | 12 | Troilite (FeS) | 29 |
| Fe3O4 (magnetite) | 8 | CaFeSiO4 (monticellite) | 19 |
| Pb3(CO3)2(OH)2 (hydrocerussite) | 1 | Fe3O4 (magnetite) | 7 |
| PbO (massicotite) | 1 | FeO (wüstite) | 2 |
| PbO (litharge) | 1 | Quartz (SiO2) | 1 |
| Pb (lead) | 0.5 | Galena (PbS) | 1 |
| Not calculated/amorphous | 5 | Lead (Pb) | 0.5 |
| Hydrocerussite (Pb3(CO3)2(OH)2) | 0.5 | ||
| Litharge (PbO) | <0.5 | ||
| Not calculated/amorphous | 5 | ||
Leaching matte and slag with EDTA
Leaching matte and slag using 0.1 mol L−1 EDTA resulted in leaching of about 64–73% of lead and 1–12% of iron, making it selective for lead over iron (Fig. 1). This favourable selectivity towards lead with minimal co-dissolution of iron significantly reduces the chemical consumption and simplifies the downstream processes. The lower leaching efficiency of iron compared to that of lead was unanticipated as EDTA usually forms a highly stable complex with iron. The equilibration reactions (eqn (1), (2) and (3)) and stability constants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KS, 25 °C and μ = 0.1) of EDTA with Fe(III), Fe(II) and Pb(II) are shown below:11
KS, 25 °C and μ = 0.1) of EDTA with Fe(III), Fe(II) and Pb(II) are shown below:11
Fe3+(aq) + H2EDTA(aq) ⇌ FeHEDTA(aq) + H+(aq), log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KS = 25.1 KS = 25.1
| (3) |
Fe2+(aq) + H2EDTA(aq) ⇌ FeH2EDTA(aq), log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KS = 14.33 KS = 14.33
| (4) |
Pb2+(aq) + H2EDTA(aq) ⇌ PbH2EDTA(aq), log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KS = 18.04 KS = 18.04
| (5) |
Based on stability constants, one could draw the wrong conclusion that most of the iron would be leached because of the high stability constant of iron–EDTA complexes and the abundance of iron in the residues. However, the leachability of metals was also influenced by the solubility of each of the metal phases. The solubility of crystalline iron oxides and iron sulfide are low and iron silicates are almost inactive with reducing agents, chelating agents or weak acids. Therefore, despite having a high stability constant for the Fe(II)–EDTA and Fe(III)–EDTA complexes, the leachability of iron was most likely inhibited by the insolubility of the iron oxides and silicates. The presence of lead in more soluble minerals, together with its high stability constant with EDTA resulted in a selective leaching of lead over iron. Independent studies by Clevenger et al. and Elles et al. already showed that EDTA can solubilize many of the common inorganic lead phases such as PbCO3, PbSO4, PbCl2, Pb(NO3)2, PbO, Pb3O4, PbO2 and Pb(OAc)2 except for PbS and PbCrO4.29,30 Thus, the main limiting factor in leaching of iron and lead from the residues was the solubility of the metal phases. The solubility product constants of the iron phases (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K at 25 °C: magnetite = 2, wustite = 0.8 and troilite = 5.25)31,32 present the residues are also significantly lower than the that of the lead phases (log
K at 25 °C: magnetite = 2, wustite = 0.8 and troilite = 5.25)31,32 present the residues are also significantly lower than the that of the lead phases (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K at 25 °C: litharge = 2, hydrocerussite = 0.8),33 supporting the discussion that the good solubility of the mineral phases is crucial in achieving a high leaching efficiency of the metals. The Cu–EDTA and Zn–EDTA complexes are also stable with stability constant (log
K at 25 °C: litharge = 2, hydrocerussite = 0.8),33 supporting the discussion that the good solubility of the mineral phases is crucial in achieving a high leaching efficiency of the metals. The Cu–EDTA and Zn–EDTA complexes are also stable with stability constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KS, 25 °C and μ = 0.1) of 18.7 and 16.44, respectively.11 However, their influence on the selective leaching of lead over iron is expected to be minimal, since their concentrations (Cu = 0.3–0.8 wt%, Zn = 0.3–0.6 wt%) were low in the residues.
KS, 25 °C and μ = 0.1) of 18.7 and 16.44, respectively.11 However, their influence on the selective leaching of lead over iron is expected to be minimal, since their concentrations (Cu = 0.3–0.8 wt%, Zn = 0.3–0.6 wt%) were low in the residues.
Optimization of leaching of lead
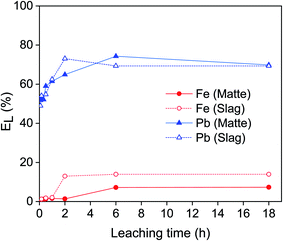
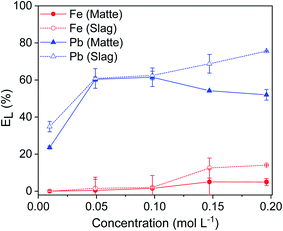
The leaching of lead and iron from matte and slag was studied as a function of the leaching time (Fig. 2). The leaching of lead was relatively fast: about ∼50% of the lead was leached from both matte and slag within the first few minutes. For the matte, it reached a maximum of 74% after 6 hours and then remained stable. For the slag, the leaching efficiency of lead increased sharply until 73% after 2 hours and then it remained constant at longer leaching time. The leaching efficiency of iron remained low for both residues: less than 1.5% after 1 hour of leaching. Forte et al. also reported fast leaching kinetics where the lead leaching efficiency reached a plateau within 2 h.9 Since the objective was to use the same condition for both matte and slag and to achieve high selectivity over iron, the optimum leaching time was chosen as 1 hour for both matte and slag. After 1 hour, about 60% of lead and 1.5% of iron were leached, making it highly selective for lead with minimal codissolution of iron.EDTA concentration stepwise from 0.01 to 0.2 mol L−1 (Fig. 3). The leaching of lead increased sharply with increasing EDTA concentration from 0.01 to 0.05 mol L−1 EDTA for both matte and slag. Further increase in the concentration led to a small decrease in leaching efficiency of lead in the matte and a gradual increase for the slag. The leaching efficiency of iron increased gradually with increasing EDTA concentration, but remained less than 14%. Due to good selectivity and the reduced cost of less concentrated EDTA solutions, the optimum concentration was chosen to be 0.05 mol L−1 at which about 60% of lead and <2% of iron were leached. However, leaching by 0.1 mol L−1 EDTA concentration was also investigated to avoid limiting the leaching efficiency of lead by the lack of sufficient EDTA molecules.
The leaching of lead and iron from matte and slag was studied as a function of pH at 0.05 and 0.1 mol L−1 EDTA concentration (Fig. 4a and b, respectively). The pH of the EDTA solution did not have a significant effect on the leaching efficiency of lead for both residues. Previous studies have also shown that pH did not influence the extraction of heavy metals by EDTA.11 As confirmed here, the pH of the EDTA solution was indeed not important for the leaching efficiency of lead. However, the leaching efficiency of iron decreased with increasing pH, and at pH = 12 there was no iron in the PLS. This was due to the precipitation of Fe(II) and Fe(III) as Fe(OH)2 and Fe(OH)3, respectively, at higher pH with a red-brown precipitate formed quickly in the PLS after filtration of the leaching residue. Fe(III) in aqueous solutions usually precipitates at much lower pH, but it is reported to be stable up to pH = 12 in EDTA solutions due to the high stability constant of Fe(III)–EDTA complexes.10 The pH of the solution was expected to remain unchanged (initial pH = 8) or to slightly decrease after equilibration due to the release of free protons by the EDTA molecule. However, the equilibration pH of the PLS increased after leaching. When pure water was used to leach matte and slag, the equilibration pH of water after leaching also increased to 10.6 and 9.5, respectively. After analyzing the PLS resulting from leaching with pure water, trace amounts of Pb, Zn, Ca, Sn and K were found to be present but one specific chemical reaction could not be linked to this pH change. Dissolution of calcium oxide in water would generate hydroxide ions but the concentration of calcium did not show positive correlation with the change in pH of the PLS. The increase in pH could be due to the release of hydroxide ions by more than one reaction taking place during leaching. The selectivity of lead over iron was better at pH 12 than pH 8. However, the iron leaching efficiency was already low and further reducing the iron leaching efficiency by increasing the pH was not a sufficient justification to select a more complicated leaching process whereby a pH adjustment of the lixiviant is required prior to the leaching. Therefore, the pH of fresh EDTA solution (∼pH 8) was chosen as the optimum pH to have simple leaching process where pH adjustment is not required.
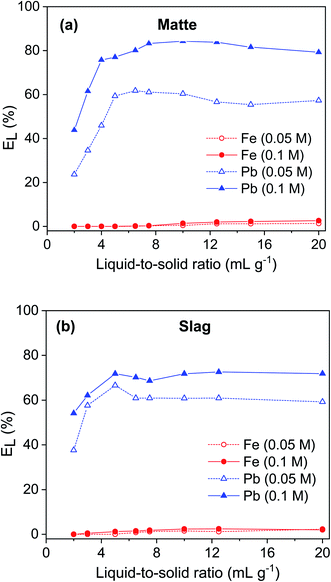
The influence of liquid-to-solid ratio (L/S) on leaching of lead and iron from matte and slag using 0.05 and 0.1 mol L−1 EDTA solution was investigated (Fig. 5). The leaching of lead increased sharply when the L/S ratio was increased up to 5. Further increase in the L/S did not significantly change the leaching efficiency of lead. The leaching of iron gradually increased but remained low with increasing L/S ratio. For matte, the leaching efficiency of lead was about 20–25% higher using 0.1 mol L−1 EDTA solution compared to that of the 0.05 mol L−1 solution. However, for slag, the lead leaching efficiency only increased by 10% when the EDTA concentration was increased from 0.05 to 0.1 mol L−1. The optimum conditions were selected to be L/S ratio of 5 and EDTA concentration of 0.1 mol L−1. At such conditions, lead and iron leaching efficiency were 77% and 0.04%, respectively, for matte and, 72% and 1.2%, respectively, for slag.
The influence of temperature on leaching of lead and iron from the residues was studied at L/S ratio 4 and 5 mL g−1 (Fig. 6). The temperature had only a small effect on the leaching efficiency of lead and iron, so room temperature (25 °C) was chosen as the optimum temperature for further experiments. Lead could not be fully leached even at higher temperatures, indicating the low reactivity of some lead phases which are still insoluble even at more severe reaction conditions. In a few studies, the lead leaching efficiency even decreased with increasing temperature, which was attributed to the precipitation of lead as a lead sulfate.8,9 In this study, the absence of lead sulfate precipitation at high temperatures may be due to the high stability constant of Pb(II)–EDTA complexes. At optimized conditions (T = 25 °C, t = 1 h, L/S = 5 mL g−1), EDTA leached about 72 to 80% of lead and less than 1% of iron from both matte and slag. Forte et al. leached 72–90% of lead and less than 3–6% of iron was co-dissolved from matte and slag at optimized conditions (T = 25 °C, t = 2 h, L/S = 20 mL g−1).9 The slightly higher leaching of lead and iron by acetic acid compared to that of EDTA could be because the lead and iron solubilizing power of acetic acid due to its acidity, is slightly higher than that of EDTA due to its chelation. Kim et al. leached 69% of lead and 14% of iron from matte using 0.5 mol L−1 nitric acid, which increased lead and iron leaching efficiency to 89% and 23%, respectively by adding ferric ion as an additional oxidant increased (T = 25 °C, t = 2 h, L/S = 10).2 In another study, Kim et al. leached 93% of lead and 0.6% of iron using 1 mol L−1 citric acid and 0.5 mol L−1 hydrogen peroxide as an oxidant.8 The authors explained that dissolution of zero-valent lead and lead sulfide would require both an acidification and an oxidization step. As a result, addition of additional oxidant had a positive effect in leaching of lead. In the process developed in this study, the leaching efficiency of lead could not be increased further than 80% and this could be due to the lack of strong acidity and oxidative power to dissolve the lead sulfide. The effect of oxidant was not investigated in this study since using oxidant usually increases the leaching efficiency of all metals, including that of iron, which would reduce the selectively toward lead.
Multi-step leaching and EDTA recovery
Since one leaching step cannot achieve 100% leaching of lead, multistep leaching was carried out where the leached residue was contacted again with a fresh EDTA solution. After contacting the residue three times with a fresh EDTA solution, 100% lead was finally leached (Fig. 7). This result is consistent with the findings of other authors. During the remediation of contaminated soils, it is commonly observed that significantly more lead was leached when the same amount of EDTA was applied in several leaching steps.11,34–37 Metals such as iron compete with heavy metals to form complexes with EDTA.11 The high lead leaching efficiency of multi-step leaching is most likely because iron interferes more strongly with lead complexation when the residues or soils were leached in single-step mode. However, a more detailed study will be needed to understand why leaching with an EDTA solution in multiple steps is more efficient and how multi-step leaching facilitates the dissolution of insoluble lead solids, like metallic lead and galena.EDTA is a rather expensive chemical and it is only slowly biodegradable. Therefore, it is crucial that EDTA can be recovered and reused to reduce cost and to avoid environmental issues. To allow the recyclability of the leaching agent, the unreacted EDTA solution in PLS of matte and slag was recovered by two precipitation steps; iron was precipitated first by increasing the pH of the PLS by adding NaOH followed by precipitation of lead by adding (NH4)2S solution. Having a 0.12 mol L−1 NaOH concentration in the PLSs was sufficient to increase their pH to 12.6 and consequently to precipitate all dissolved iron as iron hydrous oxide.5,12 The completeness of the precipitation of iron was also visually evident from the change in color of the PLS from red-brown to transparent. The red-brown color of the precipitate indicated that the precipitate was ferric hydroxide, Fe(OH)3, since ferrous hydroxide, Fe(OH)2, is either white or green. Moreover, the reddish color of PLS turns darker within few hours after leaching, indicating that the Fe(II) had been oxidized to Fe(III) by the air. Therefore, all the iron complexed with EDTA in the PLS were most likely in trivalent state, which has a higher stability constant with EDTA than the divalent state and lead. The PLS without the iron was again contacted with (NH4)2S to precipitate lead as lead sulfide (Fig. S3†). Lead was completely precipitated at 0.12 mol L−1 and 0.15 mol L−1 (NH4)2S for matte and slag, respectively (Fig. 8). Excess of unreacted (NH4)2S in the PLS was evident from the bright yellowish color of otherwise transparent PLS and the sulfurous odor. Therefore, excess of (NH4)2S must not be used during precipitation of lead. The XRD pattern of the leach residue was compared to the one of the fresh residue, and the remaining lead phases could not be identified because the diffraction peaks corresponding to lead phases were small and often overlapping with those of the iron phases. The leached residue can be used by the iron and steel industry as a secondary iron resources, because of its high iron content. Direct precipitation of lead as lead sulfide from the residues in one step using alkaline sulfide might work due to the strong affinity between lead (Pb2+) and sulfide (S2−) based on the Pearson's hard and soft acids and bases (HSAB) principle. However, direct precipitation is not always preferred since the precipitates are mixed with the leach residue and separating them can pose an even bigger challenge than a two-step process of leaching followed by precipitation.
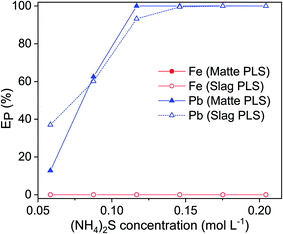 | ||
| Fig. 8 Precipitation of lead from the pregnant leach solution as lead sulfide by addition of (NH4)2S. Precipitation parameters: temperature 25 °C, stirring speed 600 rpm, time 1 h. | ||
Scale up and reusability of EDTA
The EDTA leaching of lead matte and slag at optimal conditions was tested on a larger scale in a 1 L temperature-controlled leaching reactor (Fig. 9) with overhead stirring and automatic filtration starting from 200 g of a solid residue and 1 L of 0.1 mol L−1 EDTA solution, which corresponds to 500 times upscaling compared to the screening experiments. The results show that the leaching efficiency of lead using the leaching reactor was about 15–20% lower than the small-scale experiments (Fig. 10, fresh). This may be due to ineffective stirring of the residues in a longitudinal reactor, resulting in a heterogeneous distribution of the high mass density residues. The leaching efficiency of lead in the leaching reactor can be increased to that of small-scale experiments by changing the stirring speed or reducing the amount of solids and liquid. However, the optimization of leaching in the 1 L reactor was not carried out as it was outside the scope of this study. Nevertheless, the leaching yield of lead was high with minimal co-dissolution of iron, showing potential for upscaling to a larger scale. | ||
| Fig. 9 Upscaling EDTA leaching of lead matte and slag in a temperature-controlled batch reactor (volume = 1 L). | ||
The reusability of the recovered EDTA solution was tested by reusing it to leaching of fresh matte and slag samples. The process of leaching and precipitation was repeated for two cycles and the leaching results were compared to that of fresh EDTA (Fig. 10). The leaching result between fresh and 1st recycled EDTA were quite similar. However, the leaching efficiency of lead by 2nd recycled EDTA was about 20% higher than that of the fresh and 1st recycle. The pH of the regenerated EDTA were slightly different but, as mentioned above, the pH has little influence on the leaching of lead (Table S1†). The lower leaching efficiency of lead using fresh and 1st cycle was most likely due to the fact that the leaching was carried out in larger reactors without optimization of the stirring. The 2nd cycle leaching was carried out using the same vial as the small-scale screening experiments and thus the leaching efficiency of lead was closer to the optimized small-scale experiments. Nevertheless, the leaching efficiencies were still high, indicating that the recovered EDTA solution could be reused successfully. A conceptual flowsheet of the leaching of lead matte and slag and subsequent recovery of the EDTA by two precipitation steps is shown in Fig. 11.
 | ||
| Fig. 11 Conceptual flow sheet for the selective leaching of lead from matte and slag of the secondary lead smelters by EDTA. | ||
Conclusion
EDTA was used for the leaching of lead from residues (matte and slag) of a secondary lead smelter plant. The residues were composed mainly of iron (34–66%) and lead (7–11%). The use of EDTA in leaching metals from matte and slag resulted in a highly selective leaching of lead over iron: only about 1% of iron was co-dissolved alongside about 80% of the lead. Having a PLS with lead as a major component reduces the cost of downstream processes for obtaining high purity lead and, at the same time, less lead was left in the original residue, which reduces its pollution potential when disposed. The poor leaching of iron by EDTA can be attributed to the low solubility of crystalline iron oxides, iron sulfide and iron silicates which were the major iron phases in the residues. The leaching efficiency of lead increased to 100% when the leaching residues were contacted three times by a fresh EDTA solution. The EDTA in the PLS was recovered by precipitation of iron and lead by sodium hydroxide and ammonium sulfide respectively. The recycled EDTA was successfully reused for leaching of fresh residues, making the process cheaper and environmentally friendlier.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors of this work acknowledge the Strategic Initiative Materials in Flanders (SIM) for the financial support (SBO-SMART: Sustainable Metal Extraction from Tailings) with grant no. HBC.2016.0456. The author also wish to acknowledge funding from European Commission's H2020 – Marie Sklodowska Curie Actions (MSCA) – Innovative Training Networks within the SOCRATES project under the grant agreement no. 721385 (Project website: http://etn-socrates.eu). This work reflects only the author's view, exempting the community from any liability.References
- International Lead and Zinc Study Group (ILZSG), World refined lead supply and usage, 2015–2020 Search PubMed.
- E. Kim, L. Horckmans, J. Spooren, K. C. Vrancken, M. Quaghebeur and K. Broos, Hydrometallurgy, 2017, 169, 372–381 CrossRef CAS.
- D. Pan, L. Li, X. Tian, Y. Wu, N. Cheng and H. Yu, Resour., Conserv. Recycl., 2019, 146, 140–155 CrossRef.
- A. Lassin, P. Piantone, A. Burnol, F. Bodénan, L. Chateau, C. Lerouge, C. Crouzet, D. Guyonnet and L. Bailly, J. Hazard. Mater., 2007, 139, 430–437 CrossRef CAS.
- A. Smaniotto, A. Antunes, I. do N. Filho, L. D. Venquiaruto, D. de Oliveira, A. Mossi, M. Di Luccio, H. Treichel and R. Dallago, J. Hazard. Mater., 2009, 172, 1677–1680 CrossRef CAS.
- G. De Angelis, F. Medici, M. R. Montereali and L. Pietrelli, Waste Manage., 2002, 22, 925–930 CrossRef CAS.
- M. Penpolcharoen, Cem. Concr. Res., 2005, 35, 1050–1055 CrossRef CAS.
- E. Kim, L. Horckmans, J. Spooren, K. Broos, K. C. Vrancken and M. Quaghebeur, Hydrometallurgy, 2017, 169, 290–296 CrossRef CAS.
- F. Forte, L. Horckmans, K. Broos, E. Kim, F. Kukurugya and K. Binnemans, RSC Adv., 2017, 7, 49999–50005 RSC.
- C. Kim, Y. Lee and S. K. Ong, Chemosphere, 2003, 51, 845–853 CrossRef CAS.
- N. Finzgar and D. Lestan, Chemosphere, 2007, 66, 824–832 CrossRef CAS.
- R. Sasai, H. Kubo, M. Kamiya and H. Itoh, Environ. Sci. Technol., 2008, 42, 4159–4164 CrossRef CAS.
- D. M. Heil, Z. Samani, A. T. Hanson, S. Hu and B. Rudd, Water, Air, Soil Pollut., 1999, 113, 77–95 CrossRef CAS.
- D. Lestan, C. Luo and X. Li, Environ. Pollut., 2008, 153, 3–13 CrossRef CAS.
- S. Zhang, Z. Yang, B. Wu, Y. Wang, R. Wu and Y. Liao, Clean: Soil, Air, Water, 2014, 42, 641–647 CAS.
- M. Lei, M. Zeng, P. Qin, B. Liao, B. Tie and M. Tanaka, Int. J. Environ. Sci. Technol., 2012, 15, 50–60 CAS.
- T. Deng, B. Zhang, F. Li and L. Jin, Chemosphere, 2017, 168, 450–456 CrossRef CAS.
- M. Wei and J. Chen, Environ. Sci. Pollut. Res., 2016, 23, 23123–23133 CrossRef CAS.
- M. Wei, J. Chen and X. Wang, Chemosphere, 2016, 156, 252–261 CrossRef CAS.
- S. Goel, K. K. Pant and K. D. P. Nigam, J. Hazard. Mater., 2009, 171, 253–261 CrossRef CAS.
- K. Fischer, H. P. Bipp, P. Riemschneider, P. Leidmann, D. Bieniek and A. Kettrup, Crit. Rev. Environ. Sci. Technol., 1998, 32, 2154–2161 CrossRef CAS.
- K. R. Vuyyuru, K. K. Pant, V. V. Krishnan and K. D. P. Nigam, Ind. Eng. Chem. Res., 2010, 49, 2014–2024 CrossRef CAS.
- R. W. Peters, J. Hazard. Mater., 1999, 66, 151–210 CrossRef CAS.
- Q. R. Zeng, S. Sauve, H. E. Allend and W. H. Hendershota, Environ. Pollut., 2005, 133, 225–231 CrossRef CAS.
- Q. Wang, J. Chen, A. Zheng and L. Shi, Chemosphere, 2019, 220, 1200–1207 CrossRef CAS.
- S. Gitipour, S. Ahmadi, E. Madadian and M. Ardestani, Environ. Technol., 2016, 37, 145–151 CrossRef CAS.
- R. Snellings, G. Mertens, L. Machiels and J. Elsen, Geol. Belg., 2010, 13, 183–196 CAS.
- L. Machiels, D. Garcés, R. Snellings, W. Vilema, F. Morante, C. Paredes and J. Elsen, Appl. Clay Sci., 2014, 87, 108–119 CrossRef CAS.
- M. P. Elless and M. J. Blaylock, Int. J. Phytorem., 2000, 2, 75–89 CrossRef CAS.
- T. E. Clevenger, C. Saiwan and S. R. Koirtyohann, Environ. Sci. Technol., 1991, 25, 1128–1133 CrossRef CAS.
- U. Schwertmann and R. M. Cornell, Iron Oxides in the Laboratory, VCH, Weinheim, 1991 Search PubMed.
- W. Davison, Aquat. Sci., 1991, 53, 309–329 CrossRef.
- C. Kim, Extraction of Lead Using EDTA: Factors Affecting Extraction, Effects of Amorphous Iron and Recycling of Used EDTA, PhD thesis, Iowa State University, Iowa, 1996, pp. 1–71.
- H. A. Elliott and G. A. Brown, Water, Air, Soil Pollut., 1989, 45, 361–369 CrossRef CAS.
- M. C. Steele and J. Pichtel, J. Environ. Eng., 1998, 124, 639–645 CrossRef CAS.
- P. Theodoratos, N. Papassiopi, T. Georgoudis and A. Kontopoulos, Water, Air, Soil Pollut., 2000, 122, 351–368 CrossRef CAS.
- B. Sun, F. J. Zhao, E. Lombi and S. P. McGrath, Environ. Pollut., 2001, 113, 111–120 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD patterns of matte and slag before leaching (Fig. S1 and S2), a XRD pattern of lead precipitate after adding ammonium sulfide to the PLS (Fig. S3), a table with changes in pH of PLS during recovery process (Table S1). See DOI: 10.1039/d0ra08517k |
| This journal is © The Royal Society of Chemistry 2020 |