Open Access Article
Open Access ArticleRe-evaluation of the mechanism of cytotoxicity of dialkylated lariat ether compounds†
Willy Carrasquel-Ursulaeza,
Ryan D. Reevesb,
Mahzad Dehghany b,
Corey Jonesb,
Jennifer M. Schomaker
b,
Corey Jonesb,
Jennifer M. Schomaker *b and
Baron Chanda*a
*b and
Baron Chanda*a
aDepartment of Anesthesiology, Washington University in St. Louis, St. Louis, Missouri 63110, USA. E-mail: bchanda@wustl.edu
bDepartment of Chemistry, University of Wisconsin, Madison, Wisconsin 53706, USA. E-mail: schomakerj@chem.wisc.edu
First published on 5th November 2020
Abstract
The cytotoxicity of dialkylated lariat ethers has been previously attributed to their ionophoric properties. Herein, we provide evidence that these effects are due to loss of membrane integrity rather than ion transport, a finding with important implications for the future design of synthetic ionophores.
Since the first report of their synthesis,1,2 crown ethers and their derivatives have generated wide interest due to their ability to form stable complexes with cations.2 These properties have been successfully exploited in ion transport through bulk liquid membranes3,4 as well as in sensors and scaffolds for materials,5 developments which prompted their examination as biologically relevant ionophores.6–8 The transport of ions through biological membranes underlies many key physiological processes9–18 and understanding the complexities of this phenomenon continues to be an area of active research. Crown ethers are potentially powerful tools in this pursuit, due to their binding properties and highly customizable structures. Indeed, crown ether derivatives, such as monoalkylated19 and dialkylated lariat ethers,20,21 amphiphilic benzo(crown) ether derivatives,22 hydraphiles,23 and ion shuttles24 have been demonstrated to function as ionophores. Despite these successful examples, our understanding of the mechanisms of ion transport by these crown ether derivatives remains extremely limited. An improved understanding of these processes will provide critical insights that will both advance fundamental knowledge about ion transport mechanisms and provide a framework for the rational design of synthetic ionophores with well-defined properties.
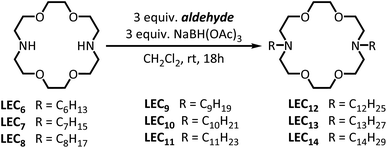
Towards this aim, we identified previously reported dialkylated diaza crown ethers20,21 as an ideal starting point. Lariat ethers are crown ether analogues with one or more sidearms attached to the macrocyclic core structure.25 The possibility of adding customized pendant groups to the crown ether core allows a high degree of selective modification to their physical parameters.25 Lariat ethers have also been reported to bind alkali cations and behave as ionophores in bulk liquid membranes and ion-selective electrodes.26–29 The dialkylated diaza(18-crown-6) ethers, a subset of the lariat class, have been reported to have toxic activity towards prokaryotic and eukaryotic cells.20 Evidence from toxicity and depolarization assays initially suggested that these compounds behave as ion carriers.20 However, experiments in asolectin bilayers revealed that dioctylated and diundecylated lariat ethers elicit discrete increases in membrane conductance,21 a result typical of ion channels, as opposed to ion carriers. Moreover, the effect of the alkyl chain lengths on the toxicity and transport implied that the interaction of these compounds with a bilayer membrane differs from their behaviour in a bulk liquid membrane. In general, the literature suggests that hydrophobic lariat ethers that bear longer alkyl chains function as more efficient cation carriers.20,30 In contrast, dialkylated lariat ethers show peak activity when a 10 carbon chain is present on the core, with the activity diminishing with increasing chain length.20 This observation prompted Leevy and co-workers to propose that dialkylated lariat ethers require a minimum hydrophobicity to act as ion carriers, but when the alkyl chains are too long, the molecules are able to nest inertly within the membrane.20 In order to test this mechanism and establish a deeper understanding of their transport behavior in membranes, we focused on a representative set of lariat ethers bearing dialkylated tails ranging from 6 to 14 carbons.
The series of dialkylated diaza(18-crown-6) ether were prepared by a simple one-step reductive amination of 4,13-diaza(18-crown-6) and the appropriate aldehyde (Fig. 1; Methods in ESI†), as opposed to previous two-step procedures.20,21 The notation LECn refers to the dialkylated lariat ether substituted with an alkyl chains of n carbons. The cytotoxicity was determined by measuring the minimum inhibitory concentrations (MIC) for this series of dialkylated lariat ethers in the Gram-negative bacteria Escherichia coli, the Gram-positive bacteria Bacillus subtilis, and human embryonic kidney (HEK293T) cells. The results showed that B. subtilis is more susceptible to LEC6–LEC14, as judged by lower MICs; LEC10 is the most toxic to E. coli (MIC = 10 μM), while LEC10, LEC11, and LEC12 are the most toxic to B. subtilis at concentrations as low as 2 μM (Fig. S1†), results that are in good agreement with similar studies20 (Fig. S1, ESI†). We also confirmed a remarkable discontinuity in the toxicity between LEC12 and LEC13, where the addition of only a single methylene group to the alkyl chain completely abrogates the toxicity towards B. subtilis from a MIC = 2 μM with LEC12 to undetectable with LEC13 at concentration as high as 400 μM. The toxicities of dialkylated lariat ethers towards HEK293T cells were more consistent than the trends with E. coli and B. subtilis; however, LEC10 (MIC = 6 μM) proved to be the most toxic lariat ether towards all three tested cellular systems.
Dialkylated lariat ethers LEC6–LEC14 were then tested for their ability to depolarize a B. subtilis membrane using the fluorescent dye 3,3-dipropylthiadicarbocyanine (DiSC3(5)), which undergoes membrane voltage-dependent partitioning between the intracellular and the extracellular medium.31 Cell hyperpolarization (more negatively charged inside the cell) results in an uptake of the dye, while cell membrane depolarization (more positively charged inside the cell) results in a release of the dye. The accumulation of the dye in the interior of the cell can be detected by a decrease in fluorescence due to self-quenching,31 which enables the dye to be utilized as an indirect reporter of changes in cell membrane voltage.32 As the resting membrane voltage in B. subtilis is approximately −120 mV,33 DiSC3(5) quickly accumulates inside intact bacteria (timepoint t2 in Fig. S2a–i†). The addition of up to 60 mM KCl (violet curve, timepoint t3 in Fig. S2a–i†) does not cause substantial membrane depolarization, as determined by the limited increase in fluorescence, mainly because the endogenous K+ transporters and channel activities34–38 are inhibited at 25 °C. However, upon addition of the dialkylated lariat ether (timepoint t4 in Fig. S2a–i†), ions move down the electrochemical gradient and give rise to membrane depolarization, as evidenced by an increase in fluorescence. The effects of the alkyl chain length on the relative DiSC3(5) release after 10 minutes following addition of the dialkylated lariat ether (Fig. S3†) were qualitatively similar to those reported previously.20 The dialkylated lariat ethers with the highest toxicities elicited faster DiSC3(5) efflux in the presence of K+, suggesting they are more efficient at transporting cations (Fig. S2a–i and S3; † violet curves). As the induction of membrane depolarization is consistent with an ionophoric mechanism, the next step was to determine the cation selectivity exhibited by this class of the compounds.
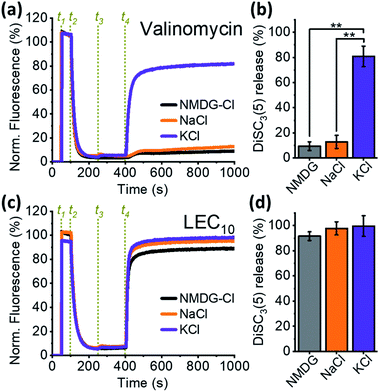
Binding to ionophores requires at least a partial substitution of water molecules in the hydration sphere by ions to achieve an ionophore-like transport mechanism. Thus, some degree of ion selectivity is expected,26–29 as observed in the case of valinomycin, a natural carrier-type ionophore that is extremely selective for K+ over Na+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39,40 and does not transport N-methyl-D-glucamine (NMDG+). When depolarization assays were performed in the presence of different cations, valinomycin promoted DiSC3(5) release only when K+ was added to the external solution, but not when Na+ or NMDG+ was added (Fig. 2a and b). Conversely, when LEC10 was tested under the same conditions, no significant differences in relative DiSC3(5) release were observed (Fig. 2c and d). Furthermore, our control experiments show that even in the absence of the alkali cation (Fig. S2a–i, and S3;† black curves), the relative DiSC3(5) release rates were similar to those observed in the presence of K+ (Fig. S2a–i and S3;† violet curve). This unexpected result forced us to consider two possible explanations. Either the dialkylated lariat ethers behave as non-selective ionophores that are capable of transporting large cations, such as NMDG+, or their primary effect is to disrupt membrane integrity, i.e. the DiSC3(5) efflux is due to the lysis of the cells, rather than ion transport across the membrane.
39,40 and does not transport N-methyl-D-glucamine (NMDG+). When depolarization assays were performed in the presence of different cations, valinomycin promoted DiSC3(5) release only when K+ was added to the external solution, but not when Na+ or NMDG+ was added (Fig. 2a and b). Conversely, when LEC10 was tested under the same conditions, no significant differences in relative DiSC3(5) release were observed (Fig. 2c and d). Furthermore, our control experiments show that even in the absence of the alkali cation (Fig. S2a–i, and S3;† black curves), the relative DiSC3(5) release rates were similar to those observed in the presence of K+ (Fig. S2a–i and S3;† violet curve). This unexpected result forced us to consider two possible explanations. Either the dialkylated lariat ethers behave as non-selective ionophores that are capable of transporting large cations, such as NMDG+, or their primary effect is to disrupt membrane integrity, i.e. the DiSC3(5) efflux is due to the lysis of the cells, rather than ion transport across the membrane.
To determine whether transport of NMDG+ can account for the observed efflux of DiSC3(5) from cells, we tested LEC10 activity in a cation-free dextrose solution (see Methods, ESI†). In this experiment, the only cation in the external solution (if any) is added at timepoint t3 (Fig. S4a†). Addition of 2 μM LEC10 produced a large DiSC3(5) release in the presence of KCl (Fig. S4a,† violet curve), but interestingly, this same effect was also observed in the absence of any external cation (Fig. S4a and b,† black curve; additional dextrose solution was added at timepoint t4). The independence of the DiSC3(5) efflux from the identity or the presence of the external cations is not compatible with an ionophore-like mechanism.
Previous studies indicated that lariat ethers reported to display the ability to collapse membrane potential in depolarization assays also exhibited ion channel-like activity in bilayers. This suggested that we might employ similar depolarization assays to serve as a convenient surrogate for measuring electrical activity. We tested our most potent compound, LEC10, for ion channel activity in planar lipid bilayers. As a control, unitary channels of the ionophore gramicidin were recorded in the presence of a KCl solution (150 mM) (Fig. S5a†). However, no ion channel activity was detected in a similar experiment performed using 2 μM LEC10 (Fig. S5b†), even after one hour of recording. Variations in the LEC10 concentration (10, 100, and 200 μM), as well as the KCl concentration (up to 500 mM), including replacing KCl with NaCl (see Methods, ESI†), resulted in no indication of ion channel activity. This lack of unitary channel formation, or even a carrier-like increase of the conductance, precludes an ionophore-like mechanism of ion transport.
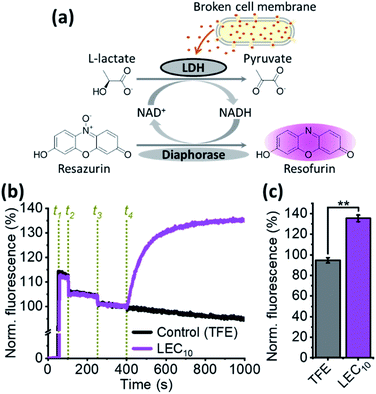
Given our findings that this class of dialkylated lariat ether derivatives do not act as typical ionophores, we next considered the possibility that the activity of these compounds results from disruption of membrane integrity. A well-established assay was utilized to measure cell lysis by monitoring the release of the enzyme lactate dehydrogenase (LDH), which is ubiquitous in the cytoplasm of all cell types. The tetrameric active form of LDH catalyzes the final step of the glycolysis in B. subtilis and has a molecular weight of approximately 146 kDa in,41,42 with a nearly globular shape of an approximated radius of 80 Å (PDB ID: 3PQD). The release of proteins of this size demonstrates the test compounds are likely to cause cell lysis but we cannot rule out the possibility that they form large pores. LDH couples two redox reactions, the first involving the interconversion of pyruvate (oxidized) and L-lactate (reduced) and the second, the interconversion of NAD+ (oxidized) and NADH (reduced). The NADH oxidation can be coupled to the diaphorase-catalyzed oxidation of resazurin to resofurin, which is highly fluorescent (Fig. 3a). When the concentrations of L-lactate, NAD+, diaphorase, and resazurin are saturated, the rate of increase in the resofurin fluorescence is limited only by the amount of available LDH in the medium. Our experiments show that the addition of LEC10 to a mixture containing B. subtilis cells and the other components necessary for the LDH assay (see Methods, ESI†) causes a rapid rise in the fluorescence (Fig. 3b and c), indicating loss of membrane integrity. In contrast, the addition of the same amount of the trifluoroethanol (TFE) solvent used to dissolve LEC10 did not produce any increase in the fluorescence (Fig. 3b and c).
In summary, we report one-step syntheses of dialkylated diaza(18-crown-6) ether derivatives and an in-depth evaluation of their behaviour as ionophores for cell membranes. The acute release of LDH, a complete lack of ion specificity, depolarization in the absence of extracellular ions, and a lack of discrete changes in the conductance in the planar lipid bilayers solidly demonstrates that biological activities of these lariat ethers are due to their membrane lytic activity, as opposed to the expected ion transport activity. Our findings warrant re-evaluation of the mechanisms of activity of many previously reported synthetic ionophores, which have been classified as such based only on cell survival assays and depolarization assays, without attention to detailed electrical activity measurements. We also note that even in cases where ionophores do not show electrical activity, due to transport via carrier type mechanisms, our control experiments are able to discriminate between ion carrier and lytic activity.
Funding
The NMR and Mass Spectrometry facilities are funded by the NSF (CHE-1048642, CHE-0342998, CHE-9304546 and CHE-9208463), the UW-Madison as well as a generous gift by Paul J. and Margaret M. Bender. We thank Prof. A Prindle and Peter Tran (Northwestern University) for generously sharing B. subtilis strains. We are grateful to Dr Jiang, Dr Chowdhury and Dr Cowgill for their generous help and training. This work was supported by funding from the UW2020 competition and the NIGMS award 5R21GM131662 (B. C. and J. M. S.) and NINDS award R35NS116850 (B. C.).Conflicts of interest
There are no conflicts to declare.Notes and references
- C. J. Pedersen, J. Am. Chem. Soc., 1967, 89, 2495–2496 CrossRef CAS.
- C. J. Pedersen and H. K. Frensdorff, Angew. Chem., Int. Ed., 1972, 11, 16–25 CrossRef CAS.
- M. Shamsipur, R. Davarkhah and A. R. Khanchi, Sep. Purif. Technol., 2010, 71, 63–69 CrossRef CAS.
- T. B. Stolwijk, E. J. Sudhoelter and D. N. Reinhoudt, J. Am. Chem. Soc., 1987, 109, 7042–7047 CrossRef CAS.
- G. W. Gokel, W. M. Leevy and M. E. Weber, Chem. Rev., 2004, 104, 2723–2750 CrossRef CAS.
- W.-W. Tso and W.-P. Fung, Inorg. Chim. Acta, 1980, 46, L33–L340 CrossRef CAS.
- W.-W. Tso and W.-P. Fung, Inorg. Chim. Acta, 1981, 55, 129–134 CrossRef CAS.
- W.-W. Tso, W.-P. Fung and M.-Y. Tso, J. Inorg. Biochem., 1981, 14, 237–244 CrossRef CAS.
- D. L. Bennett, A. J. Clark, J. Huang, S. G. Waxman and S. D. Dib-Hajj, Physiol. Rev., 2019, 99, 1079–1151 CrossRef CAS.
- E. Bourinet, C. Altier, M. E. Hildebrand, T. Trang, M. W. Salter and G. W. Zamponi, Physiol. Rev., 2014, 94, 81–140 CrossRef CAS.
- M. Endo, Physiol. Rev., 2009, 89, 1153–1176 CrossRef CAS.
- A. Horowitz, C. B. Menice, R. Laporte and K. G. Morgan, Physiol. Rev., 1996, 76, 967–1003 CrossRef CAS.
- E. Balse, D. F. Steele, H. Abriel, A. Coulombe, D. Fedida and S. N. Hatem, Physiol. Rev., 2012, 92, 1317–1358 CrossRef CAS.
- M. N. Foster and W. A. Coetzee, Physiol. Rev., 2016, 96, 177–252 CrossRef CAS.
- N. Schmitt, M. Grunnet and S. P. Olesen, Physiol. Rev., 2014, 94, 609–653 CrossRef CAS.
- L. Aguilar-Bryan, J. Bryan and M. Nakazaki, Recent Prog. Horm. Res., 2001, 56, 47–68 CrossRef CAS.
- P. Rorsman and F. M. Ashcroft, Physiol. Rev., 2018, 98, 117–214 CrossRef CAS.
- J. R. M. Harvey, A. E. Plante and A. L. Meredith, Physiol. Rev., 2020, 100, 1415–1454 CrossRef.
- Q. Xie, Y. Li, G. Gokel, J. Hernández and L. Echegoyen, J. Am. Chem. Soc., 1994, 116, 690–696 CrossRef CAS.
- W. M. Leevy, M. E. Weber, M. R. Gokel, G. B. Hughes-Strange, D. D. Daranciang, R. Ferdani and G. W. Gokel, Org. Biomol. Chem., 2005, 3, 1647–1652 RSC.
- S. Negin, M. B. Patel, M. R. Gokel, J. W. Meisel and G. W. Gokel, Chembiochem, 2016, 17, 2153–2161 CrossRef CAS.
- T. Liu, C. Bao, H. Wang, Y. Lin, H. Jia and L. Zhu, Chem. Commun., 2013, 49, 10311–10313 RSC.
- S. Negin, B. A. Smith, A. Unger, W. M. Leevy and G. W. Gokel, Int. J. Biomed. Imaging, 2013, 2013, 1–11 CrossRef.
- S. Chen, Y. Wang, T. Nie, C. Bao, C. Wang, T. Xu, Q. Lin, D. H. Qu, X. Gong, Y. Yang, L. Zhu and H. Tian, J. Am. Chem. Soc., 2018, 140, 17992–17998 CrossRef CAS.
- G. W. Gokel and L. Echegoyen, Bioorganic Chemistry Frontiers, ed. H. Dugas, Springer Verlag Inc., 1990, pp. 116–141 Search PubMed.
- J. C. Ball, J. R. Allen, J. Y. Ryu, S. Vickery, L. Cullen, P. Bukowski, T. Cynkowski, S. Daunert and L. G. Bachas, Electroanalysis, 2002, 14, 186–191 CrossRef CAS.
- P. Bühlmann, E. Pretsch and E. Bakker, Chem. Rev., 1998, 98, 1593–1688 CrossRef.
- D. M. Dishong, C. J. Diamond, M. I. Cinoman and G. W. Gokel, J. Am. Chem. Soc., 1983, 105, 586–593 CrossRef CAS.
- T. Mizuno, Y. Nakatsuji, S. Yanagida and M. Okahara, Bull. Chem. Soc. Jpn., 1980, 53, 481–484 CrossRef CAS.
- J. C. Hernandez, J. E. Trafton and G. W. Gokel, Tetrahedron Lett., 1991, 32, 6269–6272 CrossRef CAS.
- P. J. Sims, A. S. Waggoner, C. H. Wang and J. F. Hoffman, Biochemistry, 1974, 13, 3315–3330 CrossRef CAS.
- M. Wu, E. Maier, R. Benz and R. E. Hancock, Biochemistry, 1999, 38, 7235–7242 CrossRef CAS.
- A. Zaritsky, M. Kihara and R. M. Macnab, J. Membr. Biol., 1981, 63, 215–231 CrossRef CAS.
- M. Fujisawa, Y. Wada and M. Ito, FEMS Microbiol. Lett., 2004, 231, 211–217 CrossRef CAS.
- J. Gundlach, C. Herzberg, V. Kaever, K. Gunka, T. Hoffmann, M. Weiß, J. Gibhardt, A. Thürmer, D. Hertel, R. Daniel, E. Bremer, F. M. Commichau and J. Stülke, Sci. Signaling, 2017, 10, 1–9 CrossRef.
- G. Holtmann, E. P. Bakker, N. Uozumi and E. Bremer, J. Bacteriol., 2003, 185, 1289–1298 CrossRef CAS.
- J. Humphries, L. Xiong, J. Liu, A. Prindle, F. Yuan, H. A. Arjes, L. Tsimring and G. M. Süel, Cell, 2017, 168, 200–209 CrossRef CAS.
- A. Prindle, J. Liu, M. Asally, S. Ly, J. Garcia-Ojalvo and G. M. Süel, Nature, 2015, 527, 59–63 CrossRef CAS.
- G. Eisenman and O. Alvarez, in Biomembrane Structure & Function - The State of the Art, ed. B. P. Gaber and K. R. K. Easwaran, Adenine Press, Schenectady, NY, 1992, pp. 321–351 Search PubMed.
- S. Varma, D. Sabo and S. B. Rempe, J. Mol. Biol., 2008, 376, 13–22 CrossRef CAS.
- N. Richter, A. Zienert and W. Hummel, Eng. Life Sci., 2011, 11, 26–36 CrossRef CAS.
- Y. Zhang and X. Gao, Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun., 2012, 68, 63–65 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Methods, NMR and MS data, MIC determination, DiSC3(5) release assays. See DOI: 10.1039/d0ra08494h |
| This journal is © The Royal Society of Chemistry 2020 |