Open Access Article
Open Access ArticleA study on the effect of four thermoplastic elastomers on the properties of double-base propellants
Xiaobin Zou a,
Wenguo Zhanga,
Yongjun Gub,
Xiuchong Fub,
Zehao Zhanga,
Zhen Ge*a and
Yunjun Luo
a,
Wenguo Zhanga,
Yongjun Gub,
Xiuchong Fub,
Zehao Zhanga,
Zhen Ge*a and
Yunjun Luo *a
*a
aSchool of Materials Science and Engineering, Beijing Institute of Technology, Beijing, 100081, China. E-mail: gzandlsy@bit.edu.cn; yjluo@bit.edu.cn; Fax: +86-10-68911608; Tel: +86-10-68911608
bShanxi North Xing'an Chemical Industry Co., Ltd., Shanxi 030008, China
First published on 25th November 2020
Abstract
To improve the low-temperature mechanical properties of double-base propellants, glycidyl azide polymer-energetic thermoplastic elastomer (GAP-ETPE), polyether block copolyimide-thermoplastic elastomer (PEBA-TPE), 1,2-polybutadiene thermoplastic elastomer (PB-TPE), and ethylene oxide-tetrahydrofuran copolyester-thermoplastic elastomer (PET-TPE) were used to modify them. The effects of these four thermoplastic elastomers (TPEs) on the energy properties of double-base propellants were studied via theoretical calculations. The mechanical properties of double-base propellants at high, low, and room temperature were compared, and their thermal properties were analyzed. It was found that GAP-ETPE had a significant effect on improving the low-temperature mechanical properties of double-base propellants, with the maximum tensile strength and maximum elongation at a low temperature for the double-base propellant reaching 43.30 MPa and 6.24%, respectively.
1 Introduction
A double-base propellant is a kind of solid propellant that is widely used in free-loading missiles and rocket engines.1In general, the main components of a double-base propellant are nitrocellulose (NC) and nitroglycerin (NG), along with ballistic stabilizers, combustion catalysts, and other additives.2,3 Nitrocellulose is the main structural material of double-base propellants,4,5 and nitroglycerin acts as the plasticizer for nitrocellulose. Double-base propellants show viscoelasticity above their glass transition temperature.6–8 However, double-base propellants are in the glassy state at a lower temperature, which would become brittle and easy to crack. As a result, the mechanical properties of double-base propellants are very poor at low temperatures. Therefore, the application range of double-base propellants is limited.
There are two main methods to improve the low-temperature mechanical properties of solid propellants: addition of polymer materials with good low-temperature mechanical properties (mostly a thermoplastic elastomer) and low-temperature plasticizer, respectively. The first is the most effective way. Thermoplastic elastomers (TPEs) are also called artificial rubber or synthetic rubber. TPEs have numerous characteristics of convenient processing and wide processing methods of ordinary plastics as well as various excellent properties such as resistant to oil and aging, and high elasticity of traditional cross-linked vulcanized rubber.9 TPE as a solid propellant binder was proposed in the late 1970s, and there had been a lot of research so far.10–12
Guo13 used varied proportions of GAP-ETPE/NC as binders to improve the thermal and mechanical properties of a nitramine propellant. The study found that after introducing GAP-ETPE into the binder system, the thermal stability of the propellant greatly improved. When the ratio of GAP-ETPE/NC in the binder system was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the mechanical properties of the nitramine propellant was relatively excellent, and the maximum impact strength increased from 3.58 kJ m−2 to 7.593.58 kJ m−2 compared with the formula without GAP-ETPE.
2, the mechanical properties of the nitramine propellant was relatively excellent, and the maximum impact strength increased from 3.58 kJ m−2 to 7.593.58 kJ m−2 compared with the formula without GAP-ETPE.
Bozic14 researched the introduction of a polyether block copolyimide-thermoplastic elastomer (PEBA-TPE) into solid propellants, which showed that the energy was higher than that of active HTPB-TPE/AP propellants, and it had appropriate mechanical properties as well as excellent processing performance and aging characteristics at a low cost. The properties of 1,2-polybutadiene are related to its crystallinity. When the crystallinity is 10–40%, 1,2-butadiene became a thermoplastic elastomer, namely 1,2-polybutadiene thermoplastic elastomer (PB-TPE). PB-TPE is a homopolymer with a crystalline phase as a hard segment and an amorphous phase as a soft segment. The application of PB-TPE to solid propellants have been reported.15,16 The mechanical properties and processability have also been improved.
Chen17 synthesized an ethylene oxide-tetrahydrofuran copolyester-thermoplastic elastomer (PET-TPE) with PET and polyethylene glycol (PEG) mixed polyether as the soft segment. When the molecular weight and content of PEG were controlled to 4000 g mol−1 and 6% of soft segments, respectively, the property of PET-TPE was optimal. The propellant prepared with the thermoplastic elastomer had good processing temperature and excellent mechanical properties. The normal (20 °C), high (50 °C), and low (−40 °C) temperature tensile strengths were 1.14 MPa, 0.51 MPa, and 9.94 MPa, respectively. The combustion properties showed that the maximum impulse reaches up to 265–270 s which was slightly higher than that of the HTPB propellant. Also, the combustion of the propellant was easy to control.
In order to solve the problem of double-base propellant's brittleness at a lower temperature, four TPEs (PET-TPE, GAP-ETPE, PEBA-TPE, and PB-TPE) were selected as modifiers of the double-base propellant. In this study, the change in the theoretical properties of the propellant was compared by theoretical calculations, and the change in mechanics and α transition temperature were measured by a static tensile mechanical property test and dynamic thermomechanical analysis, respectively. The influence of TPEs on the thermal decomposition behavior of a double-base propellant was analyzed by a thermogravimetry test. Combined with the influence of the double-base propellant on mechanics and energy, the best type of thermoplastic elastomer was determined.
2 Experimental
2.1 Materials
Tetrahydrofuran (THF), AR, was purchased from Beijing Chemical Reagent Factory. Deionized water and PET-thermoplastic polyurethane elastomer (PET-TPE),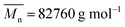 , were prepared in the laboratory.18 GAP-energetic thermoplastic polyurethane elastomer (GAP-ETPE),
, were prepared in the laboratory.18 GAP-energetic thermoplastic polyurethane elastomer (GAP-ETPE), 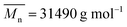 , was synthesized in the laboratory.19 Polyether block copolyimide elastomer (PEBA-TPE),
, was synthesized in the laboratory.19 Polyether block copolyimide elastomer (PEBA-TPE), 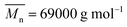 , was purchased from Arkema, France. 1,2-Polybutadiene thermoplastic elastomer (PB-TPE);
, was purchased from Arkema, France. 1,2-Polybutadiene thermoplastic elastomer (PB-TPE); 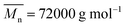 , was purchased from JSR, Japan. A compound of nitrocellulose and nitroglycerin (NG: 47.5%, NC: 51.5%, dimethyl diphenyl urea: 1.0%), NC (nitrogen content: 12.6%), dinitrotoluene (DNT), titanium dioxide (TiO2); adipic acid copper (d-Cu), purchased from Shanxi North Xing'an Chemical Industry Co., Ltd, China, were of technical grade.
, was purchased from JSR, Japan. A compound of nitrocellulose and nitroglycerin (NG: 47.5%, NC: 51.5%, dimethyl diphenyl urea: 1.0%), NC (nitrogen content: 12.6%), dinitrotoluene (DNT), titanium dioxide (TiO2); adipic acid copper (d-Cu), purchased from Shanxi North Xing'an Chemical Industry Co., Ltd, China, were of technical grade.
2.2 Characterization and analysis
(1) Theoretical calculationNASA “propellant property computer” software calculated the specific impulse (Is), oxygen balance (OB), combustion temperature (Tc), explosion heat (Q), and density (ρ) of each formula, and the test pressure was 7 MPa.
(2) Static tensile mechanical property test
The static tensile mechanical property test was performed on an AGS-J, Shimadzu, Japan. Test method is referenced ASTMD638-2003. The stretch rate was 10 mm min−1, and the test temperature was −40 °C, 20 °C, and 50 °C.
(3) Dynamic thermomechanical analysis (DMA)
The dynamic thermomechanical analysis was performed on a DMA/SDTA861e, METTLER-TOLEDO, Switzerland. The sample size was 4 × 4 mm, the frequency was 1 Hz, the amplitude was 5 μm, the heating rate was 2 K min−1, and the range of temperature was –100 to 120 °C.
(4) Thermogravimetric analysis (TG)
The thermogravimetric analysis was performed on a TGA/DSC1SF/417-2, METTLER-TOLEDO, Switzerland. The sample mass was less than 1 mg, the test temperature was 30–600 °C, the heating rate was 10 K min−1, and the N2 flow rate was 40 mL min−1.
2.3 Preparation of a thermoplastic elastomer-modified double-base propellant
| Propellant formula | NC | NG | Elastomer | DNT | TiO2 | d-Cu | C2 |
|---|---|---|---|---|---|---|---|
| DB-0 | 52.3 | 39.2 | 0 | 2.2 | 1.5 | 3 | 1.8 |
| DB-1 | 50.3 | 37.2 | 4 | 2.2 | 1.5 | 3 | 1.8 |
| DB-2 | 50.3 | 37.2 | 4 | 2.2 | 1.5 | 3 | 1.8 |
| DB-3 | 50.3 | 37.2 | 4 | 2.2 | 1.5 | 3 | 1.8 |
| DB-4 | 50.3 | 37.2 | 4 | 2.2 | 1.5 | 3 | 1.8 |
In the formulas, dinitrotoluene (DNT) could increase the energy of the propellant and act as a cosolvent; TiO2 acts as a ballistic stabilizer; copper adipate (d-Cu) as a catalyst; dimethyl diphenyl urea (C2) plays a stabilizing role.
3 Results and discussion
3.1 Theoretical calculation of the properties of the thermoplastic elastomer-modified double-base propellant
According to the molecular formula, density, and enthalpy of formation of each thermoplastic elastomer, the theoretical values of density (ρ), specific impulse (Is), oxygen balance (OB), combustion temperature (Tc), and explosion heat (Q) for double-base propellant formulas were calculated by the NASA “prospect property computer” software, as shown in Table 2.| Parameter | DB-0 | DB-1 | DB-2 | DB-3 | DB-4 |
|---|---|---|---|---|---|
| ρ/g cm−3 | 1.620 | 1.599 | 1.583 | 1.565 | 1.580 |
| Is/s | 237.9 | 234.7 | 232.6 | 223.5 | 225.8 |
| OB | 0.645 | 0.623 | 0.594 | 0.569 | 0.598 |
| Tc/K | 2656.9 | 2590.0 | 2516.5 | 2346.2 | 2431.3 |
| Q/kJ kg−1 | 3758 | 3659 | 3569 | 3306 | 3441 |
It can be seen from Table 2 that the theoretical values of ρ, Is, OB, Tc, and Q of double-base propellants was reduced with the addition of four thermoplastic elastomers. In terms of density, GAP-ETPE had the smallest impact on the propellant, whereas PB-TPE had the largest impact. In terms of energy, GAP-ETPE was the only energetic thermoplastic material among four TPEs, so the theoretical specific impulse of the double-base propellant decreased relatively less with the addition of GAP-ETPE, and the energy level of the propellant was not greatly reduced. The other three TPEs caused the specific impulse, combustion temperature, and explosion heat of double-base propellants to be reduced to a certain extent. From another aspect, the introduction of TPEs reduced the combustion temperature, which improved the ablation resistance of the propellant engine. Thus, the above analysis shows that it is not suitable to add too much elastomer.
3.2 Static mechanical properties of the thermoplastic elastomer-modified double-base propellant
The static mechanical results of the original formula and the four thermoplastic elastomer-modified double-base propellants at room temperature, low temperature, and high temperature are shown in Fig. 2, 3, and 4, respectively.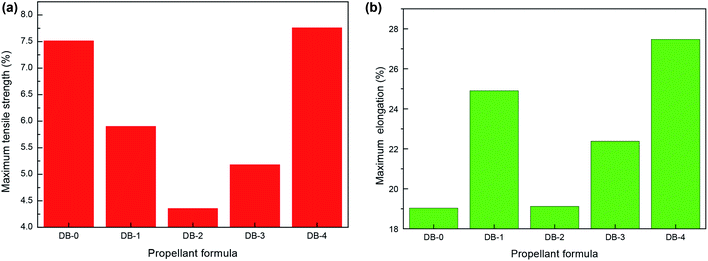 | ||
| Fig. 2 The static mechanical properties of double-base propellants at room temperature (20 °C). (a) Maximum tensile strength. (b) Maximum elongation. | ||
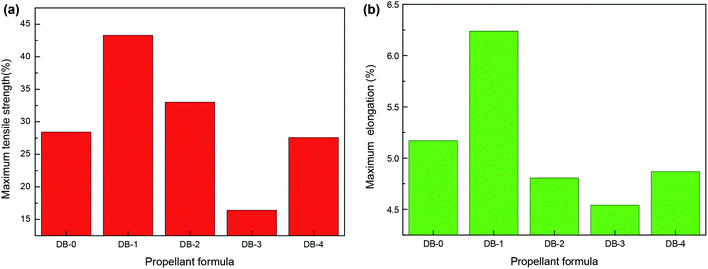 | ||
| Fig. 3 The static mechanical properties of double-base propellants at low temperature (−40 °C). (a) Maximum tensile strength. (b) Maximum elongation. | ||
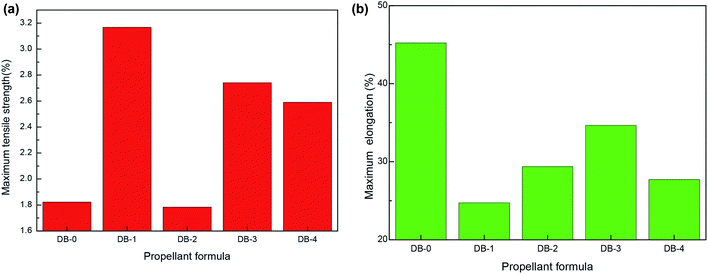 | ||
| Fig. 4 The static mechanical properties of double-base propellants at high temperature (50 °C). (a) Maximum tensile strength. (b) Maximum elongation. | ||
It can be seen from Fig. 2–4 that the mechanical properties of DB-2 had decreased at room temperature and high temperature, but the maximum tensile strength was 33.03 MPa at a low temperature, which was slightly improved compared with DB-0. For DB-3, the mechanical properties of the propellant decreased at a low temperature and room temperature except for the small increase at a high temperature, where the maximum tensile strength reached 2.74 MPa. For DB-4, the addition of PET-TPE improved the mechanical properties at room temperature and the maximum tensile strength at a high temperature.
The maximum tensile strength of DB-1 at a low temperature increased from 28.42 MPa to 43.30 MPa, and the maximum elongation increased from 5.17% to 6.24% as compared to DB-0. The mechanical properties of double-base propellants were improved by adding GAP-ETPE for the following reasons: on the one hand, as an energetic binder, GAP-ETPE itself had good mechanical properties, which interacted with the double-based component, particularly the nitrocellulose molecular chain as a skeleton structure, through a secondary bond. In addition, since both nitrocellulose and GAP-ETPE have longer molecular chains, the entanglement would occur between the molecular chains, and the bonding effect occurred. On the other hand, GAP-ETPE played a role of “intramolecular plasticization” in semi-rigid cellulose macromolecules, the molecular chain spacing was widened, and thus the elongation of NC was increased.20 However, at a high temperature (50 °C), as the thermal movement of the molecular chains strengthened, the distance between the molecular chains increased, and the hydrogen bonding effect weakened, the adhesion of GAP-ETPE to the double-based components was reduced. Besides, the reduction of nitroglycerin was not conducive to plasticizing nitrocellulose and GAP-ETPE, so the maximum tensile strength of DB-1 a high temperature was lower than that of DB-0.
Based on the mechanical properties at room temperature, low temperature, and high temperature as a reference, it can be seen from Fig. 2–4 and analysis above that DB-1 has good mechanical properties at the room temperature, low temperature, and high temperature. To summarize, among the four thermoplastic elastomers, GAP-ETPE had the best effect on improving the mechanical properties of the double-base propellant.
3.3 Dynamic thermomechanical analysis of the thermoplastic elastomer-modified double-base propellant
Since the double-base propellant is a heterogeneous system, its relaxation process is complicated. Two relaxation processes could be obtained by the DMA test. The one at a lower temperature is called β relaxation, and the other at a higher temperature is called α relaxation. It is widely believed that polymer materials are in a rigid glassy state below the glass transition temperature (Tg). However, below Tg, a double-base propellant could still undergo a forced high-elastic deformation. Therefore, the double-base propellant still maintained a certain elasticity between α and β transition temperature. Some researchers have used β transition as the glass transition temperature of double-base propellants in the past.21 However, during the β transition of the double-base propellant, in addition to the modulus, there were some complicated relaxation behaviors such as sudden changes of specific heat capacity and thermal expansion coefficient. The β transition temperature was in a wide range, so it could not be accurately used as the glass transition temperature of the double-base propellant. In the DMA test, at low frequencies, the loss modulus E′′ of the α relaxation of the double-base propellant was a shoulder peak corresponding to β relaxation, which was difficult to measure accurately, but the peak of loss tangent tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of α relaxation was the largest and clearest, and it had good repeatability and reproducibility by many experiments. Therefore, in the DMA test, the α-transition temperature of the loss tangent was usually used as Tg of the double-base propellant by some researchers,22,23 which was also a common reference in the present study.
δ of α relaxation was the largest and clearest, and it had good repeatability and reproducibility by many experiments. Therefore, in the DMA test, the α-transition temperature of the loss tangent was usually used as Tg of the double-base propellant by some researchers,22,23 which was also a common reference in the present study.
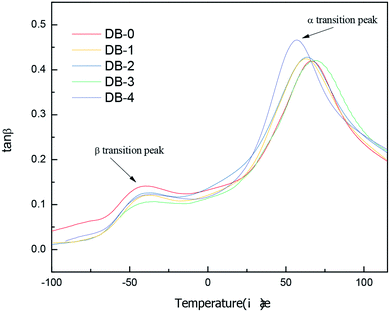
In this study, DB-0, DB-1, DB-2, DB-3, and DB-4 were tested by DMA. The change in the loss tangent of the elastomer was studied, and the corresponding α transition temperature was obtained. The results are shown in Fig. 5.
Table 3 shows the loss tangent α transition temperature and peak value results of double-base propellants by the DMA test.
| Propellant formula | α transition peak value | α transition temperature/°C |
|---|---|---|
| DB-0 | 0.419 | 66.9 |
| DB-1 | 0.424 | 62.9 |
| DB-2 | 0.428 | 63.9 |
| DB-3 | 0.420 | 69.3 |
| DB-4 | 0.466 | 56.5 |
It can be seen from Fig. 5 that the tangent curve of double-base propellants has two-loss peaks. The peak position of the β transition was between −60 °C and 10 °C and was related to the joint movement of NG and NC molecular side groups. The position of the α transition peak was at about 50–70 °C, which showed that TPE-modified double-base propellants were in the glassy state above room temperature. Compared with DB-0, the peak value of the α transition increased with the addition of TPEs. DB-4 had the highest peak value of α-transition. The α transition temperature of DB-0, DB-1, DB-2, DB-3, and DB-4 were 66.9 °C, 62.9 °C, 63.9 °C, 69.3 °C, and 56.5 °C, respectively. Compared with DB-0, the addition of PB-TPE increased the α transition temperature of the double-base propellant, while the addition of PEBA-TPE, GAP-ETPE, and PET-TPE decreased Tg, particularly the formula containing PET-TPE, which decreased Tg to 10 °C.
From the glassy state to the high elastic state, the motion of the small units occurred first, and then the chain segmental motion of the molecular main chains occurred. The addition of thermoplastic elastomers reduced the α transition temperature of the propellant system and increased the operating temperature range of the propellant. It can be seen from Section 3.2 that the addition of thermoplastic elastomers not only improved the tensile strength of the propellant at a low temperature but also ensured that the tensile strain was almost unchanged. Therefore, the mechanical properties of the double-base propellant at the low temperature were improved, which was consistent with the increase of the peak value of α transition and the decrease in the α transition temperature in the DMA curve.
3.4 Thermogravimetric analysis of the thermoplastic elastomer-modified double-base propellant
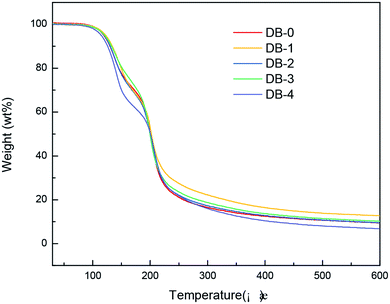
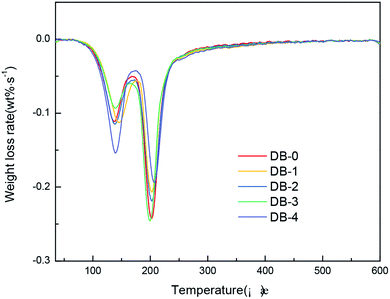
The thermogravimetric tests of the five propellant formulas were carried out. The results are shown in Fig. 6 and 7.Table 4 showed initial decomposition temperature, maximum decomposition rate, and temperature of the maximum decomposition rate of double-base propellants obtained by the TG test.
| Propellant formula | Temperature of initial decomposition | Stage 1 | Stage 2 | ||
|---|---|---|---|---|---|
| Maximum decomposition rate/% s−1 | Temperature of maximum decomposition rate/°C | Maximum decomposition rate/% s−1 | Temperature of maximum decomposition rate/°C | ||
| a The initial decomposition temperature is referred to as the temperature when the weight loss rate of the sample was 5%. | |||||
| DB-0 | 119.4 | −0.11 | 138.2 | −0.24 | 202.3 |
| DB-1 | 120.6 | −0.09 | 138.8 | −0.25 | 199.3 |
| DB-2 | 119.9 | −0.11 | 138.6 | −0.22 | 202.5 |
| DB-3 | 122.4 | −0.11 | 144.8 | −0.21 | 202.6 |
| DB-4 | 114.8 | −0.15 | 139.2 | −0.19 | 206.6 |
The thermal decomposition reaction of NC and NG in the double-base propellant was autocatalytic. The decomposition products played a catalytic role in the decomposition, and the decomposition released a lot of heat to accelerate the chemical reaction of the system. The thermal decomposition of the double-base propellant was divided into two stages, the first stage was the decomposition of nitroglycerin, and the second stage was the decomposition of nitrocellulose. It can be seen from TG and DTG curves that the addition of four thermoplastic elastomers did not change the thermal decomposition behavior of the double-base propellant. The change in the initial decomposition temperature of four elastomer-modified double-base propellants were within 5 °C. In terms of the decomposition rate and the temperature of maximum decomposition rate in the two stages, the four elastomers had a minimal effect.
4 Conclusion
In this study, the theoretical calculation properties of different formulas were compared, and the effects of thermoplastic elastomers on the dynamic and static mechanical and thermal properties of the double-base propellant were studied. The optimal formula was comprehensively determined. The main results are as follows:(1) The theoretical calculation showed that the addition of four thermoplastic elastomers would result in a loss of the energy level of the double-based propellant; therefore, it was not suitable to add too much elastomer. The energy loss of the double-base propellant with GAP-ETPE was the least.
(2) The dynamic thermal-mechanical analysis was used to study the changes in the thermal transition behavior of four TPE-modified double-base propellants as compared to the original formula. It was found that the other three thermoplastic elastomers could reduce α transition temperature of double-base propellant except for PB-TPE. The thermogravimetric analysis showed that the addition of four thermoplastic elastomers did not change the thermal decomposition.
(3) By a comprehensive consideration of mechanical properties and energy level, GAP-ETPE had the best effect on improving the low-temperature mechanical properties of double-base propellant. Its maximum tensile strength and maximum elongation at low temperature could reach 43.30 MPa and 6.24%, respectively, which was 52.4% and 20.7% higher than that of the original formula.
Conflicts of interest
There are no conflicts to declare.References
- M. K. Boulkadid, M. H. Lefebvre and L. Jeunieau, et al., Burning rate of artificially aged solid double-base gun propellants, J. Energ. Mater., 2019, 38, 1–19 CrossRef.
- M. A. Zayed, E. M. El-Begawy Salah and E. S. Hassan Hossam, Mechanism study of stabilization of double-base propellants by using zeolite stabilizers (nano- and micro-clinoptilolite), Arabian J. Chem., 2017, 10, 573–581 CrossRef CAS.
- M. M. Bhalerao, G. K. Gautam and G. V. Subramanian, et al., Nitramine double base propellants, Def. Sci. J., 1996, 46, 207–214 CrossRef CAS.
- L. Torbjörn, Reactions in Stabilizer and Between Stabilizer and Nitrocellulose in Propellants, Propellants, Explos., Pyrotech., 2002, 27, 197–208 CrossRef.
- A. Chin, S. Ellison Daniel and K. Poehlein Sara, et al., Investigation of the Decomposition Mechanism and Thermal Stability of Nitrocellulose/Nitroglycerine Based Propellants by Electron Spin Resonance, Propellants, Explos., Pyrotech., 2007, 32, 117–126 CrossRef CAS.
- L. Qi, Z. Ma and J. Liang, et al., Thermomechanical investigation on the effect of nitroguanidine on the thermal expansion coefficient and glass transition temperature of double-base gun propellant, J. Mater. Res. Technol., 2019, 8, 4264–4272 CrossRef CAS.
- J. B. Zhang, J. Yu Tao and C. S. Zhou, Research on Creep Characteristics of the Double-base Solid Propellant, Adv. Mater. Res., 2012, 591–593, 1062–1066 CAS.
- C. Sun, J. Xu and C. Xiong, et al., Strain rate and temperature dependence of the compressive behavior of a composite modified double-base propellant, Mech. Mater., 2015, 89, 35–46 CrossRef.
- G. Daniel Figuly and M. B. Goldfinger, Computer Simulation of Polymeric Materials. Thermoplastic Elastomers, Springer, Singapore, 2016 Search PubMed.
- S. Y. Lee, Y. H. Kim and S. B. Son, Azide-based elastomer useful as solid propellant binder, comprises elastic body containing glycidyl azide polymer, Korea, KR1955499-B1, 2019 Search PubMed.
- Z. Zhang, N. Luo and J. Deng, et al., A kind of bonding functional energetic thermoplastic elastomers based on glycidyl azide polymer, J. Elastomers Plast., 2016, 48, 728–738 CrossRef CAS.
- H. C. Joon, G. L. Young, J. L. Hyung, et al., Solid propellant composition used in fuel rocket, comprises thermoplastic binder, oxidizing agent, plasticizer, metal compound, where plasticizer comprises dioctyl adipate, and n-ethyltoluene sulfonamide and dioctyl phthalate, Korea, KR1807432-B1, 2017 Search PubMed.
- M. Guo, Z. Ma and L. He, et al., Effect of varied proportion of GAP-ETPE/NC as binder on thermal decomposition behaviors, stability and mechanical properties of nitramine propellants, J. Therm. Anal. Calorim., 2017, 130, 909–918 CrossRef CAS.
- V. Bozic and I. Krakovsky, Analysis of thermoplastic propellants based on a PEBA-TPE binder system, Int. J. Energ. Mater. Chem. Propul., 2010, 9, 455–465 Search PubMed.
- E. D. Brown, K. M. Nelson and G. L. Smith, Process for preparing solid propellant grains using thermoplastic binders and product thereof, US Pat., US4764316A, 1988 Search PubMed.
- B. D. Nahlovsky and G. A. J. Zimmerman, Thermoplastic castable composite rocket propellant, US Pat., US4985094A, 1991 Search PubMed.
- F. Chen, D. Yingquan and Y. Luo, et al., Novel Segmented Thermoplastic Polyurethanes Elastomers Based on Tetrahydrofuran Ethylene Oxide Copolyethers as High Energetic Propellant Binders, Propellants, Explos., Pyrotech., 2003, 28, 7–11 CrossRef CAS.
- Y. B. Jiu, Y. Luo and Z. Ge, et al., Synthesis and Properties of IPDI-Based TPU and HMDI-Based TPU, Polym. Mater.: Sci. Eng., 2011, 27, 19–22 CAS.
- Z. Zhang, G. Wang and Z. Wang, et al., Synthesis and characterization of novel energetic thermoplastic elastomers based on glycidyl azide polymer (GAP) with bonding functions, Polym. Bull., 2015, 72, 1835–1847 CrossRef CAS.
- Z. Wang, T. Zhang and B. Zhao, et al., Effect of nitrocellulose (NC) on morphology, rheological and mechanical properties of glycidyl azide polymer based energetic thermoplastic elastomer/NC blends, Polym. Int., 2017, 66, 705–711 CrossRef CAS.
- A. Schwartz, Glass-transition temperatures of polymer materials, measured by thermomechanical analysis - influence of rates of heating and cooling, J. Therm. Anal. Calorim., 1978, 13, 489–497 CrossRef CAS.
- S. M. Musanic and M. Suceska, Artificial ageing of double base rocket propellant effect on dynamic mechanical properties, J. Therm. Anal. Calorim., 2009, 96, 523–529 CrossRef.
- M. M. Sanja and S. Muhamed, Dynamic Mechanical Properties of Artificially Aged Double Base Rocket Propellant and the Possibilities for the Prediction of Their Service Lifetime, Cent. Eur. J. Energ. Mater., 2013, 10, 225–244 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |