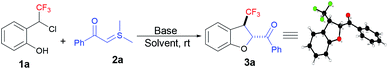Open Access Article
Open Access ArticleDiastereoselective synthesis of CF3-dihydrobenzofurans by [4+1] annulation of in situ-generated CF3-o-quinone methides and sulfur ylides†
Babli K. Jhaab,
Jaggaraju Prudhviraja,
Prathama S. Mainkar ab,
Nagender Punna
ab,
Nagender Punna *ab and
Srivari Chandrasekhar
*ab and
Srivari Chandrasekhar *ab
*ab
aDepartment of Organic Synthesis and Process Chemistry, CSIR-Indian Institute of Chemical Technology, Hyderabad 500007, India. E-mail: srivaric@iict.res.in; nagenderpunna@iict.res.in
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad, 201002, India
First published on 20th October 2020
Abstract
An efficient and highly diastereoselective synthesis of CF3-dihydrobenzofurans by the reaction of in situ-generated CF3-oQMs in the presence of a base with sulphur ylides is put forward. The generality of the present developed method was well studied with diverse substrates to access the corresponding products in excellent yields. The highly reactive CF3-oQM has been utilized first time for the annulation reaction.
Fluorine or fluoroalkyl group-containing organic molecules occupy a vital position in drug discovery due to the unique impact of fluorine atom in terms of lipophilicity, permeability, and protein-binding.1 In the last two years, 45% of FDA-approved small molecule pharmaceuticals are fluorinated, which denotes the importance of synthesizing fluorinated molecules, with special emphasis on medicinal chemistry, for the identification of new scaffolds. Among the fluorinated functional groups, the trifluoromethyl group has emerged as one of the imperative fluoroalkyl groups to enhance the bio-efficacy and metabolic stability of the corresponding motifs, which is needed for the identification of lead compounds.2 Thus, finding new methods for the inclusion of the CF3 group into novel biological entities is always desirable and challenging.
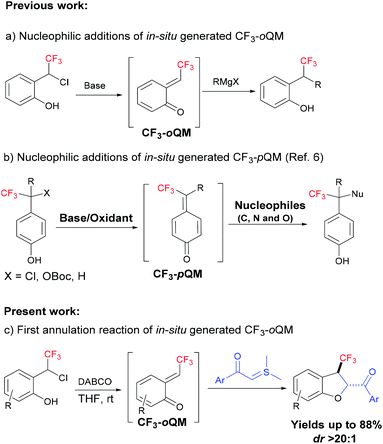
In this context, ortho-quinone methides (oQMs) are powerful reactive intermediates in synthetic organic chemistry to construct complex medium sized rings.3,4 Since oQM was first observed in 1907, it created a large impact in the synthesis of oxygen-containing benzannulated rings, which are of interest as photochromic materials and biologically active compounds.3 However, the reactions of oQMs were restricted to electron-rich substrates due to their high electrophilic nature. However, annulation reactions of diversely substituted oQMs (substitution on the exocyclic double bond) were explored extensively for the construction of oxygen-containing complex heterocyclic structures;3 however, it is quite surprising that annulation reactions involving trifluoromethyl-substituted oQM (CF3-oQM) have not been reported yet, especially because CF3-oQM is like a gold mine and could open the realm to construct versatile fluorinated oxygen architectures.
Kato et al. were the first to report the nucleophilic addition of Grignard reagents and amines to the in situ-generated trifluoromethyl-substituted ortho- and para-quinone methides (Fig. 1a).5 After that, there were no reports of this in situ-generated CF3-oQM for any nucleophilic additions and annulations. In 2020, several papers were published on in situ-generated CF3-para-quinone methides (CF3-pQM) by quickly trapping them with different carbon and hetero atom-centered nucleophiles (Fig. 1b).6 Notably, Waser et al. demonstrated that CF3-pQM has higher electrophilicity parameter (E) when compared to other substituted para-quinone methides.6d Similarly, we hypothesized that CF3-oQMs may also have higher “E” in comparison with the corresponding oQMs. Thus, the utilization of this highly reactive CF3-oQM for annulation reactions is extremely challenging and equally desirable towards the synthesis of novel organofluorine molecules. In continuation of our research interest on oQM-based annulations and the development of new fluorinating methodologies,7 herein, we report for the first [4 + 1] annulation of in situ-generated CF3-oQM with sulphur ylide to access trifluoromethyl-substituted dihydrobenzofurans with high diastereoselectivity.
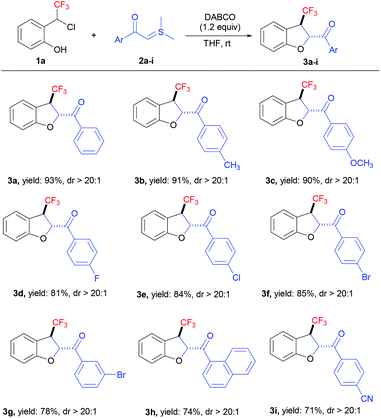
The study was initiated by exposing 2-(1-chloro-2,2,2-trifluoroethyl) phenol 1a and sulphur ylide 2a to 1.2 equiv. of Cs2CO3 at room temperature in THF. Delightedly, the in situ-generated CF3-oQM was successfully trapped with sulphur ylide to afford the corresponding trifluoromethyl-substituted dihydrobenzofuran 3a in 80% yield (Table 1, entry 1) with high diastereoselectivity. The investigation of a variety of solvents revealed that THF was the optimal solvent for the [4 + 1] annulation reaction (Table 1, entries 2–5). A quick survey was then conducted with different bases, and organic base DABCO was found to be the best to deliver 3a in 93% yield (Table 1, entries 6–9). The control experiment showed that the reaction in the absence of a base failed to produce the [4 + 1] annulation product (Table 1, entry 10). The reaction conditions in entry 6 (Table 1) were optimal and gave the product in 93% yield with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. The configuration of the obtained product was confirmed as trans from the X-ray crystallographic structure of compound 3a (CCDC 2023269).
1 dr. The configuration of the obtained product was confirmed as trans from the X-ray crystallographic structure of compound 3a (CCDC 2023269).
| Entry | Base | Solvent | Yieldb | drc |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.6 mmol), 2a (0.5 mmol), base (0.6 mmol) in solvent (2 mL) at rt.b Isolated yields.c dr was determined by 19F NMR. | ||||
| 1 | Cs2CO3 (1.2 equiv.) | THF | 80% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | Cs2CO3 (1.2 equiv.) | DCM | 62% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | Cs2CO3 (1.2 equiv.) | CH3CN | 51% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | Cs2CO3 (1.2 equiv.) | Toluene | Trace | — |
| 5 | Cs2CO3 (1.2 equiv.) | MeOH | Trace | — |
| 6 | DABCO (1.2 equiv.) | THF | 93% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | DBU (1.2 equiv.) | THF | 65% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | Et3N (1.2 equiv.) | THF | Trace | — |
| 9 | K2CO3 (1.2 equiv.) | THF | 45% | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | — | THF | NR | — |
With the determination of optimized conditions, the substrate scope for the [4 + 1] annulation reaction was scrutinized by the reaction of compound 1a with a broad array of sulphur ylides (Table 2). First, the reaction of electron-rich substrates 2b (CH3) and 2c (OCH3) afforded CF3-dihydrobenzofurans 3b (91%) and 3c (90%) in very good yields, respectively. The halogen-containing sulphur ylides 2d, 2e, and 2f (F, Cl, and Br) also proceeded smoothly to furnish the required products 3d–f in excellent yield (up to 85%) and we observed a slight improvement in the yield from fluoro to bromo substrates. Further, the reaction of naphthalene-derived sulphur ylide 2h also participated well in the reaction to deliver the required CF3-dihydrobenzofuran 3h in 74% yield. The electron-deficient substrate 2i (CN) also underwent the [4 + 1] annulation reaction very well to give CF3-dihydrobenzofuran 3i in 71% yield. CF3-oQM generated in situ from compound 1a, was trapped with a wide range of sulphur ylides without any effect on the substituents to yield the required trifluoromethyl-substituted dihydrobenzofurans in good yields with high diastereoselectivity (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
| a Reaction conditions: 1 (0.6 mmol), 2 (0.5 mmol), DABCO (0.6 mmol), THF (2 mL). Isolated yield. dr was determined by 19F NMR. |
|---|
 |
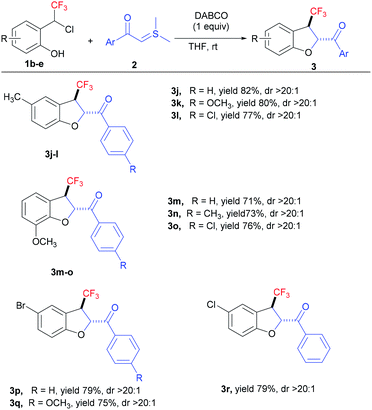
Next, we investigated the substrate scope with respect to ortho-hydroxy-CF3-benzyl chlorides 1b–d to delineate the generality of the present [4 + 1] annulation under the standard reaction conditions (Table 3). The reactions with CF3-benzyl chlorides having electronically dissimilar groups as substituents, proceeded well to furnish the desired products in good yields. The reaction of methyl (1b)- and methoxy (1c)-substituted CF3-benzyl chlorides with a variety of sulphur ylides delivered the corresponding CF3-dihydrobenzofurans 3j–o in excellent yields with good diastereoselectivity. Further, CF3-oQMs generated from the bromo (1d)- and chloro (1e)-substituted CF3-benzyl chlorides were also successfully trapped to deliver the desired products 3p–r in good yields (up to 79% with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).
| a Reaction conditions: 1 (0.6 mmol), 2 (0.5 mmol), DABCO (0.6 mmol), THF (2 mL) at rt. Isolated yield. dr was determined by 19F NMR. |
|---|
 |
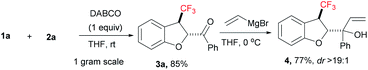
To determine the synthetic utility of the present transformation, we executed a gram scale [4 + 1] annulation reaction of compound 1a with 2a under applied reaction conditions, which gave CF3-dihydrobenzofuran 3a in 85% yield (Scheme 1). Later, we exposed compound 3a to vinyl magnesium bromide at 0 °C in THF to furnish the corresponding alcohol 4 in good yield with excellent diastereoselectivity (dr > 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
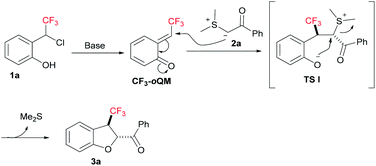
The plausible reaction mechanism for the base-catalyzed [4 + 1] annulation of ortho-hydroxy-CF3-benzyl chloride 1a with compound 2a is depicted in Fig. 2. Initially, CF3-oQM was generated in the presence of a stoichiometric amount of base.5,8 This highly electrophilic CF3-oQM undergoes nucleophilic addition with compound 2a to form a new C–C bond in TS I; the diastereoselectivity in TS I arises due to the favourable steric repulsions between the trifluoromethyl group and sulphur ylide, resulting in the final compound with trans configuration. Finally, the intramolecular nucleophilic substitution in TS I by oxygen with a sulphonium moiety furnishes the desired CF3-dihdrobenzofuran 3a in good yield.
Conclusions
In conclusion, we demonstrated a novel method for the synthesis of CF3-dihdrobenzofurans 3 via [4 + 1] annulation of ortho-hydroxy-CF3-benzyl chlorides 1 with sulphur ylides 2 under basic conditions in good yields (up to 93%) and diastereoselectivities (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The highly reactive CF3-oQM, due to the electron-withdrawing nature of the CF3 group, was trapped successfully in the present [4 + 1] annulation. This annulation is the first example for the trapping of trifluoromethyl-substituted oQM. The core skeleton of dihydrobenzofuran obtained in the present protocol has received huge attention in literature,9 and CF3 present at a strategic position may improve the biological activities of molecules tremendously. Further, the expansion of annulation reactions via in situ-generated CF3-oQMs is in progress in our laboratory to construct versatile trifluoromethyl-substituted oxygen-containing heterocycles.
1). The highly reactive CF3-oQM, due to the electron-withdrawing nature of the CF3 group, was trapped successfully in the present [4 + 1] annulation. This annulation is the first example for the trapping of trifluoromethyl-substituted oQM. The core skeleton of dihydrobenzofuran obtained in the present protocol has received huge attention in literature,9 and CF3 present at a strategic position may improve the biological activities of molecules tremendously. Further, the expansion of annulation reactions via in situ-generated CF3-oQMs is in progress in our laboratory to construct versatile trifluoromethyl-substituted oxygen-containing heterocycles.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank the Council of Scientific and Industrial Research (CSIR), Ministry of Science and Technology, Government of India for research facilities. B. J. thanks Department of Science & Technology (DST), Government of India for Inspire fellowship (IF170776). S. C. thanks the Science and Engineering Research Board, Government of India for J C Bose fellowship (SB/S2/JCB-002/2015). We gratefully acknowledge fruitful scientific discussions with Dr Balasubramanian Sridhar, Laboratory of X-ray Crystallography, CSIR-IICT, for X-ray analysis. CSIR-IICT manuscript communication no: IICT/Pubs./2020/243.Notes and references
- (a) I. Ojima, in Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, Chichester, 2009 CrossRef; (b) V. A. Petrov, Fluorinated Heterocyclic Compounds; Synthesis, Chemistry and Applications, John Wiley & Sons, Inc., Hoboken: New Jersey, 2009 Search PubMed; (c) K. L. Kirk, J. Fluorine Chem., 2006, 127, 1013–1029 CrossRef CAS; (d) C. Isanbor and D. O. Hagan, J. Fluorine Chem., 2006, 127, 303–319 CrossRef CAS; (e) F. Menaa, B. Menaa and O. N. Sharts, J. Mol. Pharm. Org. Process Res., 2013, 1, 1000104 Search PubMed; (f) H. Kawai and N. Shibata, Chem. Rec., 2014, 14, 1024–1040 CrossRef CAS; (g) J. Wang, M. S. Rosello, J. L. Acena, C. d. Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS.
- (a) Chemistry: Principles and Commercial Applications, ed. R. E. Banks, B. E. Smart and J. C. Tatlow, Plenum, New York, 1994 Search PubMed; (b) Biomedical Frontiers of Fluorine Chemistry, ed I. Ojima, J. R. McCarthy and J. T. Welch, ACS, Washington, 1996 Search PubMed; (c) H. Hiyama, Organofluorine Compounds: Chemistry and Applications, Springer, Berlin, 2000 CrossRef; (d) P. Kirsch, Modern Fluoroorganic Chemistry, Wiley-VCH, Weinheim, 2004 CrossRef; (e) Y. Ozoe, Adv. Insect Physiol, 2013, 44, 211–286 CrossRef; (f) X.-H. Xu, K. Matsuzaki and N. Shibata, Chem. Rev., 2015, 115, 731–764 CrossRef CAS.
- For selected recent reviews, see: (a) H. Amouri and J. L. Le Bras, Acc. Chem. Res., 2002, 35, 501–510 CrossRef CAS; (b) T. P. Pathak and M. S. Sigman, J. Org. Chem., 2011, 76, 9210–9215 CrossRef CAS; (c) N. J. Willis and C. D. Bray, Chem.–Eur. J., 2012, 18, 9160–9173 CrossRef CAS; (d) W.-J. Bai, J. G. David, Z.-G. Feng, M. G. Weaver, K.-L. Wu and T. R. R. Pettus, Acc. Chem. Res., 2014, 47, 3655–3664 CrossRef CAS; (e) C. L. Caruana, M. Fochi and L. Bernardi, Molecules, 2015, 20, 11733–11764 CrossRef; (f) Z. Wang and J. Sun, Synthesis, 2015, 47, 3629–3644 CrossRef; (g) A. A. Jaworski and K. A. Scheidt, J. Org. Chem., 2016, 81, 10145–10153 CrossRef CAS; (h) B. Yang and S. Gao, Chem. Soc. Rev., 2018, 47, 7926−–7953 RSC.
- For some recent examples, see: (a) E. Alden-Danforth, M. T. Scerba and T. Lectka, Org. Lett., 2008, 10, 4951–4953 CrossRef CAS; (b) H. Lv, L. You and S. Ye, Adv. Synth. Catal., 2009, 351, 2822–2826 CrossRef CAS; (c) J. Izquierdo, A. Orue and K. A. Scheidt, J. Am. Chem. Soc., 2013, 135, 10634–10637 CrossRef CAS; (d) C.-C. Hsiao, S. Raja, H.-H. Liao, I. Atodiresei and M. Rueping, Angew. Chem., Int. Ed., 2015, 54, 5762–5765 CrossRef CAS; (e) L. Caruana, M. Mondatori, V. Corti, S. Morales, A. Mazzanti, M. Fochi and L. Bernardi, Chem.–Eur. J., 2015, 21, 6037–6041 CrossRef CAS; (f) B. Wu, X. Gao, Z. Yan, W.-X. Huang and Y.-G. Zhou, Tetrahedron Lett., 2015, 56, 4334–4338 CrossRef CAS; (g) S. K. Alamsetti, M. Spanka and C. Schneider, Angew. Chem., Int. Ed., 2016, 55, 2392–2396 CrossRef CAS; (h) Y. Zhu, L. Zhang and S. Luo, J. Am. Chem. Soc., 2016, 138, 3978–3981 CrossRef CAS; (i) B. Wu, Z. Yu, X. Gao, Y. Lan and Y.-G. Zhou, Angew. Chem., Int. Ed., 2017, 56, 4006–4010 CrossRef CAS; (j) J. Zhou, M.-L. Wang, X. Gao, G.-F. Jiang and Y.-G. Zhou, Chem. Commun., 2017, 53, 3531–3534 RSC; (k) Y. Zhu, W.-Z. Zhang, L. Zhang and S. Luo, Chem.–Eur. J., 2017, 23, 1253–1257 CrossRef CAS.
- (a) Y.-F. Gong and K. Kato, Synlett, 2002, 431 CrossRef; (b) Y.-F. Gong and K. Kato, J. Fluorine Chem., 2003, 121, 141 CrossRef CAS.
- (a) X. Pan, Z. Wang, L. Kan, Y. Mao, Y. Zhu and L. Liu, Chem. Sci., 2020, 11, 2414–2419 RSC; (b) K. Terashima, T. Kawasaki-Takasuka, T. Agou, T. Kubota and T. Yamazaki, Chem. Commun., 2020, 56, 3031–3034 RSC; (c) K. Terashima, T. Kawasaki-Takasuka, T. Agou, T. Kubota and T. Yamazak, Org. Biomol. Chem., 2020, 18, 4638 RSC; (d) M. Winter, R. Schütz, A. Eitzinger, A. R. Ofial and M. Waser, Eur. J. Org. Chem., 2020, 2020, 3812 CrossRef CAS.
- (a) P. Gouthami, L. N. Chavan, R. Chegondi and S. Chandrasekhar, J. Org. Chem., 2018, 83, 3325–3332 CrossRef CAS; (b) N. Punna, P. Das, V. Gouverneur and N. Shibata, Org. Lett., 2018, 20, 1526–1529 CrossRef CAS; (c) P. Das, S. Gondo, N. Punna, H. Uno, E. Tokunaga and N. Shibata, Chem. Sci., 2018, 9, 3276–3281 RSC; (d) N. Punna, K. Harada and N. Shibata, Chem. Commun., 2018, 54, 7171–7174 RSC; (e) N. Punna, K. Harada, J. Zhou and N. Shibata, Org. Lett., 2019, 21, 1515–1520 CrossRef CAS.
- (a) M.-W. Chen, L.-L. Cao, Z.-S. Ye, G.-F. Jiang and Y.-G. Zhou, Chem. Commun., 2013, 49, 1660–1662 RSC; (b) B. Wu, M.-W. Chen, Z.-S. Ye, C.-B. Yu and Y.-G. Zhou, Adv. Synth. Catal., 2014, 356, 383–387 CrossRef CAS; (c) X. Lei, C.-H. Jiang, X. Wen and Q.-L. H. Sun, RSC Adv., 2015, 5, 14953 RSC; (d) N. Meisinger, L. Roiser, U. Monkowius, M. Himmelsbach, R. Robiette and M. Waser, Chem.–Eur. J., 2017, 23, 5137–5142 CrossRef CAS; (e) Q.-Q. Yang and W.-J. Xiao, Eur. J. Org. Chem., 2017, 233–236 CrossRef CAS; (f) L. Liu, Z. Yuan, R. Pan, Y. Zeng, A. Lin, H. Yao and Y. Huang, Org. Chem. Front., 2018, 5, 623 RSC; (g) X.-M. Chen, K.-X. Xie, D.-F. Yue, X.-M. Zhang, X.-Y. Xu and W.-C. Yuan, Tetrahedron, 2018, 74, 600–605 CrossRef CAS; (h) X. He, M. Xie, Q. Tang, Y. Zuo, R. Li and Y. Shang, J. Org. Chem., 2019, 84, 11623–11638 CrossRef CAS.
- (a) R. J. Nevagi and S. N. Dighe, Eur. J. Med. Chem., 2015, 97, 561–581 CrossRef CAS; (b) A. Radadiya and A. Shah, Eur. J. Med. Chem., 2015, 97, 356–376 CrossRef CAS; (c) H. K. Shamsuzzaman, Eur. J. Med. Chem., 2015, 97, 483–504 CrossRef; (d) L. N. Qin, D. D. Vo, A. Nakhai, C. D. Andersson and M. Elofsson, ACS Comb. Sci., 2017, 19, 370–376 CrossRef CAS; (e) M. M. Heravi, V. Zadsirjan, H. Hamidi and P. H. T. Amiri, RSC Adv., 2017, 7, 24470–24521 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2023269. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra08289a |
| This journal is © The Royal Society of Chemistry 2020 |