Open Access Article
Open Access ArticleNew Span-PEG-composited Fe3O4-CNT as a multifunctional ultrasound contrast agent for inflammation and thrombotic niduses
Jie Zhang *a,
Jinzi Yanga,
Huiming Zhangb,
Ming Hu*c,
Jinjing Lia and
Xiangyu Zhanga
*a,
Jinzi Yanga,
Huiming Zhangb,
Ming Hu*c,
Jinjing Lia and
Xiangyu Zhanga
aPharmacy College, Jiamusi University, Jiamusi 154007, China. E-mail: zjie612@163.com; Tel: +86 18045411988
bCollege of Basic Medicine, Jiamusi University, Jiamusi 154007, China
cCollege of Materials Science & Engineering, Jiamusi University, Jiamusi 154007, China. E-mail: minghu02@jmsu.edu.cn; Tel: +86 13846158051
First published on 20th October 2020
Abstract
By attaching ferroferric oxide (Fe3O4) to drug-carrying carbon nanotubes (CNTs), we generated a new Span-PEG composite with Fe3O4-CNT multifunctional microbubbles for inflammation and thrombus niduses. The Fe3O4-CNT magnetic targeting complex was prepared by in situ synthesis, and then acetylsalicylic acid (ASA) and gentamicin (GM) were loaded onto the Fe3O4-CNT complex by physical methods to produce Fe3O4-CNT-ASA and Fe3O4-CNT-GM complexes, respectively. Span-PEG-composited Fe3O4-CNT-ASA or Fe3O4-CNT-GM microbubbles were synthesized with Span and PEG as the membrane materials by the acoustic cavitation method. The obtained composite microbubbles were smooth, hollow spheres with an average particle size of 425 nm. The ASA and GM loading rates in Span-PEG-composited Fe3O4-CNT-ASA and Fe3O4-CNT-GM microbubbles were 1.12% and 19.05%, respectively. Span-PEG-composited Fe3O4-CNT-ASA microbubbles inhibited thrombosis and demonstrated an anticoagulation effect in vitro. Additionally, Span-PEG-composited Fe3O4-CNT-ASA microbubbles showed significantly enhanced ultrasound imaging of rabbit abdominal aorta and extended the signal time under the action of an external magnetic field. Thus, Span-PEG-composited Fe3O4-CNT-GM microbubbles inhibited Escherichia coli and Staphylococcus aureus, enhanced the ultrasound imaging of rabbit abdominal uterus and had better stability and fluidity.
1 Introduction
Ultrasound molecular imaging is achieved using targeted microbubble contrast agents, which are highly reflective ultrasound materials that can target specific tissue imaging using specific biomarkers.1 The imaging of targeted microbubble ultrasound contrast agents in thrombosis and inflammation has attracted increased research attention, and the targeted specificity and binding stability of the contrast agent are beneficial for early diagnosis.2,3 Thrombus formation is a common condition of the cardiovascular system. For example, deep vein thrombosis is often called a “silent killer” because of the lack of obvious symptoms until the late stage.4,5 Deep vein thrombosis most often occurs in the legs, but it can gradually invade the deep veins of the arms, visceral veins, and cerebral veins.6 Therefore, early diagnosis and appropriate treatment are very important to reduce the morbidity and mortality of thrombosis. As a result, ultrasound has become an important detection method presently. Angiography can improve the resolution of the vascular intima, and ultrasound contrast agents can more accurately determine intravascular occlusion and increase measurement accuracy. Johri et al.7 determined the activity of blood flow by observing microbubble movement in intracavitary blood vessels after vasodilation so that intravascular conditions could be observed. Pelvic inflammation is a gynecological disease that threatens women's health. Accurate assessments of the morphology and function of the uterus and fallopian tubes are the keys to diagnosis and treatment. Recently, some studies have shown that microbubble ultrasound contrast agents could be used to assess the patency performance of fallopian tubes; this finding has been increasingly recognized by clinicians and patients in recent years due to their high accuracy, noninvasiveness, and low cost. Dreyer et al.8 conducted a study to explore the influence of the choice of hysterosalpingian tube contrast media on the pregnancy rate. In that study, 1119 women were randomly assigned to use oil-based contrast media or water-based contrast media for hysterosalpingography. The pregnancy rate of women who received oil-based contrast hysterosalpingography was as high as 29.1%, slightly higher than that of women who received water-based contrast hysterosalpingography.As one of the most advanced nanocarriers for the efficient delivery of drugs, CNTs can achieve transmembrane transportation by noncovalent or covalent binding with different drugs, biomolecules and nanoparticles. Zhang et al.9 prepared a nanometer drug-loaded complex with CNTs covalently binding with the anticancer drug doxorubicin (DOX), significantly improving the drug loading capacity and drug release kinetics; additionally, the obtained CNT-DOX complex showed a multistimulus response induced by pH, temperature and a reducing agent. CNTs are an emerging diagnostic imaging tool. The application of CNTs in ultrasound imaging can enhance the role of ultrasound imaging, increase the drug loading and stability of ultrasound contrast agents, and reduce the burst release of drugs.
Fe3O4 nanoparticles have the characteristics of stability, controllability, and strong targeting due to the unique magnetic property and have broad application prospects in the field of biomedicine. Attari et al.10 delivered methotrexate coupled with Fe3O4 to cancer cells under the guidance of an external magnetic field, which enhanced the delivery of the therapeutic complex at their action sites.
In this study, the excellent drug-carrying ability and imaging function of CNTs were effectively combined with the superparamagnetic property of Fe3O4 nanoparticles, and they were applied as ultrasound contrast agents. A new Span-PEG composited with Fe3O4-CNT multifunctional ultrasound contrast agent was obtained that demonstrated multiple effects in ultrasound imaging, targeting and adjuvant therapy by loading ASA and GM onto the Fe3O4-CNT complexes, which could be applied to early prevention and intervention in thrombus and inflammation.
2 Experiment
2.1 Preparation of Span-PEG-composited drug-Fe3O4-CNT microbubbles
First, 0.45 g of CNTs was dispersed in distilled water under ultrasound and transferred into a three-necked flask. Next, 0.47 g of FeCl3, 0.23 g of FeCl2 and an appropriate amount of PEG1500 were added to this three-necked flask. NaOH solution (4 mg mL−1) was slowly added to the above system at 60 °C until the reaction system was alkaline, stirring for 20 min and aging for 30 min at 80 °C. After the reaction, the sediment was gathered at the bottom of the flask by the magnet, repeatedly washing with triple-distilled water, and the Fe3O4-CNT magnetic targeting complex was obtained by freeze-drying.Second, 20 mg of the Fe3O4-CNT complex was dispersed in an appropriate amount of distilled water. Next, 80 mg of gentamicin (GM) and 80 mg of aspirin (ASA) were dissolved in the appropriate amount of distilled water and absolute ethanol, respectively. Additionally, the two obtained drug liquids were added to the Fe3O4-CNT suspension, stirring at 30–40 °C for 120–150 min. After the above system was filtered, washed and freeze-dried, the Fe3O4-CNT-GM and Fe3O4-CNT-ASA complexes were obtained.
Finally, 30 mg of Fe3O4-CNT-GM complex and 30 mg of Fe3O4-CNT-ASA complex were dispersed into 10 mL of PBS under ultrasound. Next, 450 mg of Span60, 450 mg of PEG1500 and 900 mg of NaCl were ground well and transferred to a beaker with 40 mL of PBS, stirring for 30 min at 80 °C. The above complex dispersion was then added into this system, and the stirring process was continued for 15 min. The obtained mixture was subjected to an ultrasonic cell disruption instrument at a power of 600 W for 6 min, while nitrogen gas was introduced. After washing 3–4 times with PBS, the middle layer of the microbubble suspension was collected and freeze-dried. Finally, Span-PEG-composited Fe3O4-CNT-GM and Fe3O4-CNT-ASA microbubbles were prepared.
2.2 Determination of the drug content in the composite microbubbles
Span-PEG-composited Fe3O4-CNT-ASA microbubbles (50 mg) was dissolved in an appropriate amount of anhydrous ethanol and was treated to release the drug under ultrasound for 2 h. The obtained mixture was filtered using a microporous membrane, and 25 mL of the filtrate was subjected to ultraviolet spectrophotometry at 276.0 nm. According to the ASA standard curve, the loading rate of ASA in the composite microbubbles was calculated.
Span-PEG-composited Fe3O4-CNT-GM microbubbles (20 mg) was dissolved in an appropriate amount of triple-steamed water and was treated to release drug under ultrasound for 2 h. The obtained mixture was filtered using a microporous membrane, and 20 mL of the filtrate was subjected to ultraviolet spectrophotometry at 320.0 nm. According to the above method, the loading rate of GM in the composite microbubbles was obtained.
2.3 In vitro antibacterial analysis of Span-PEG-composited Fe3O4-CNT-GM microbubbles
Physiological saline suspensions of Span-PEG microbubbles, Span-PEG-composited Fe3O4-CNT microbubbles, Span-PEG-composited CNT-GM microbubbles and Span-PEG-composited Fe3O4-CNT-GM microbubbles were treated under ultrasound to release the drug. Additionally, filter paper was placed into each sample solution and allowed to soak for 12 h.Drug-sensitive papers of Span-PEG-composited Fe3O4-CNT-GM microbubbles at different concentrations and with different composite microbubbles were prepared. Six pieces per drug-sensitive paper were prepared and attached to the surfaces of Escherichia coli and Staphylococcus aureus agar plates. The plates with the drug-sensitive papers were placed into an incubator at 37 °C for 12 h, and the diameter of the inhibition zone was measured using a Vernier caliper.
2.4 In vitro antithrombotic experiment of Span-PEG-composited Fe3O4-CNT-ASA microbubbles
As mentioned in section 2.3 of this study, the different microbubble suspension groups were treated to release the drug under ultrasound. Span-PEG microbubbles, Span-PEG-composited CNT microbubbles, Span-PEG-composited Fe3O4-CNT microbubbles, Span-PEG-composited CNT-ASA microbubbles and Span-PEG-composited Fe3O4-CNT-ASA microbubbles were prepared. Three parallel samples were set for each group, and each tube was fitted with 0.5 mL of blood. The formed clot was removed and weighed after 1 h of drug action.After different microbubbles interacted with the blood, the following blood coagulation parameters were measured using an automatic blood coagulation analyzer: prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT) and international normalized ratio (INR).
2.5 Ultrasound imaging of Span-PEG-composited Fe3O4-CNT-GM microbubbles
The New Zealand white rabbit was used as an experimental subject. After anesthesia, the uterus of the rabbit was perfused with Span-PEG composited with Fe3O4-CNT-GM microbubbles (60 mg mL−1) in physiological saline by uterine intubation. Ultrasound imaging of the rabbit uterus before and after injection was observed and recorded using a Doppler color ultrasound imaging system.2.6 Ultrasound imaging of Span-PEG-composited Fe3O4-CNT-ASA microbubbles
The experimental groups were as follows: normal saline control group, Span-PEG microbubbles, Span-PEG-composited CNT microbubbles and Span-PEG-composited Fe3O4-CNT-ASA microbubbles. Freeze-dried powders of different microbubbles were added to the suspensions at a concentration of 60 mg mL−1 with degassed physiological saline.The rabbit abdominal aorta thrombosis model was established. First, filter paper was immersed with 34% ferric chloride aqueous solution. Next, a healthy rabbit was anesthetized according to its weight, and the underbelly hair was shaved. An abdominal aorta section of 2 cm was dissociated by abdominal surgery and covered by the above-treated filter paper to induce the formation of the thrombosis model. Thereafter, the normal saline group and each microbubble suspension group were injected with the materials via the marginal ear vein. Ultrasound inspection of the abdominal aorta was performed using a Doppler color ultrasound instrument.
3 Results and discussion
Fig. 1 displays the hysteresis curves of Fe3O4-CNT magnetic targeting complexes and Span-PEG-composited Fe3O4-CNT microbubbles at room temperature. These two hysteresis curves showed similar magnetic behavior, no coercive magnetic field and remanence at the zero point, indicating that the prepared Fe3O4-CNT magnetic targeting complexes and Span-PEG-composited Fe3O4-CNT microbubbles had good magnetic properties.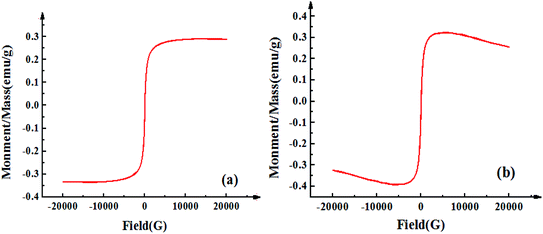 | ||
| Fig. 1 Hysteresis curves of the Fe3O4-CNT complex (a) and Span-PEG-composited Fe3O4-CNT microbubbles (b) at room temperature. | ||
SEM and TEM of Span-PEG-composited Fe3O4-CNT microbubbles are shown in Fig. 2. The prepared Span-PEG-composited Fe3O4-CNT microbubbles had a smooth surface and a uniform particle size (Fig. 2(a)). Span-PEG-composited Fe3O4-CNT microbubbles showed a hollow structure, the size was nanoscale, and the outer membrane layer of the composite microbubbles showed a black shaded area (Fig. 2(b)). Combined with Fig. 2(b), the structure simulation of Span-PEG-composited Fe3O4-CNT microbubbles is shown in Fig. 2(c). In the preparation process of composite microbubbles, using water as the dispersing phase, Span and PEG as the surfactant molecules formed stable microbubbles that wrapped N2 inside the acoustic cavitation. Incidentally, the hydrophilic end of Span was outward while its hydrophobic end was inward, and PEG macromolecules were interspersed among the Span60 molecules. Additionally, due to the hydrophilicity of the carboxylated CNTs in the Fe3O4-CNT complex, the Fe3O4-CNT complex combined with the outer wall of the composite microbubbles. Under a higher preparation temperature, the carboxyl group on the Fe3O4-CNTs complex could form ester bonds with the hydroxyl group of Span by dehydration, and part of the Fe3O4-CNT complex could also bind to the outer wall of the composite microbubble. Thus, most of the Fe3O4-CNT complexes existed on the outside of the microbubble. Additionally, a small amount of the Fe3O4-CNT complex could be encapsulated inside the composite microbubble under acoustic vibration.
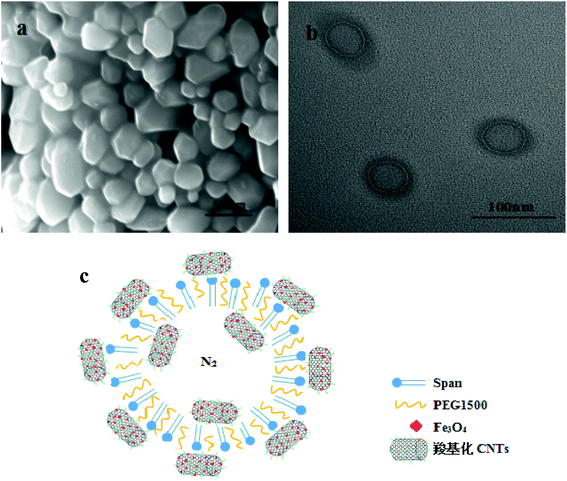 | ||
| Fig. 2 SEM (a), TEM (b) and structure simulation diagram (c) of Span-PEG-composited Fe3O4-CNT microbubbles. | ||
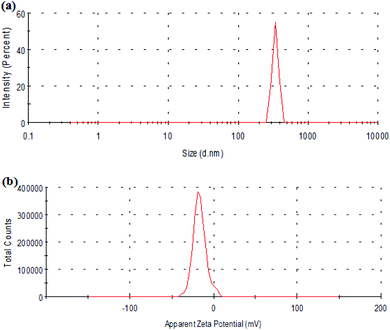
Fig. 3 shows the particle size distribution and zeta potential of Span-PEG-composited Fe3O4-CNT microbubbles. The size of the composite microbubbles was uniform, the average particle size was 425 nm, and the particle size distribution manifested as the unimodal form (Fig. 3). The zeta potential was −27.5 mV, indicating that the composite microbubbles had good stability and met the requirements as an ultrasound contrast agent.
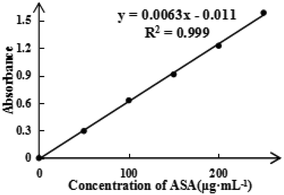
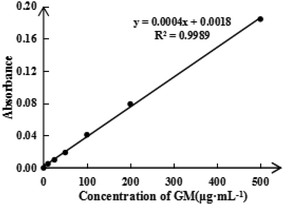
To detect the ASA load rate in Span-PEG-composited Fe3O4-CNT-ASA microbubbles, the ASA standard curve (Fig. 4) and ASA absorbance in composite microbubbles (Table 1) were obtained according to the experimental method in Section 2.2.1 of this study. Thus, the ASA load rate in Span-PEG-composited Fe3O4-CNT-ASA microbubbles was 1.12%. Similarly, the GM standard curve (Fig. 5) and GM absorbance in composite microbubbles (Table 2) were obtained according to the experimental method in section 2.2.2 of this study. The GM load rate in Span-PEG-composited Fe3O4-CNT-GM microbubbles was calculated as 19.05%.
| Times | 1 | 2 | 3 | 4 | 5 | Average |
|---|---|---|---|---|---|---|
| ASA absorbance | 0.112 | 0.150 | 0.099 | 0.156 | 0.132 | 0.130 |
| ASA loading rate (%) | 0.97 | 1.28 | 0.88 | 1.32 | 1.14 | 1.12 |
| Times | 1 | 2 | 3 | 4 | 5 | Average |
|---|---|---|---|---|---|---|
| GM absorbance | 0.070 | 0.076 | 0.088 | 0.072 | 0.083 | 0.078 |
| GM loading rate (%) | 16.98 | 18.58 | 21.66 | 17.61 | 20.37 | 19.05 |
Fig. 6 shows the inhibitory effects of Span-PEG-composited Fe3O4-CNT-GM microbubbles in different concentrations on Escherichia coli and Staphylococcus aureus. The inhibitory effect of the composite microbubbles on Escherichia coli and Staphylococcus aureus showed a concentration-dependent trend; its bacteriostatic effect gradually increased as the concentration of the composite microbubbles increased (Fig. 6). Specifically, for Escherichia coli, the concentration of Span-PEG-composited Fe3O4-CNT-GM microbubbles was 0 mg mL−1 and the diameter of the inhibition zone was 6.9 ± 0.2 mm. When the concentration was 20–40 mg mL−1, the diameter range was 10.5 ± 0.5–13.4 ± 0.8 mm, indicating medium drug sensitivity. When the concentration was 60–80 mg mL−1, the diameter range was 16.8 ± 0.9–19.7 ± 0.4 mm, indicating high drug sensitivity. Additionally, when the concentration was > 80 mg mL−1, the drug sensitivity was extremely sensitive. For Staphylococcus aureus, when the concentration of Span-PEG-composited Fe3O4-CNT-GM microbubbles was 20 mg mL−1, the diameter of the inhibition circle was 9.6 ± 0.4 mm, indicating low drug sensitivity. When the concentration was 40–60 mg mL−1, the diameter range was 11.7 ± 0.8–14.4 ± 0.7 mm, indicating medium drug sensitivity. Additionally, when the concentration > 80 mg mL−1, high drug sensitivity was noted. In summary, the antibacterial effect of Span-PEG-composited Fe3O4-CNT-GM microbubbles on Escherichia coli and Staphylococcus aureus was dose-dependent, and the inhibitory effect on Escherichia coli was higher than that of Staphylococcus aureus. When the concentration of Span-PEG-composited Fe3O4-CNT-GM microbubbles reached 60 mg mL−1 or higher, both bacteria demonstrated favorable antibacterial effects.
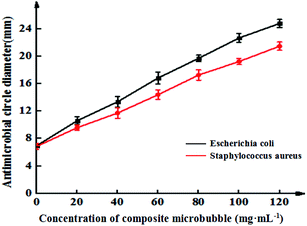 | ||
| Fig. 6 Inhibition curves of Span-PEG-composited Fe3O4-CNT-GM microbubbles at different concentrations for the effect on Escherichia coli and Staphylococcus aureus. | ||
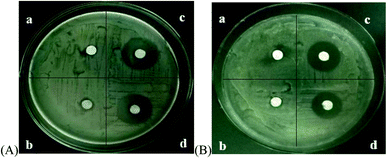
The antimicrobial effects of the different microbubbles on Escherichia coli and Staphylococcus aureus are shown in Fig. 7. Span-PEG microbubbles and Span-PEG-composited Fe3O4-CNTs demonstrated no antibacterial activity. Span-PEG-composited CNT-GM microbubbles and Span-PEG-composited Fe3O4-CNT-GM microbubbles manifested good antibacterial activity against Escherichia coli and Staphylococcus aureus, and their bacteriostatic activity on Escherichia coli was stronger.
The bacteriostatic circle diameters of different composite microbubbles on Escherichia coli and Staphylococcus aureus are shown in Table 3. For Escherichia coli, the bacteriostatic circle diameter of the Span-PEG microbubble group was 6.8 ± 0.42 mm and that of the Span-PEG-composited Fe3O4-CNT microbubble group was 7.2 ± 0.56 mm, with no significant difference between the groups (P > 0.05). Span-PEG-composited CNT-GM microbubbles (21.2 ± 1.02 mm) showed a significant difference compared with Span-PEG microbubbles (P < 0.01), and Span-PEG-composited Fe3O4-CNT-GM microbubbles (18.6 ± 0.81 mm) also showed a significant difference compared with Span-PEG microbubbles (P < 0.01). For Staphylococcus aureus, Span-PEG microbubbles and Span-PEG-composited Fe3O4-CNT microbubbles showed no antibacterial activity. The diameter of the bacteriostatic circle of Span-PEG-composited CNT-GM microbubbles was 18.1 ± 0.73 mm, showing a significant difference compared with Span-PEG microbubbles (P < 0.01). The diameter of the Span-PEG-composited Fe3O4-CNT-GM microbubbles was 15.8 ± 0.65 mm, showing a significant difference compared with Span-PEG microbubbles (P < 0.05). In summary, Span-PEG membrane material and Fe3O4-CNTs as the targeted drug carrier showed no bacteriostatic activity. Both Span-PEG-composited CNT-GM microbubbles and Span-PEG-composited Fe3O4-CNT-GM microbubbles exhibited good antibacterial activity on Escherichia coli and Staphylococcus aureus, indicating that the antibacterial effect of Span-PEG-composited Fe3O4-CNT-GM microbubbles originated from the loading drug GM, and the inhibitory effects of Span-PEG-composited CNT-GM microbubbles and Span-PEG-composited Fe3O4-CNT-GM microbubbles on Escherichia coli were better than those on Staphylococcus aureus.
![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) ± s)a
± s)a
| Group | Diameter of the bacteriostatic circle D mm−1 | |
|---|---|---|
| Escherichia coli | Staphylococcus aureus | |
| a “*” for P < 0.05,“#” for P < 0.01. | ||
| Span-PEG microbubbles | 6.8 ± 0.42 | 6.5 ± 0.3 |
| Span-PEG-composited Fe3O4-CNTs microbubbles | 7.2 ± 0.56 | 6.4 ± 0.35 |
| Span-PEG-composited CNT-GM microbubbles | 21.2 ± 1.02# | 18.1 ± 0.73# |
| Span-PEG-composited Fe3O4-CNT-GM microbubbles | 18.6 ± 0.81# | 15.8 ± 0.65* |
The thrombosis inhibition results of Span-PEG-composited Fe3O4-CNT-ASA microbubbles with different concentrations are shown in Table 4. With the increase in the composite microbubble concentration, the wet and dry weights of the thrombus were both reduced. When the concentration of Span-PEG-composited Fe3O4-CNT-ASA was from 40 mg mL−1 to 80 mg mL−1, the wet weight and dry weight of the thrombus showed little change, with no statistical significance (P > 0.05) compared with the concentration at 0 mg mL−1. When the concentration of Span-PEG-composited Fe3O4-CNT-ASA microbubbles was 120 mg mL−1, a significant difference was found in the wet and dry weights of the thrombus compared with that at the concentration of 0 mg mL−1 (P < 0.05), indicating that the composite microbubbles inhibited the thrombosis in vitro at this time. When the concentration of Span-PEG-composited Fe3O4-CNT-ASA microbubbles was 160 mg mL−1, the wet weight of the thrombus was significantly different from that at 0 mg mL−1 (P < 0.05), and the dry weight of the thrombus showed a very significant difference compared with that at 0 mg mL−1 (P < 0.05). When the concentration of Span-PEG-composited Fe3O4-CNT-ASA microbubbles was 200 mg mL−1, the wet and dry weights of the thrombus were significantly different from those at 0 mg mL−1 (P < 0.05). Thus, Span-PEG-composited Fe3O4-CNT-ASA microbubbles inhibit thrombosis in vitro and show a dose-dependent trend.
![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) ± s)a
± s)a
| Number | Concentration of composite microbubbles (mg mL−1) | Wet weight of thrombus (mg) | Dry weight of thrombus (mg) |
|---|---|---|---|
| a “*” for P < 0.05,“#” for P < 0.01. | |||
| 1 | 0 | 126.17 ± 10.90 | 53.60 ± 6.95 |
| 2 | 40 | 122.63 ± 12.22 | 51.00 ± 9.54 |
| 3 | 80 | 105.30 ± 9.54 | 39.70 ± 2.29 |
| 4 | 120 | 93.93 ± 13.40* | 36.17 ± 6.67* |
| 5 | 160 | 84.37 ± 11.14* | 25.87 ± 4.85# |
| 6 | 200 | 63.10 ± 9.88# | 18.33 ± 5.82# |
The thrombosis inhibition effects in vitro for different microbubble groups at the concentration of 120 mg mL−1 and the normal saline group are shown in Table 5. The wet and dry weights of the thrombus in the saline control group were 128.43 ± 8.11 mg and 50.27 ± 1.33 mg, respectively. Those of the Span-PEG microbubble group and Span-PEG-composited Fe3O4-CNT microbubble group were not statistically significant compared with those of the control group (P > 0.05), indicating that Span-PEG membrane material and the Fe3O4-CNT targeted drug-loading complex could not inhibit the thrombosis in vitro. The wet and dry weights of the thrombus for Span-PEG-composited CNT-ASA microbubbles showed a very significant difference compared with those of the saline control group (P < 0.01). Compared with the control group, the wet and dry weights of the thrombus for Span-PEG-composited Fe3O4-CNT-ASA microbubbles showed significant differences (P < 0.05 and P < 0.01, respectively). Span-PEG-composited CNT-ASA microbubbles and Span-PEG-composited Fe3O4-CNT-ASA microbubbles could effectively inhibit the thrombosis in vitro, and this inhibition effect was mainly derived from the drug ASA in the composite microbubbles. No significant difference was found between the wet and dry weights of the thrombus for Span-PEG-composited CNT-ASA microbubbles and Span-PEG-composited Fe3O4-CNT-ASA microbubbles, indicating the effect of Fe3O4 particles on the CNT surface on the drug loading performance of CNTs was not obvious.
![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) ± s)a
± s)a
| Group | Wet weight of thrombus (mg) | Dry weight of thrombus (mg) |
|---|---|---|
| a Compared with the normal saline control group, “*” for P < 0.05,“#” for P < 0.01. | ||
| Normal saline | 128.43 ± 8.11 | 54.97 ± 5.04 |
| Span-PEG microbubbles | 121.30 ± 3.99 | 50.27 ± 1.33 |
| Span-PEG-composited Fe3O4-CNTs microbubbles | 123.00 ± 8.41 | 53.10 ± 7.31 |
| Span-PEG-composited CNT-ASA microbubbles | 92.43 ± 6.64# | 32.30 ± 5.31# |
| Span-PEG-composited Fe3O4-CNT-ASA microbubbles | 97.97 ± 8.97* | 36.00 ± 4.80# |
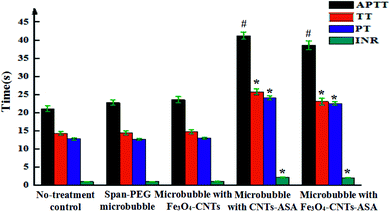
Fig. 8 shows the coagulation indexes of different composite microvesicles. APTT, TT, PT, and INR are commonly used to determine the coagulation index. APTT reflects the total coagulation status of each coagulation component in the endogenous coagulation system. PT and INR reflect the coagulation status of the exogenous coagulation system, and TT reflects the coagulation status of plasma fibrinogen converting into fibrin. Span-PEG microbubbles and Span-PEG-composited Fe3O4-CNT microbubbles did not influence APTT, TT, PT, and INR, with no statistical significance compared with the control group (Fig. 8; P > 0.05). Span-PEG-composited CNT-ASA microbubbles and Span-PEG-composited Fe3O4-CNT-ASA microbubbles significantly increased the value of APTT, which was significantly different from that of the control group (P < 0.01), indicating that the two composite microbubbles affect the endogenous coagulation system. At the same time, Span-PEG-composited CNT-ASA microbubbles and Span-PEG-composited Fe3O4-CNT-ASA microbubbles increased the values of TT, PT, and INR, which were significantly different from those of the control group (P < 0.05), indicating that these two microbubbles had a good effect on the exogenous coagulation system and the conversion of plasma fibrinogen to fibrin. An INR value at the range of 2.1–3 indicates moderate anticoagulation. The INR values of Span-PEG-composited CNT-ASA microbubbles and Span-PEG-composited Fe3O4-CNT-ASA microbubbles were 2.23 and 2.15, respectively, indicating that the prepared Span-PEG-composited Fe3O4-CNT-ASA microbubbles demonstrated anticoagulant activity and could play a role in adjuvant therapy.
The ultrasound imaging effect of Span-PEG-composited Fe3O4-CNT-GM microbubbles in the rabbit uterus is shown in Fig. 9. Before perfusion of the composite microbubbles (Fig. 9(a)), the uterus was not displayed in the ultrasound image because the echo signal was weak, leading to a challenging ultrasound diagnosis of the uterine lesion. After perfusion of the composite microbubbles, the uterus shape was clear and a strong echo existed across the entire uterus. Moreover, no obvious resistance and no reflux during the injection were detected; the composite microbubbles could easily flow throughout the uterus. The composite microbubbles showed good stability and fluidity and generated strong sound scattering. Thus, the image contrast increased and effective imaging was achieved.
 | ||
| Fig. 9 Ultrasonic imaging effect of the rabbit uterus before (a) and after (b) perfusion with Span-PEG-composited Fe3O4-CNT-GM microbubbles. | ||
Fig. 10 displays the ultrasound imaging results of a rabbit aortic thrombus after the injection of normal saline, Span-PEG microbubbles, Span-PEG-composited CNT microbubbles and Span-PEG-composited Fe3O4-CNT-ASA microbubbles. The echo signal was very low, and the obtained imaging contrast was low for the saline group, making it impossible to effectively diagnose the lesion (Fig. 10(a)). The imaging clarity was enhanced because the blood appeared as a low-density echo signal, and effective imaging of the Span-PEG microbubble group was achieved (Fig. 10(b)). Fig. 10(c) represents the ultrasound imaging of the rabbit abdominal aorta after the injection of Span-PEG-composited CNT microbubbles. After a few seconds of injection, the blood vessels of the rabbit abdominal aorta rapidly filled up and the echo signal rapidly brightened; the enhanced contrast effect was more evident than that of Span-PEG microbubbles. For Span-PEG-composited Fe3O4-CNT-ASA microbubbles (Fig. 10(d)), the blood vessels of the abdominal aorta rabbit rapidly filled up after a few seconds of injection, and the signal rapidly increased and became brighter. The ultrasound signal of Span-PEG-composited Fe3O4-CNT-ASA microbubbles was similar to that of Span-PEG-composited CNT microbubbles, and the thrombosis location of the abdominal aorta was clear. Thus, Span-PEG-composited Fe3O4-CNT-ASA microbubbles could effectively diagnose the lesion.
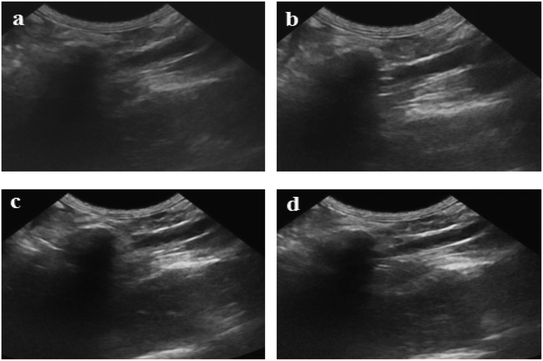
Fig. 11 shows ultrasound imaging of the rabbit abdominal aorta in an external magnetic field for Span-PEG-composited Fe3O4-CNT-ASA microbubbles. The blood vessels of the rabbit abdominal aorta quickly filled up after a few seconds of injection, and Span-PEG-composited Fe3O4-CNT-ASA microbubbles aggregated under the action of an external magnetic field because of Fe3O4 as a magnetic targeting factor, causing the signal to gradually increase and effectively extending the time of ultrasound imaging (Fig. 11). Compared with the non-applied magnetic field (Fig. 10(d)), the obtained ultrasound image had higher contrast. Thus it can be seen that the Fe3O4 particles on Fe3O4-CNT complex played a very good magnetic targeting role. Additionally, it was sufficient to meet the clinical requirements for the contrast time of ultrasound contrast agents. The above results indicated that the prepared Span-PEG-composited Fe3O4-CNT-ASA microbubbles could achieve targeted ultrasound imaging of thrombosis under the action of an external magnetic field.
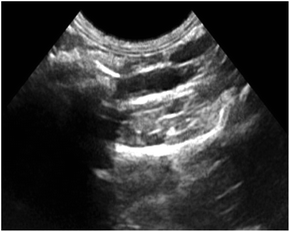 | ||
| Fig. 11 Ultrasound imaging of the rabbit abdominal aorta after the instillation of Span-PEG composite Fe3O4-CNT-ASA microvesicles in an external magnetic field. | ||
4 Conclusion
In this study, Span-PEG-composited Fe3O4-CNTs, a new multifunctional microbubble type, were designed and prepared by an in situ, liquid-phase synthesis method and acoustic cavitation. Here, CNT was used as the carrier of the magnetic targeting factor and drug loading, the magnetic targeting complex Fe3O4-CNT was respectively loaded with ASA and GM. The obtained composite microbubbles could realize targeted ultrasound imaging and adjuvant therapy for thrombosis and inflammation. We believe that this new multifunctional ultrasound contrast agent will have a good application prospect.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51671096 and 81601616), Scientific Research Initiation Found of Postdoctoral Researchers Settled down in Heilongjiang (31303120), Excellent Innovation Team based on the basic scientific research vocational cost for the provincial undergraduate Universities in Heilongjiang (No. 2018-KYYWF-0914) and Heilongjiang Health Department Project (2019-291).References
- D. Hyun, L. Abou Elkacem and R. Bam, et al., Nondestructive Detection of Targeted Microbubbles Using Dual-Mode Data and Deep Learning for Real-Time Ultrasound Molecular Imaging, IEEE Trans. Med. Imag., 2020,(2), 3079–3088 Search PubMed.
- Y. Zhang, Y. Zhong and M. Ye, et al., Polydopamine-modified dual-ligand nanoparticles as highly effective and targeted magnetic resonance/photoacoustic dual-modality thrombus imaging agents, Int. J. Nanomed., 2019, 14, 7155–7171 CrossRef CAS.
- N. Beziere, C. von Schacky and Y. Kosanke, et al., Optoacoustic Imaging and Staging of Inflammation in a Murine Model of Arthritis, Int. J. Nanomed., 2014, 66(8), 2071–2078 CAS.
- G. Lippi, M. Franchini and G. Targher, Arterial thrombus formation in cardiovascular disease, Nat. Rev. Cardiol., 2011, 8(9), 502–512 CrossRef.
- S. P. Jackson, Arterial thrombosis–insidious, unpredictable and deadly, Nat. Med., 2011, 17(11), 1423–1436 CrossRef CAS.
- M. Di Nisio, N. van Es and H. R. Büller, Deep vein thrombosis and pulmonary embolism, Lancet, 2016, 388(10063), 3060–3073 CrossRef.
- A. Johri, T. Li and O. Yau, et al., Novel Ultrasound Methods to Investigate Carotid Artery Plaque Vulnerability, J. Am. Soc. Echocardiogr., 2017, 30(2), 139–148 CrossRef.
- D. Kim, R. Joukje van and M. Velja, et al., Oil-Based or Water-Based Contrast for Hysterosalpingography in Infertile Women., N. Engl. J. Med., 2017, 376(21), 2043–2052 CrossRef.
- R. Zhang, Z. Liu and Y. Luo, et al., Tri-stimuli responsive carbon nanotubes covered by mesoporous silica graft copolymer multifunctional materials for intracellular drug delivery, J. Ind. Eng. Chem., 2019, 80, 431–443 CrossRef CAS.
- E. Attari, H. Nosrati and H. Danafar, et al., Methotrexate anticancer drug delivery to breast cancer cell lines by iron oxide magnetic based nanocarrier, J. Biomed. Mater. Res., Part A, 2019, 107(11), 2492–2500 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |