Open Access Article
Open Access ArticleHighly efficient hamburger-like nanostructure of a triadic Ag/Co3O4/BiVO4 photoanode for enhanced photoelectrochemical water oxidation†
Xintong Gaoab,
Zhiqun Baia,
Shuai Zhangc,
Jingchao Liu *a and
Zenghe Li
*a and
Zenghe Li *b
*b
aState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: Jingchaoliu1992@163.com
bKey Laboratory of Environmentally Harmful Chemical Analysis, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029, China. E-mail: lizh@mail.buct.edu.cn
cKey Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190, China
First published on 21st December 2020
Abstract
The combination of a semiconductor heterojunction and oxygen evolution cocatalyst (OEC) is an important strategy to improve photoelectrochemical (PEC) water oxidation. Herein, a novel hamburger-like nanostructure of a triadic photoanode composed of BiVO4 nanobulks, Co3O4 nanosheets and Ag nanoparticles (NPs), that is, Ag/Co3O4/BiVO4, was designed. In our study, an interlaced 2D ultrathin p-type Co3O4 OEC layer was introduced onto n-type BiVO4 to form a p–n Co3O4/BiVO4 heterojunction with an internal electric field (IEF) in order to facilitate charge transport. Then the modification with Ag NPs can significantly facilitate the separation and transport of photogenerated carriers through the surface plasma resonance (SPR) effect, inhibiting the electron–hole recombination. The resulting Ag/Co3O4/BiVO4 photoanodes exhibit largely enhanced PEC water oxidation performance: the photocurrent density of the ternary photoanode reaches up to 1.84 mA cm−2 at 1.23 V vs. RHE, which is 4.60 times higher than that of the pristine BiVO4 photoanode. The IPCE value is 2.83 times higher than that of the pristine BiVO4 at 400 nm and the onset potential has a significant cathodic shift of 550 mV for the ternary well-constructed photoanode.
1. Introduction
Photoelectrochemical (PEC) water splitting is of great significance to mitigate global environmental problems by converting abundant solar energy into chemical fuels in the form of H2 or hydrocarbon compounds.1 The photoanodes have low efficiency compared to the photocathodes since the water oxidation half-reaction occurring in the photoanode is recognized as a speed-limiting step in the overall water splitting reaction. Therefore, it is important to develop an efficient and practical anode system for construction of high-performance PEC cells.2–4 BiVO4 is a highly promising photoanode, which exhibits proper band gap (∼2.4 eV) for solar light absorption and favorable valence band very near the thermodynamic oxygen evolution potential.5–7 However, poor charge transport, excessive electron–hole recombination, slow hole kinetics of oxygen evolution reaction and limited quantum efficiency are major challenges with the BiVO4 photoanodes.8,9 To overcome these challenges, various strategies such as elemental doping,10,11 construction of heterojunctions12,13 and coupling with various oxygen evolution cocatalysts (OECs)14–16 have been employed to improve the photoactivity of BiVO4.Formation of BiVO4-based nanostructured heterojunction photoanodes was recognized as an attractive route to improve PEC water splitting performances because it enlarges the interfacial area, promotes efficient charge separation, and improves the optical absorption.17–19 Among the rest, staggered (type-II) band structure alignment and complementary solar light harvesting contribute to further improve the PEC property of BiVO4. Especially, p-Co3O4 is not only expected to form a p–n junction with n-BiVO4, but also acted as an effective OEC. Thus among the various BiVO4-based heterojunction photoanodes, p–n Co3O4/BiVO4 photoanode exhibits the synergetic enhancement of surface reaction kinetics and bulk charge separation. For instance, Wang et al. synthesized powdered composite of BiVO4/Co3O4 with O2 evolution of 11 mmol g−1 h−1, which is 17 times greater than for unmodified BiVO4.20 Hou et al. also reported Co3O4@C/BiVO4 photoanode derived from MOF-template with the enhanced photocurrent density, which is approximately tenfold higher with respect to bare BiVO4.21
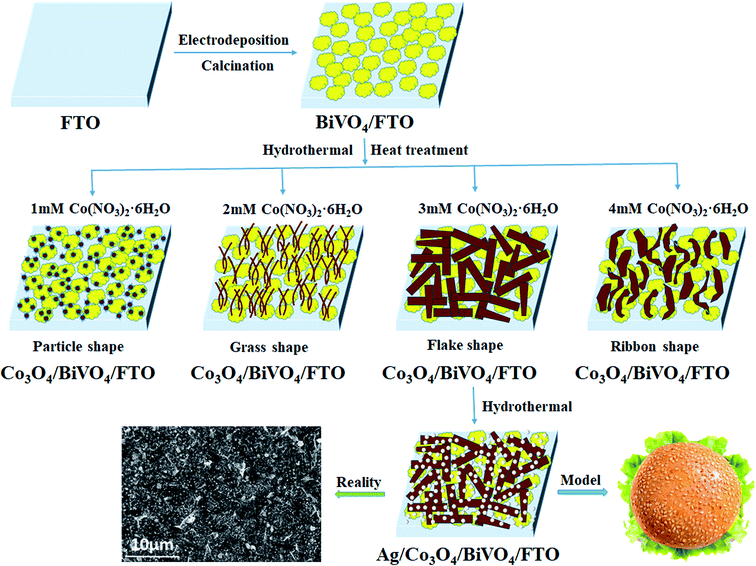
Although it has been well recognized the surface catalytic effect of Co3O4, the modification of Co3O4 may introduce more surface recombination centres that hamper the PEC performance inversely, in consideration of the junction formed at the photoanode/OEC interface. In this work, firstly, BiVO4 photoanodes with a nanoporous morphology were synthesized to reduce the travel distance of photogenerated holes, decreasing the chance of recombination. Secondly, p-Co3O4 OECs with interlaced nanosheets were dispersed on the surface of n-BiVO4 for constructing heterojunctions,8 enhancing charge separation by an internal electric field (IEF) and accelerate the kinetics of oxygen evolution reaction by the active site of cobalt. Lastly, the small particle size and high dispersion of Ag NPs accelerated interfacial electrons transport due to the formation of Schottky barrier, effectively improving the charge separation and greatly reducing the recombination of photogenerated electron hole pairs. Thus, highly efficient hamburger-like nanostructure of triadic Ag/Co3O4/BiVO4 photoanode was fabricated, as illustrated in Fig. 1. Considering that the loading amount would change the size or morphology of Co3O4 OECs, the effects of Co3O4 loading amount on PEC performance for Co3O4/BiVO4 heterojunction photoanode are discussed in detail. A working mechanism was also proposed to understand the improved separation of the photogenerated carriers by energy band structure, and the accelerated kinetics of oxygen evolution reaction at the interface of ternary components. In conclusion, the novel hamburger-like nanostructure of triadic Ag/Co3O4/BiVO4 photoanode designed in this work is proven to represent a highly effective pathway to improve the PEC conversion efficiency.
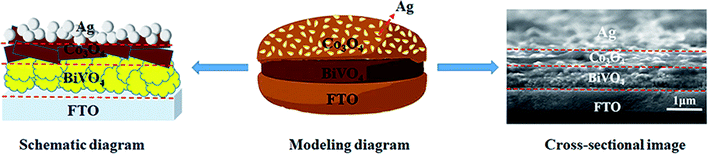 | ||
| Fig. 1 Schematic diagram, modeling diagram and cross-sectional image of triadic Ag/Co3O4/BiVO4 photoanode. | ||
2. Experimental sections
2.1 Preparation of pristine BiVO4 photoanode
The pristine BiVO4 photoanode was prepared from a Bi metal precursor reported previously.19 In a typical synthesis, a Bi metal film was first electrodeposited in a three-electrode quartz cell system on the electrochemical workstation. Then the Bi metal film was converted into BiVO4 after VO(acac)2 DMSO solution were dipped onto the surface of the Bi metal film and heated at 450 °C for 2 h in air. Finally, the residual V2O5 was removed by 1 M NaOH solution. Thus, the BiVO4 photoanode was obtained after washing and drying.2.2 Preparation of Co3O4/BiVO4 heterojunction photoanode
The Co3O4/BiVO4 photoanode was fabricated after Co3O4 was loaded onto the surface of BiVO4 photoanode by combing hydrothermal with heat treatment process. In order to investigate the effects of Co3O4 loading amount on PEC performance, the Co3O4/BiVO4 photoanodes with different Co3O4 loading amount were prepared by adjusting the reactant concentration of Co precursor. Taking the optimal reactant concentration as an example, the BiVO4/FTO substrates were transformed into Teflon-lined stainless steel autoclave containing 3 mM Co(NO3)2·6H2O, 10 mM NH4F and 25 mM urea. The autoclave was sealed and maintained at 120 °C for 5 h and then cooled down to room temperature. The obtained composite photoanode was then calcined at optimum 350 °C for 3 h, thus the Co3O4/BiVO4 photoanode was prepared accordingly. Similarly, the Co3O4/BiVO4 photoanodes with different Co3O4 loading amount were prepared according to the Co(NO3)2·6H2O concentration of 1 mM, 2 mM, 3 mM and 4 mM, keeping the concentration of 10 mM NH4F and 25 mM urea unchangeably.2.3 Preparation of Ag/Co3O4/BiVO4 triadic photoanode
The FTO substrate coated with Co3O4/BiVO4 heterojunction was immersed in a mixture solution containing 0.5 mM silver nitrate and 1 mM sodium citrate at 100 °C for 2 h. Afterwards, the Ag/Co3O4/BiVO4 triadic photoanode22 was rinsed with deionized water and dried in air. The complete synthesis process was sketched in Fig. 2. Additional details of chemicals, characterizations, PEC measurements and supporting data are available in ESI.†3. Results and discussion
The phase composition of the samples was detected by XRD technique, and the corresponding results are shown in Fig. 3. In the XRD pattern of pure BiVO4 photoanode (the red line), all diffraction peaks can be easily indexed to those of a monoclinic BiVO4 cell (JCPDS No. 75-1866). The peaks at 2θ value of 19.00, 28.97 and 30.55 indexed to (011), (112) and (004) plane respectively, and no other crystalline phase like V2O5 or Bi2O3 was detected. Furthermore, it can be seen that weak diffraction peaks are consistent with cubic spinel Co3O4 phase (cell parameters: a = 8.084 Å; JCPDS No. 74-2120), as shown in the blue line. Even higher calcination temperature cannot improve the crystallinity of Co3O4 due to the low loading amount and high dispersion (Fig. S1†). Based on some experimental results and discussion (Fig. S2 and S3†), 350 °C was determined to the optimal calcining temperature for the formation of the Co3O4/BiVO4 photoanode. After Ag NPs loading onto the Co3O4/BiVO4/FTO, all monoclinic BiVO4, cubic Co3O4 and Ag phases appeared simultaneously (the pink line in Fig. 3). In order to differentiate the lapped peaks of FTO and Ag NPs, the XRD patterns of 2θ values from 33 to 47 are enlarged partially. (111) and (200) with 2θ values around 38.11° and 44.30° preferred orientation diffraction peaks appeared, consistent with the crystal planes of metallic silver.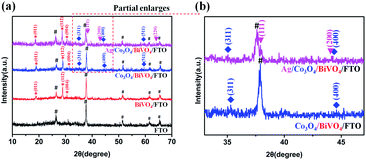 | ||
| Fig. 3 (a) XRD patterns of FTO, BiVO4/FTO, Co3O4/BiVO4/FTO and Ag/Co3O4/BiVO4/FTO. (b) Enlarged view of the area marked with the red dots in the (a) XRD patterns. | ||
To further confirm the composition of the composite sample, XPS measurement was carried out as shown in Fig. 4. From the survey XPS spectrum (Fig. 4a), C 1s peak is located at 284.6 eV,23 and except C, Bi, V, O, Co and Ag, no peaks of other elements could be observed, which indicates the presence of Co and Ag species on BiVO4, compared with pristine BiVO4. Fig. 4b and d present the high resolution XPS scan spectra of Bi 4f, V 2p, and O 1s. The Bi 4f XPS spectra in Fig. 4b show that the binding energies of Bi 4f7/2 and Bi 4f5/2 occur at 158.7 and 164.05 eV, respectively.24 The V 2p peak in Fig. 4c can be fitted with two peaks at 523.9 and 516.3 eV, which are assignable to V 2p1/2 and V 2p3/2 signals, respectively.25 Compared with the pure BiVO4, an obvious negative shift in V 2p peaks and a slight negative shift in Bi 4f peaks are observed for Ag/Co3O4/BiVO4, confirming the electron transfer from Co3O4 to BiVO4.26 At the same time, the O 1s spectra of pristine BiVO4 and Ag/Co3O4/BiVO4 shown in Fig. 4d exhibit the binding energies for O 1s (529.1 eV) core levels, while in the O 1s XPS spectrum of Ag/Co3O4/BiVO4, a new peak (531.1 eV) appears as compared to pristine BiVO4, which is probably caused by the Co loading and also indicates that the loaded Co species is exist in oxides form. The peaks located at 529.1 and 531.1 eV can be assigned to the oxygen species of lattice oxygen of layer-structured Bi2O22+ and Co3O4, respectively. The high-resolution spectrum for Co 2p (Fig. 4e) shows two major peaks with binding energies at 779.3 and 795.1 eV, corresponding to Co 2p3/2 and Co 2p1/2, respectively, which is the characteristic of a Co3O4. The spectra in the Co 2p1/2 region can be further deconvoluted into two peaks. The peaks at 793.6 eV and 795.4 eV are ascribed to Co3+ and Co2+ species respectively, which indicates the Co2+ and Co3+ species co-exist in the as-grown Co3O4 layer.27 Fig. 4f shows that Ag 3d binding energies are 367.2 and 373.2 eV, confirming the existence of Ag.28 Based on the XPS data, the examined sample was confirmed as Ag/Co3O4/BiVO4 composite, and a strong interface interaction between photocatalyst BiVO4 and cocatalyst Co3O4 would influence the resulting PEC properties, which will be discussed in later sections.
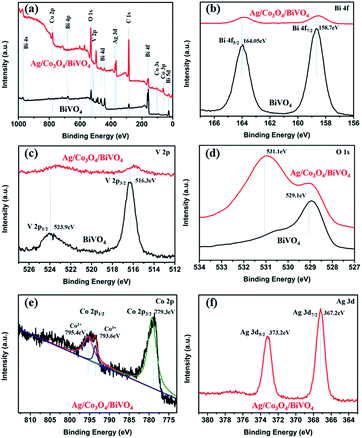 | ||
| Fig. 4 XPS survey spectra of pristine BiVO4 and Ag/Co3O4/BiVO4: (a) survey scan; (b) Bi 4f; (c) V 2p; and (d) O 1s. (e) Co 2p and (f) Ag 3d scan spectra of Ag/Co3O4/BiVO4. | ||
The morphology and microstructure of triadic Ag/Co3O4/BiVO4 photoanode was characterized by FESEM and HRTEM in Fig. 5. The triadic Ag/Co3O4/BiVO4 photoanode was fabricated by BiVO4 nanobulks deposited with Co3O4 nanosheets and Ag nanoparticles as shown in Fig. 5a. It can be observed that BiVO4 nanobulks were covered under the Co3O4 nanosheets, and Ag nanoparticles were evenly distributed on the surface of Co3O4 in Fig. 5b. The microstructure and elemental distribution of Ag/Co3O4/BiVO4 are further revealed by the TEM and energy dispersive X-ray (EDX) mappings. EDX image of Ag/Co3O4/BiVO4 photoanode indicates the presence of the Bi, V, Co, Ag and O (Fig. 5d). The elemental mappings of Fig. 5c display that the BiVO4 nanobulks are well-distributed under Co3O4 nanosheets and Ag nanoparticles, and the EDX mapping analyses are consistent with the SEM observation. From TEM image in Fig. 5e, three components of the triadic photoanode were uniformly distributed. The HRTEM image in Fig. 5f shows the distinct lattice fringe of Ag/Co3O4/BiVO4 complex. The fringes spacing is measured to be 0.310 nm, which corresponded to the (−121) lattice spacing of BiVO4. In addition, the Co3O4 displays a lattice spacing of 0.241 nm, corresponding to the (311) plane.8 A typical lattice spacing of 0.236 nm indexed to (111) plane of Ag, revealing that the Ag nanoparticles were loaded on the Co3O4 nanosheets closely.29
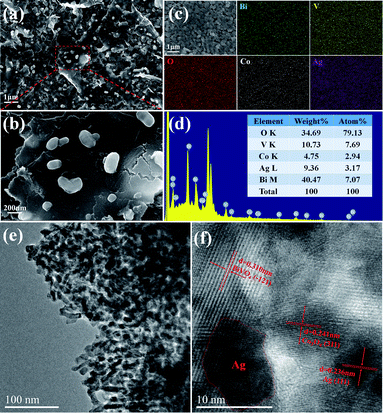 | ||
| Fig. 5 SEM images (a and b) of Ag/Co3O4/BiVO4, EDX mappings (c and d) for Ag/Co3O4/BiVO4, TEM (e) and HRTEM (f) images of Ag/Co3O4/BiVO4. | ||
To give a deep insight into the loading amount for changing the nanostructure of Co3O4 layer, the effects of Co3O4 loading on BiVO4 photoanodes were further investigated by controlling the reactant concentration of Co(NO3)2·6H2O. As illustrated in Fig. 6, it is interesting to note that various nanostructured Co3O4 cocatalysts were obtained in the reaction solutions with different cobalt precursor concentrations. As shown in Fig. 6a and b, when the cobalt precursor was 1 mM, Co3O4 can only be dispersed in particle shape on BiVO4 nanobulks. When the cobalt precursor concentration was adjusted to 2 mM, the one-dimensional Co3O4 nanowires with a diameter of ∼50 nm in grass shape were obtained, as shown by the SEM images in Fig. 6c and d. When the cobalt precursor concentration continued to rise to 3 mM, the two-dimensional Co3O4 nanosheets in flake shape were observed in Fig. 6e and f, which are interwoven with each other and laid flat on BiVO4 nanobulks. As illustrated in Fig. 6g and h, the solvothermal syntheses in the solutions with 4 mM cobalt precursor resulted in the growth of three-dimensional Co3O4 nanosheets in ribbon shape. The as-prepared Co3O4/BiVO4 heterojunction photoanodes with different Co3O4 loading amount have obvious effects on PEC properties, which will be discussed in detail next.
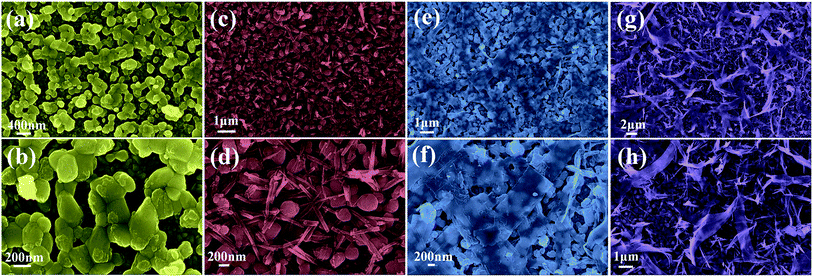 | ||
| Fig. 6 SEM images (a and b) of 1 mM Co3O4/BiVO4; (c and d) of 2 mM Co3O4/BiVO4; (e and f) of 3 mM Co3O4/BiVO4; (g and h) of 4 mM Co3O4/BiVO4. | ||
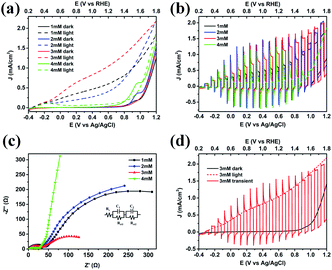
The PEC measurements were achieved to disclose the functional roles of Co3O4 when coupled with BiVO4 (Fig. 7). The current–voltage behaviors of the photoanodes under illumination and in the dark were studied as shown in Fig. 7a. In the dark, the current densities of the four photoanodes are almost zero, until the electrocatalytic oxidation peaks of cobalt appear at 0.95 V vs. Ag/AgCl. It can be observed that with the increase of Co3O4 loading amount, the electrocatalytic oxidation of water is stronger, that is, the peak strength increases and the onset potential shifts negatively. However, the difference is that 3 mM Co3O4/BiVO4 heterojunction electrode has no obvious oxidation peak, which may be ascribed to highly dispersed Co2+/Co3+ species oxidizing more easily to higher valence Co3+/Co4+, expediting the kinetics.30 Under illumination, 3 mM Co3O4/BiVO4 photoanode exhibited the highest photocurrent response, with the increase of Co3O4 loading amount. However, with the increase of excessive Co3O4 loading on 4 mM Co3O4/BiVO4 photoanode, the corresponding light response is particularly poor, even shows no obvious response compared to the behaviour in the dark. Nevertheless, an in-depth investigation of the current–potential curves measured under chopped light illumination in Fig. 7b reveals that the 4 mM Co3O4/BiVO4 photoanode is not nonresponsive but severely suppressed. When the photoanode was exposed to incident light, an anodic photocurrent spike was observed and then rapidly dropped to nearly zero, which is associated with the sudden generation of charge carriers and the subsequent recombination process.31 Electrochemical impedance spectroscopy (EIS) studies were further carried out to investigate the charge transport behaviour at the electrode/electrolyte interface in these photoanodes. The obtained EIS data is fitted to an equivalent circuit shown in Fig. 7c, and the parameters extracted from the Nyquist fit are listed in Table S1.† Rs is defined as the series resistance, Rct1 in low impedance (high frequency) represents charge transfer resistance across the interface between the semiconductors, Rct2 in high impedance (low frequency) is the charge transfer resistance across the electrode/electrolyte interface.32–34 A smaller semidiameter of the semicircle represents a better charge transfer ability (i.e., faster surface reaction kinetics).35 4 mM Co3O4/BiVO4 photoanode exhibited the largest semidiameter and the highest low-frequency impedance Rct2 in agreement with its worse PEC activity, which also suggests the severe aggregation and recombination of photogenerated carriers, rather than favourable migration to involve in water oxidation reaction.32 3 mM Co3O4/BiVO4 photoanode presented the lowest low-frequency impedance Rct2 and the best PEC behaviour, which may be also attributed to its interlaced 2D nanosheet structure for facilitating the carrier transportation and surface water oxidation reaction. Current–potential curves of the 3 mM Co3O4/BiVO4 photoanode under different illumination conditions are shown in Fig. 7d. The dark and light behaviours are very consistent with current–potential curve under chopped illumination upon turning on or off the light, but the sharp photocurrent spikes can still be clearly observed, which requires further improvement of its PEC performance. The above results indicate that the PEC properties of Co3O4/BiVO4 composite photoanode are affected by the loading amount and nanostructure of Co3O4 layer.
Diffuse reflectance UV-visible spectra (DRS) were collected for BiVO4, Co3O4 and Co3O4/BiVO4 photoanodes to understand the influence of the heterostructure on light harvesting capability and to discuss the mechanism of charge separation for p-Co3O4/n-BiVO4 heterojunction. As demonstrated in Fig. S4a,† pristine BiVO4 photoelectrode shows a typical semiconductor absorption, whose absorption edge is located approximately at 500 nm. After loading the Co3O4 layer, the absorption edge of Co3O4/BiVO4 obviously red-shifts and the absorption can be significantly enhanced in the whole visible region, which implies that the Co3O4/BiVO4 composite photoanode has better visible light absorption capacity. Moreover, it is not difficult to find that the trend of light absorption enhancement for p-Co3O4/n-BiVO4 photoanode is very consistent with the light absorption characteristics of Co3O4 film, which is attributed to Co3O4 as a p-type semiconductor with narrower bandgap width. The direct band gap energy (Eg) of BiVO4 and Co3O4 are evaluated to be 2.50 and 2.06 eV respectively, as shown in Fig. S4b.† The Eg of the composite photoanode is further narrowed to 2.0 eV, which suggests the formation of heterojunction with the strong interaction between n-BiVO4 and p-Co3O4, consistent with the aforementioned XPS results. The band positions of pure BiVO4 and Co3O4 before contact were predicted theoretically from the absolute electronegativity using eqn (1) and (2):
| EV = X − Ee + 0.5Eg | (1) |
| EC = EV − Eg | (2) |
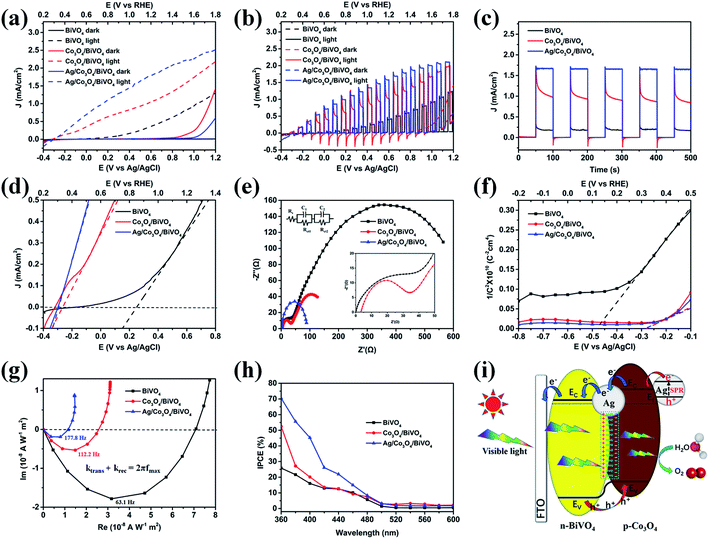
Although the as-prepared Co3O4/BiVO4 heterojunction photoanode has played a good role in charge separation, carrier transport is still not ideal, as can be seen from the transient current curve in Fig. 7d. Considering the excellent electron conductivity and catalytic performance of silver nanoparticles, we tried to further improve the PEC performance of composite photoanode by loading Ag NPs. As shown in Fig. 8a, pristine BiVO4 photoanode displays a relatively low photoresponse with a photocurrent density of 0.40 mA cm−2 at 1.23 V vs. RHE. A largely enhanced photocurrent density (1.08 mA cm−2) is observed for the Co3O4/BiVO4 heterojunction photoanode. In the case of triadic Ag/Co3O4/BiVO4 photoanode, the photocurrent density further reaches to 1.84 mA cm−2 at 1.23 V vs. RHE, 4.60 and 1.70 times higher than that of the pristine BiVO4 and Co3O4/BiVO4 samples, respectively. Moreover, the photoconversion efficiency (PCE) of each photoanode was calculated based on the photocurrent response as shown in Fig. S5.† The PCE of triadic Ag/Co3O4/BiVO4 photoanode reached 0.483% at 0.7 V vs. RHE, 28.4 times higher than the pristine BiVO4 (0.017%) and 1.8 times higher than Co3O4/BiVO4 photoanode (0.267%). In addition, Fig. 8b shows the transient photocurrent response of the photoanodes under chopped light illumination. The instantaneous current when the light is switched ON is a measure of the flux of holes into the surface. The subsequent decay towards the steady state current results from the capture of build-up holes in surface states inducing an electron flux associated with recombination.36 In particular, the cathodic current overshoot observed when the light is switched OFF is due to the continuing flux of electrons from Co3O4 into the surface, correspondingly, no cathodic current was observed in the pristine BiVO4 photoanode. When Ag NPs were loaded onto the surface of Co3O4, and interlayers between Co3O4 and BiVO4, the surface plasma resonance (SPR) effect and excellent electrical conductivity of Ag NPs inhibited the recombination of surface electrons and holes, which further improved the photocurrent response.37 The effect of Ag NPs was further confirmed by the chronoamperometry measurement under chopped light illumination in Fig. 8c. Amperometric I–t curves also depict the fast response toward irradiation stimulation in five cycles of switching light ON and OFF. The current density of these electrodes under illumination follows a descending order as: Ag/Co3O4/BiVO4 > Co3O4/BiVO4 > pristine BiVO4. This is in high accordance with the LSV results (Fig. 8a) and further demonstrates the improvement in PEC performance provided by Co3O4 and Ag NPs. Moreover, the square profiles and steady photocurrent density of transient photocurrent response in Ag/Co3O4/BiVO4 indicate a fast surface oxidation kinetics, suppressed electron–hole recombination and excellent photochemical stability.38 Fig. 8d shows an enlarged image of the current–voltage performance of the photoanodes under illumination based on Fig. 8a. Compared to pristine BiVO4, the onset potential of the Co3O4/BiVO4 heterojunction significantly reduces from 0.86 V to 0.35 V with a cathodic shift of 510 mV. This cathodic shift value is the highest amongst all reported ones, in which BiVO4 photoanodes were composited with a p-type semiconductor Co3O4, NiO or WO3.8,39–41 Furthermore, the onset potential is further negatively shifted 40 mV after modification of Ag NPs. The above results indicate that the triadic Ag/Co3O4/BiVO4 photoanode further inhibited the electron–hole recombination and enhanced the photoresponse, after Ag NPs were introduced into the p-Co3O4/n-BiVO4 heterojunction.
To gain further insight into the mechanism of the triadic Ag/Co3O4/BiVO4 photoanode, the charge transfer at the photoanodes and water oxidation kinetics at the photoanode/electrolyte interface are evaluated using EIS plots. As shown in Fig. 8e, the obtained EIS data is fitted to an equivalent circuit,32–34 and the parameters are listed in Table S2.† As reported, the first arc (high frequency) represents the charge transfer step and the second arc (low frequency) corresponds to the surface water oxidation step.26 Compared to the pristine BiVO4 photoanode, Co3O4/BiVO4 photoanode exhibits smaller diameter of both two arcs, indicating that Co3O4 can boost the charge separation/transfer and surface reaction kinetics simultaneously, which can be attributed to the formation of Co3O4/BiVO4 p–n junction and great catalytic activity of Co3O4 cocatalysts toward water oxidation. After introducing Ag NPs, the semicircle in the high-frequency region is eliminated and the semicircle in the low-frequency region is further reduced, indicating that the triadic Ag/Co3O4/BiVO4 photoanode has better charge separation and transportation performance. Moreover, the flat band potential is obtained by extrapolating the Mott–Schottky plot to 1/C2 = 0 (Fig. 8f). Compared to the pristine BiVO4, an evidently positive shift is observed for the Co3O4/BiVO4 photoanode. The positive shift of the flat band potential for Co3O4/BiVO4 suggests a decrease in the band bending, thus facilitating electrode/electrolyte interface charge transfer.
Intensity modulated photocurrent spectroscopy (IMPS) is a useful method to study semiconductor charge transport properties and is widely used in electron transport characterization of PEC devices.42 Fig. 8g shows typical IMPS responses of pristine BiVO4, Co3O4/BiVO4 and Ag/Co3O4/BiVO4 photoanodes in the complex plane. The semicircle observed in the fourth quadrant provides information on the combination of charge transfer and relaxation inside the photoanode.43 Accordingly, the frequency of the maximum imaginary corresponds to the sum of the charge transfer (ktrans) and recombination (krec) rate constants (ktrans + krec = 2πfmax). The transient time of the electron (τd) was calculated according to the characteristic frequency apex of IMPS imaginary components. According to Fig. 8g, τd values of BiVO4, Co3O4/BiVO4 and Ag/Co3O4/BiVO4 were calculated to be 2.52, 1.42 and 0.90 ms, respectively. This result indicates that the Co3O4/BiVO4 photoanode has a higher electron transfer rate than that of pristine BiVO4 photoanode, which results from band bending at the interface in heterojunction, leading to a directional flow of electron. The time constant further decrease to 0.90 ms for Ag NPs modified Co3O4/BiVO4 photoanode, indicating the enhanced carries transport and better surface reaction kinetics for water oxidation, which supports the EIS and Mott–Schottky results very well. The incident photon-to-current efficiency (IPCE) value is a critical index to study solar energy conversion efficiency. The Co3O4/BiVO4 photoanode presents a higher IPCE value than BiVO4 from UV extending to the visible region (Fig. 8h). Moreover, the Ag/Co3O4/BiVO4 has higher IPCE profile than that of Co3O4/BiVO4 until 500 nm, which is attributed to their corresponding SPR absorption peaks in the visible region.38 This SPR wavelength dependent IPCE feature signifies that excitation of the Ag SPR is also responsible for the improved PEC performance of the triadic Ag/Co3O4/BiVO4 photoanode. As a consequence, the IPCE value of ternary photoanode at 400 nm is up to 45.3%, which is 2.83 and 2.25 times higher than that of BiVO4 and Co3O4/BiVO4 respectively. The largely enhanced IPCE of the triadic Ag/Co3O4/BiVO4 photoanode further confirms the integrated contribution of Co3O4 layer and Ag NPs to the PEC performance.
Given the discussion above, a mechanism for the enhanced PEC water oxidation performance in this triadic Ag/Co3O4/BiVO4 photoanode is proposed and shown in Fig. 8i. Firstly, BiVO4 with a small bandgap can utilize a significant portion of visible light to generate electrons and holes, and its valence band (VB) edge is positive enough to provide the holes with sufficient overpotential for the water oxidation reaction. When p-Co3O4 and n-BiVO4 come into contact, a p–n junction is formed at their interface. Then the photoexcited holes on the VB of BiVO4 can transfer to Co3O4, while the electrons on the CB of Co3O4 transfer to BiVO4 that is driven by an IEF at the Co3O4/BiVO4 interface. Furthermore, the unique interwoven nanostructure composed of 2D Co3O4 nanosheets could shorten the hole diffusion distance and facilitate the transport of holes to the surface. Besides, the highly dispersed Co2+/Co3+ species within the Co3O4 ultrathin nanosheets can be oxidized to higher valence Co3+/Co4+, which are believed to catalyse water oxidation and expedite the kinetics. Finally, the interlaminar Ag induced surface potential at Co3O4/BiVO4 interface facilitates rapid carrier separation and transport.44 Meanwhile, under the irradiation of simulated solar light, the hot electrons generated by the SPR effect of Ag NPs can transfer to the conduction band (CB) of Co3O4.37,45 Then the oscillation of the Ag NPs facilitates electron movement from Co3O4 to BiVO4 and improves the photocurrent response.37 Besides, the Schottky barrier existing at the interface of Ag and Co3O4 can efficiently prevent the hot electrons accumulated in Co3O4 from returning back to Ag nanoparticles, which is confirmed by the XPS and IPCE results.45,46 Consequently, the significant enhancement in PEC performance of the triadic Ag/Co3O4/BiVO4 photoanode can be ascribed to the 2D structure/p–n junction/SPR effect triple functionality.
4. Conclusions
The work demonstrates that the novel hamburger-like nanostructure of triadic Ag/Co3O4/BiVO4 photoanode based on ingenious design is successfully prepared, where the Co3O4 plays a synergistic role of OECs and p–n heterojunction, meanwhile Ag NPs enhance carrier transport and inhibit electron–hole recombination due to the SPR effect. The resulting ternary photoanode exhibit significantly enhanced efficiency in PEC water splitting: the photocurrent density enhances 4.60 times than pristine BiVO4 and higher than other BiVO4 composites reported in literatures. The IPCE of Ag/Co3O4/BiVO4 reaches 2.83 times higher than BiVO4 due to the charge transit time shorten trebly than BiVO4 from IMPS spectra. The onset potential of the ternary well-constructed photoanode has an astonishing cathodic shift of 550 mV compared to the pristine BiVO4 photoanode. Besides, the positive shift of flat band potential, the enhancement of carrier density and the decrease in energy gap are also driven by synergistic action of p–n junction and SPR effect. It is expected that this strategy can be extended to other multilevel heterostructures for advanced performance in the fields of energy conversion and storage.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Central University Basic Scientific Research Operating Expenses Project and National College Student Innovation and Entrepreneurship Training Program from Beijing University of Chemical Technology.References
- Y. Cui, L. Pan, Y. Chen, N. Afzal, S. Ullah, D. Liu, L. Wang, X. Zhang and J.-J. Zou, RSC Adv., 2019, 9, 5492–5500 Search PubMed.
- M. G. Lee, D. H. Kim, W. Sohn, C. W. Moon, H. Park, S. Lee and H. W. Jang, Nano Energy, 2016, 28, 250–260 Search PubMed.
- S. Kumar, S. Ahirwar and A. K. Satpati, RSC Adv., 2019, 9, 41368–41382 Search PubMed.
- J. Liu, S.-M. Xu, Y. Li, R. Zhang and M. Shao, Appl. Catal., B, 2020, 264, 118540 Search PubMed.
- W. Jiang, Y. Jiang, J. Tong, Q. Zhang, S. Li, H. Tong and L. Xia, RSC Adv., 2018, 8, 41439–41444 Search PubMed.
- S. J. A. Moniz, S. A. Shevlin, D. J. Martin, Z.-X. Guo and J. Tang, Energy Environ. Sci., 2015, 8, 731–759 Search PubMed.
- J. Qi, D. Kong, D. Liu, L. Pan, Y. Chen, X. Zhang and J.-J. Zou, RSC Adv., 2019, 9, 15629–15634 Search PubMed.
- X. Chang, T. Wang, P. Zhang, J. Zhang, A. Li and J. Gong, J. Am. Chem. Soc., 2015, 137, 8356–8359 Search PubMed.
- J. Liu, J. Li, M. Shao and M. Wei, J. Mater. Chem. A, 2019, 7, 6327–6336 Search PubMed.
- S. Byun, G. Jung, S.-Y. Moon, B. Kim, J. Y. Park, S. Jeon, S.-W. Nam and B. Shin, Nano Energy, 2018, 43, 244–252 Search PubMed.
- K. Okuno, H. Kato, J. J. M. Vequizo, A. Yamakata, H. Kobayashi, M. Kobayashi and M. Kakihana, RSC Adv., 2018, 8, 38140–38145 Search PubMed.
- J. H. Kim and J. S. Lee, Adv. Mater., 2019, 31, 1806938 Search PubMed.
- C. Ràfols i Bellés, S. Selim, N. M. Harrison, E. A. Ahmad and A. Kafizas, Sustainable Energy Fuels, 2019, 3, 264–271 Search PubMed.
- T. W. Kim and K.-S. Choi, Science, 2014, 343, 990 Search PubMed.
- S. Wang, T. He, J.-H. Yun, Y. Hu, M. Xiao, A. Du and L. Wang, Adv. Funct. Mater., 2018, 28, 1802685 Search PubMed.
- Y. Hermans, S. Murcia-López, A. Klein, R. van de Krol, T. Andreu, J. R. Morante, T. Toupance and W. Jaegermann, Phys. Chem. Chem. Phys., 2019, 21, 5086–5096 Search PubMed.
- X. Zhao, W. Luo, J. Feng, M. Li, Z. Li, T. Yu and Z. Zou, Adv. Energy Mater., 2014, 4, 1301785 Search PubMed.
- Z.-F. Huang, L. Pan, J.-J. Zou, X. Zhang and L. Wang, Nanoscale, 2014, 6, 14044–14063 Search PubMed.
- S. Bai, J. Liu, M. Cui, R. Luo, J. He and A. Chen, Dalton Trans., 2018, 47, 6763–6771 Search PubMed.
- J. Wang and F. E. Osterloh, J. Mater. Chem. A, 2014, 2, 9405–9411 Search PubMed.
- C.-C. Hou, T.-T. Li, Y. Chen and W.-F. Fu, ChemPlusChem, 2015, 80, 1465–1471 Search PubMed.
- Y. Tang, R. Wang, Y. Yang, D. Yan and X. Xiang, ACS Appl. Mater. Interfaces, 2016, 8, 19446–19455 Search PubMed.
- S. Bai, J. Liu, J. Guo, R. Luo, D. Li, Y. Song, C. C. Liu and A. Chen, Sens. Actuators, B, 2017, 249, 22–29 Search PubMed.
- P. Guan, H. Bai, F. Wang, H. Yu, D. Xu, B. Chen, T. Xia, W. Fan and W. Shi, ChemCatChem, 2018, 10, 4927–4933 Search PubMed.
- B. He, Y. Wang, X. Liu, Y. Li, X. Hu, J. Huang, Y. Yu, Z. Shu, Z. Li and Y. Zhao, J. Mater. Chem. A, 2019, 7, 6747–6752 Search PubMed.
- R. Zhang, M. Shao, S. Xu, F. Ning, L. Zhou and M. Wei, Nano Energy, 2017, 33, 21–28 Search PubMed.
- X. Dang, X. Zhang, X. Dong, W. Ruan, H. Ma and M. Xue, RSC Adv., 2014, 4, 54655–54661 Search PubMed.
- W. Zhao, J. Zhang, F. Zhu, F. Mu, L. Zhang, B. Dai, J. Xu, A. Zhu, C. Sun and D. Y. C. Leung, Chem. Eng. J., 2019, 361, 1352–1362 Search PubMed.
- T. Hong, Z. Liu, X. Zheng, J. Zhang and L. Yan, Appl. Catal., B, 2017, 202, 454–459 Search PubMed.
- W. He, R. Wang, L. Zhang, J. Zhu, X. Xiang and F. Li, J. Mater. Chem. A, 2015, 3, 17977–17982 Search PubMed.
- Y. Zhu, J. Ren, X. Yang, G. Chang, Y. Bu, G. Wei, W. Han and D. Yang, J. Mater. Chem. A, 2017, 5, 9952–9959 Search PubMed.
- G. Liu, Y. Zhao, K. Wang, D. He, R. Yao and J. Li, ACS Sustainable Chem. Eng., 2018, 6, 2353–2361 Search PubMed.
- S. S. M. Bhat, S. A. Lee, T. H. Lee, C. Kim, J. Park, T.-W. Lee, S. Y. Kim and H. W. Jang, ACS Appl. Energy Mater., 2020, 3, 5646–5656 Search PubMed.
- X. Zhang, X. Wang, X. Yi, J. Ye and D. Wang, ACS Sustainable Chem. Eng., 2019, 7, 5420–5429 Search PubMed.
- B. Klahr, S. Gimenez, F. Fabregat-Santiago, T. Hamann and J. Bisquert, J. Am. Chem. Soc., 2012, 134, 4294–4302 Search PubMed.
- C. Y. Cummings, F. Marken, L. M. Peter, A. A. Tahir and K. G. U. Wijayantha, Chem. Commun., 2012, 48, 2027–2029 Search PubMed.
- Y. Ren, Q. Xu, X. Zheng, Y. Fu, Z. Wang, H. Chen, Y. Weng and Y. Zhou, Appl. Catal., B, 2018, 231, 381–390 Search PubMed.
- F. Ning, M. Shao, S. Xu, Y. Fu, R. Zhang, M. Wei, D. G. Evans and X. Duan, Energy Environ. Sci., 2016, 9, 2633–2643 Search PubMed.
- M. Zhong, T. Hisatomi, Y. Kuang, J. Zhao, M. Liu, A. Iwase, Q. Jia, H. Nishiyama, T. Minegishi, M. Nakabayashi, N. Shibata, R. Niishiro, C. Katayama, H. Shibano, M. Katayama, A. Kudo, T. Yamada and K. Domen, J. Am. Chem. Soc., 2015, 137, 5053–5060 Search PubMed.
- L. Li, X. Yang, Y. Lei, H. Yu, Z. Yang, Z. Zheng and D. Wang, Chem. Sci., 2018, 9, 8860–8870 Search PubMed.
- S. S. Kalanur, I.-H. Yoo, J. Park and H. Seo, J. Mater. Chem. A, 2017, 5, 1455–1461 Search PubMed.
- J. Su, L. Guo, N. Bao and C. A. Grimes, Nano Lett., 2011, 11, 1928–1933 Search PubMed.
- F. Yu, F. Li, T. Yao, J. Du, Y. Liang, Y. Wang, H. Han and L. Sun, ACS Catal., 2017, 7, 1868–1874 Search PubMed.
- X. Zhang, Y. Li, J. Zhao, S. Wang, Y. Li, H. Dai and X. Sun, J. Power Sources, 2014, 269, 466–472 Search PubMed.
- S. Kim, Y. Yu, S. Y. Jeong, M. G. Lee, H. W. Jeong, Y. M. Kwon, J. M. Baik, H. Park, H. W. Jang and S. Lee, Catal. Sci. Technol., 2018, 8, 3759–3766 Search PubMed.
- C. Clavero, Nat. Photonics, 2014, 8, 95–103 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08102g |
| This journal is © The Royal Society of Chemistry 2020 |