Open Access Article
Open Access ArticleInsight into the enhanced photocatalytic activity of Mo and P codoped SrTiO3 from first-principles prediction
Yueqin Wang a,
Jingyu Wangb,
Wei Lianb and
Yin Liu*b
a,
Jingyu Wangb,
Wei Lianb and
Yin Liu*b
aSchool of Mechanics and Optoelectronic Physics, Anhui University of Science and Technology, Huainan 232001, China. E-mail: yqwang1025@126.com
bSchool of Materials Science and Engineering, Anhui University of Science and Technology, Huainan 232001, China. E-mail: yinliu@aust.edu.cn
First published on 4th November 2020
Abstract
In this study, the synergistic effect of cation codoping (Mo and the cation P) on the band structure of SrTiO3 is demonstrated to enhance its photocatalytic activity. The electronic structure and optical properties of (Mo + P) codoped SrTiO3 are examined by performing GGA + U calculations. The results show that the strong hybridization between the Mo 4d states and the O 2p states assisted by the non-metal P leads to the formation of fully occupied and delocalized intermediate states (IBs) near the valence band of SrTiO3. The proximity of IBs to the valence band resulted in the ability to separate photo-excited electrons from reaction holes, which helps to ensure efficient electron replenishment reducing the probability of trapping electrons from the CB. This kind of metal Mo and non-metal P-compensated codoping can efficiently narrow the band gap and enhance the visible-light absorption. Moreover, the positions of the band edges after codoping (Mo + P) are found to be thermodynamically favorable for water splitting.
1. Introduction
Perovskite, SrTiO3, has attracted intense attention as a promising material in the fields of photocatalysis and water splitting, since it presents excellent properties such as low cost and long term stability.1,2 Due to the large band gap of SrTiO3 (3.25 eV),3 it is, however, not able to show photocatalytic activity in the visible-light region, and can absorb only ultraviolet light (only 5% of solar energy). Besides the long term stability, an ideal photocatalyst should also have a narrow band gap to utilize cheaper visible-light, and appropriate band edge positions to meet the requirements for water splitting.4,5 As such, there has been ever-growing interest in the band structure engineering of SrTiO3. Enabling SrTiO3 to absorb the more abundant visible-light is a crucial prerequisite for enhancing the photoconversion efficiency by reducing its band gap below 2.0 eV.6–8In exploring the enhanced photocatalytic activity of SrTiO3-based materials to the visible-light region, there have been many reports that have adopted different strategies for optimizing the band gap and extending the light absorption of SrTiO3 by different doping schemes.9–15 Among them, the codoping approach is an effective means for lowering the band gap and improving the visible-light response, involving either cation–anion,16–18 cation–cation,19–21 or anion–anion22,23 pairs. In the case of cation–cation codoping, the substitution of transition metal cations (Cr, Fe, Rh, Mo, W, etc.) at the host Ti sites in SrTiO3 has shown to lower the band gap by introducing localized 3d impurity states, shifting the absorption spectra threshold towards the visible region.24–27 Recently, the substitution of non-metal and metal cations at the Ti sites in TiO2 has stimulated great interest in enhancing the visible-light absorption and photocatalytic activity by including (Fe + Si), (Mo + P) and (W + P) codoping.28–30 However, to the best of our knowledge, there is no reported experimental or theoretical work focusing on the synergistic effects of (Mo + P) codoped SrTiO3 on the band gap engineering.
The present work investigates the role of Mo and P cation codoping in SrTiO3 using first-principles calculations. The substitutional positions of both the Mo and P dopants are incorporated at the Ti sites. The stabilities and optical properties of codoped systems were first determined. The electronic structure of the codoped system was analyzed and compared to gain insight into the intermediate states for lowering the band gap. To explore the synergistic effects on the redox capacity for photocatalytic water splitting, the band edge alignments were carefully carried out. These prediction results indicate that the band structure engineering of SrTiO3 by codoping with metal and non-metal cations (Mo + P) is a promising strategy for enhancing photocatalytic activity.
2. Computational methods
All the spin-polarized calculations were performed using the CASTEP package in Materials Studio 8.0.31 The exchange and correlation interaction was treated with the generalized gradient approximation (GGA) of Perdew–Burke–Ernzerhof (PBE). The interactions between the valence electrons and ionic core were approximated by the ultra-soft pseudopotentials (USPP). To reproduce the experimental band gap of 3.25 eV in SrTiO3, the GGA + Up + Ud approach was adopted to describe the correlation effects, which can predict the corrected electronic structure and optical properties.32–34 The on-site corrections are typically applied to the d or f orbitals of transition metal oxides; however, the band gaps are still underestimated with respect to the experimental values. Theoretical studies have revealed that the corrected band electronic structures of metal oxides can be obtained by simultaneously using the on-site Coulomb corrections on the d states of transition metals and the 2p orbital of oxygen atoms. Ma et al. theoretically found that the simultaneous application of the on-site Coulomb corrections on the Zn-3d (Ud) and O-2p (Up) orbitals lead to a corrected band structure of ZnO.32 It has been also reported that the pair of Ud (10 eV) and Up (7 eV) was identified as an optimal choice for the correct band structure of W-doped ZnO; in this work, we chose the GGA + Ud + Up method to discuss the correction effects on the electronic structure of SrTiO3 oxide materials. Based on the previous theoretical predictions, we firstly fit the Ud (2.3 eV) of Ti-3d states,35 then discuss the effects of different Up values on the band structures. It was found that the band gaps were 3.11, 3.22 and 3.48 eV for bulk SrTiO3 system as using the Up values (7, 8 and 9 eV) for O-2p orbitals, respectively. The correct band gap was 3.22 eV within Up = 8 eV, which agrees well with the experimental results. We note that a Up value of 8 eV is the best choice, which is somewhat larger than the U value used in previous work.32 Taking into account the transition metal Mo dopant, we also calculated the band gap of Mo-doped SrTiO3 by using Coulomb corrections on the Mo-4d. The indirect spin-down band gap is about 1.75 eV within Ud = 4 eV, which is very close to the band gap (1.86 eV) calculated by the HSE hybrid density functional.36 Therefore, for a better description of the localized transition, we used the on-site repulsion Ueff = 2.3 eV and 4 eV on the Ti 3d and Mo 4d states as in previous theoretical investigations,35,37,38 and the U of the O 2p states was set to 8 eV. Spin-polarized calculations were performed for pure and different doped SrTiO3 systems. The energy cut-off for the wave function expanded in plane waves was chosen to be 400 eV, and k-point mesh with 3 × 3 × 3 was used for the supercell calculation. Both the lattice parameters and the atomic positions were fully relaxed until the residual forces were smaller than 0.03 eV Å−1, and the convergence tolerance for the optimized electronic energy was set to 1 × 10−5 eV.To explore the optical properties, the absorption coefficients (αabs(ω)) were simulated according to the following relation:39
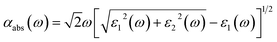 | (1) |
3. Results and discussion
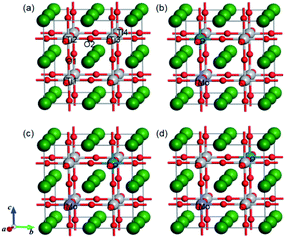
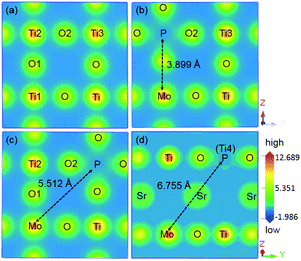
The presence of one electron and one hole per dopant is essential for modeling the charge compensation synergistic effect. Intense attention has been paid to B–B sites for metal–metal elements codoping (i.e., Cr/Ta, Ta/Ni, and W/Cd),40,41 which provide one electron as the donor dopant and one hole as the acceptor dopant, thus keeping the charge balance in codoping systems. The substitution of metal and non-metal dopants on the Ti sites can also introduce the charge balance. To model the Mo and P dopants codoping in SrTiO3, a 2 × 2 × 2 periodic supercell within 40 atoms was constructed, as shown in Fig. 1. Before investigating the electronic and optical properties of the (Mo + P) codoped SrTiO3 systems, we first optimized the primitive SrTiO3. The optimized lattice parameters a, b, c were 3.952 Å, consistent with previous theoretical and experimental results.42 The GGA + Up + Ud calculated band gap of SrTiO3 was 3.22 eV, which also agrees well with the experimental (3.25 eV) and HSE calculation values (3.19 eV) due to the partial correction of the band gap by suitable U values. These results show that the parameters and computational methods (GGA + U) employed here are suitable for describing different monodoped and codoped SrTiO3 systems.The monodoped and codoped systems were constructed from the relaxed 2 × 2 × 2 supercell. Considering the effect of the oxidation states of Mo and P cations on the ground states of the codoped SrTiO3, we calculated the total energy of different oxidation state configurations with the pairs of (Mo5+, P3+), (Mo5+, P5+), (Mo6+, P3+) and (Mo6+, P5+) codoping. For simplicity, the energy of the (Mo5+, P3+) codoped configuration was set to zero, and the energies of (Mo5+, P5+), (Mo6+, P3+) and (Mo6+, P5+) codoped configurations were 6.7, 31.6 and 32.3 meV, respectively. It is noted that the (Mo5+, P3+) codoped configuration possesses the lowest total energy, which is considered to be the ground state. We used one Mo5+ cation to substitute for a Ti4+ host site to produce an electron per supercell, while one P3+ cation substituted for a Ti4+ site to introduce a hole per supercell. For the Mo or P-monodoped SrTiO3 system, there was only one doping configuration due to the symmetry of the cubic crystal, thus the Mo or P was substituted at the Ti1 site as labeled in Fig. 1(a). According to the relative substitute positions of Mo and P dopants, there are three different configurations for the (Mo + P) codoping. For the convenience of discussion, the three configurations are defined as the “near”, “medium” and “far” configurations, respectively, as shown in Fig. 1(b)–(d). For the near configuration, the Mo and P dopants occupy the nearest neighboring Ti sites at Ti1 and Ti2 sites, respectively. While for the medium and far configurations, the (Mo, P) dopant pairs substitute at (Ti1, Ti3) and (Ti1, Ti4), respectively. For the cases of three (Mo + P) codoped SrTiO3 systems, the near configuration was found to be energetically more stable by 0.07 and 0.22 eV than the medium and the far configurations, respectively. Although the nearest neighbour codoping configuration was found to be more energetically attainable in experimental techniques such as hydrothermal and sol–gel methods,43,44 the dopants will enter the lattice sites randomly in the supersonic cluster beam deposition techniques.45 Therefore, to provide a theoretical basis for the design and fabrication of new photocatalysts, in the following sections, we discuss the electronic structure and optical properties of all three codoped configurations.
To obtain detailed insight into the doping effect of the Mo/P on the electronic structure of SrTiO3, we first calculated the total DOS and partial DOS (PDOS) of pure and monodoped models, as shown in Fig. 2. For the pure SrTiO3, the valence band maximum (VBM) and conduction band minimum (CBM) were mainly contributed by the O 2p states and Ti 3d states, respectively, as shown in Fig. 2(a). The Mo monodoped SrTiO3 was modeled by introducing one Mo dopant at the host Ti1 site. The highest occupied orbital energy level was shifted to near the CBM after Mo doping, which is ascribed to the downward shift of the VBM and CBM. Here, the Fermi level was set at zero energy (CASTEP takes the energy of the highest occupied orbitals as the energy zero by default). That is to say, the Mo-doped SrTiO3 system shows a typical n-type semiconductor nature due to the electron doping. The CBM was significantly downward shifted to lower energy, and some mid-gap impurity states appeared as isolated states within the forbidden gap, which can greatly narrow the band gap of SrTiO3, as shown in Fig. 2(b). Analysis of PDOS showed that these localized impurity states originated from the strong hybridization of the Mo 4d and O 2p states. The lower energy of the Mo 4d orbital than the Ti 3d orbital induced the spin-up partially occupied states 0.74 eV above the VB and unoccupied states 0.23 eV below the CB, which can be interpreted by the different valence electron configurations of Mo (5s24d4) and Ti (4s23d2). Substitution of a Ti4+ site with a Mo5+ dopant leads to the intrinsic donor defects in SrTiO3, which typically acts as trapping centers for charge carriers to depress the photocatalytic activity. In the case of P cation doping, there was no variation in the VBM but a slight upward shifting of the CBM, as shown in Fig. 2(c), which displays p-type doping character. In this case, the band gap expands slightly to 3.34 eV. Some spin states lying above the CBM do not originate from the P 3p impurity orbitals but from the Ti 3d states due to lattice distortion. Due to the large differences in ionic radius between the dopant P (0.44 Å) and the host Ti (0.605 Å) elements, the perturbation in the lattice structure is relatively large. Based on these results, we considered the charge compensation effects of the Mo and P codoping on SrTiO3 to solve the above problems, and then enhance the photocatalytic performance.
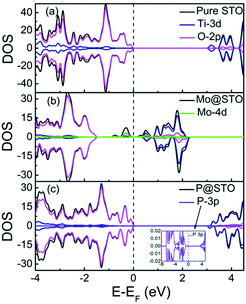 | ||
| Fig. 2 TDOS and PDOS of (a) pure SrTiO3, (b) Mo monodoped SrTiO3, and (c) P monodoping. The Fermi level is set at zero energy. | ||
To evaluate the relative difficulty for the incorporation of dopants into the host lattice in experiments, we calculated the formation energies (Ef) of different (Mo + P) codoping configurations. The smaller the formation energy, the easier the substitutional doping is;46 that is to say, the stability of the doped SrTiO3 with different substitutional sites is determined by the relative formation energy. The formation energies of monodoped Mo and P, and the codoped (Mo + P) SrTiO3 systems are defined as follows:
| Ef = Emonodoped − Epure + μTi − μMo/P | (2) |
| Ef = Ecodoped − Epure + 2μTi − μMo − μP | (3) |
| μSr + μTi + 3μO = μSrTiO3 | (4) |
| Dopants | EO-richf (eV) | ETi-richf (eV) | Eb (eV) |
|---|---|---|---|
| Mo | −8.987 | 3.599 | — |
| P | −15.349 | −2.762 | — |
| (Mo + P)-near | −24.768 | 0.405 | 0.433 |
| (Mo + P)-medium | −24.696 | 0.478 | 0.359 |
| (Mo + P)-far | −24.549 | 0.625 | 0.213 |
To examine the coupling strength between the Mo–P dopant pairs in the codoped models, we calculated the defect pair binding energy (Eb), according to the following equation:
| Eb = EMo + EP − EMo+P − Epure | (5) |
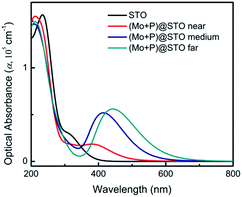
The optical absorption of the codoped SrTiO3 is strongly related to its photocatalytic activity. Here, we calculated the optical absorption coefficients of three codoped SrTiO3 systems and compared them with the results of pure SrTiO3; the results are shown in Fig. 3. The calculated optical absorption spectra of pure SrTiO3 is consistent with previous experimental and theoretical results.48,49 The pure SrTiO3 can only respond to UV light, which has no absorption activity in the visible-light region. The gradient curves with their absorption edges all extend into the visible-light region for three (Mo + P) codoped SrTiO3 configurations, and a new optical absorption peak was observed in the wavelength range of 365–650 nm. In comparison, the medium configuration and the far configuration had even higher absorption efficiencies than that in the near case, due to narrower band gaps. The maximum absorption edges can be extended to 649 nm for the far configuration. The (Mo + P) codoped SrTiO3 system showed significantly improved absorption performance, which indicates its potential for application as a visible-light-driven photocatalyst. These prominent visible absorptions from our predicted calculations are comparable to previous works on SrTiO3 with different codoping.50
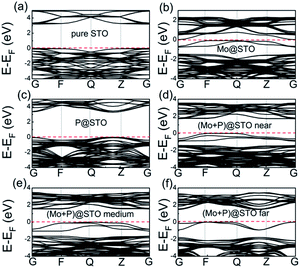
In general, the optical properties of codoped SrTiO3 are mainly determined by their electronic band structures.51 As shown in Fig. 4, we first calculated the band structure of monodoped Mo/P, codoped (Mo + P) SrTiO3 systems, and compared them with that of pure SrTiO3. For the Mo monodoped case, there are several spin-up partially occupied impurity bands just below the CB, as shown in Fig. 4(b), which can be ascribed to the presence of one more electron in the shell of the Mo dopant (5s24d3) than that in the host Ti (4s23d2). Since donor states are localized near to the CBM, we assumed that the band gap was calculated as the direct gap between the impurity states and the VBM of about 0.74 eV. Attractive candidates for photocatalytic water splitting must have a suitable band gap ranging from 1.5 to 3.0 eV, although the Mo monodoped SrTiO3 can efficiently narrow the band gap; note that a relatively smaller band gap is not suitable for water splitting. For the P monodoped case, one P3+ cation is substituted at the host Ti4+ of perovskite SrTiO3, which will introduce one hole into the system. From Fig. 4(c), we see that the bandwidth of the P monodoped SrTiO3 is even higher than that of pure SrTiO3, which is also not suitable for water splitting. In view of the band structure engineering, the formation of the charge non-compensation defects and the partially occupied impurity bands in Mo or P monodoped SrTiO3 can facilitate the formation of recombination centers, which will decrease the photocatalytic performance.
To overcome these problems, we studied the effect of (Mo + P) compensated codoping on the band gap of SrTiO3. The obtained energy band structures of (Mo + P) codoped SrTiO3 configurations are plotted and shown in Fig. 4. For the near configuration, there is a spin-up intermediate bands (IBs) with a width of 0.86 eV, which is located below the Fermi level, as shown in Fig. 4(d). The intrinsic forbidden gap is divided into two sub-gaps by the presence of such IBs. In this case, the host band gap decreased significantly to 1.73 eV. Compared with the narrow band gap of about 0.74 eV in Mo monodoped SrTiO3, the (Mo + P) codoped system exhibited an indirect band gap with an ideal value. On the one hand, the IBs can be stepping stones to improving the excitation of electrons to the CB under low photon energy, which will promote the absorption efficiency. On the other hand, the IBs can also act as a trapping center, which will promote the electron and hole recombination. However, the excitation or recombination depends on the energy difference between the VB and IBs or CB and IBs. Usually, the shallow impurity states near the VB are beneficial for the charge separation; in contrast, the deep impurity states near the CB can easily become recombination centers. As seen from Fig. 5, the energy difference between the VBM and the IBs minimum is much smaller than that between the CBM and the IBs maximum. This means that the probability for a VB hole to pump up into the IBs will be greater than the probability for a CB electron to combine with a hole in the IBs;52 i.e., the IBs can facilitate electron excitation and enhance the photocatalytic efficiency. We also noted that the IBs that appeared below the Fermi level in (Mo + P) codoped system are fully occupied states. By the introduction of Mo and P dopants, these fully filled IBs near the VBM can lead to photo-excitation from the IBs to the CB directly, reducing the chance of electron–hole recombination. Here, we also find that the partially occupied bands of Mo monodoped SrTiO3 preferred to be located near the CBM, which can act as a carrier-trapping center. For the (Mo + P) codoped cases, the fully occupied IBs were moved to the nearby VBM due to the synergistic effect of charge compensation codoping. As shown in Fig. 4(e) and (f), for the medium and far configurations cases, there are also some fully occupied IBs below the energy zero level. Moreover, the CBMs of both configurations showed slight downward shifts as compared with the near configuration, which further narrowed the band gap.
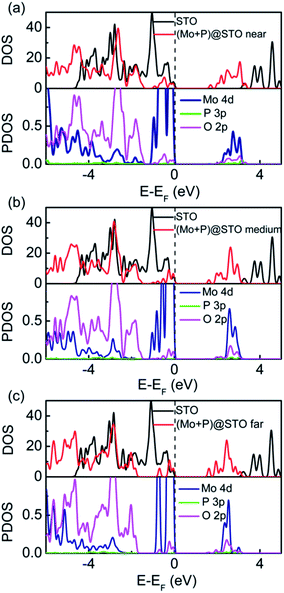
To further understand the origin of the enhanced optical performance of SrTiO3, we calculated the total DOS and partial DOS of (Mo + P) codoped SrTiO3 and compared them with that of pure SrTiO3, as shown in Fig. 5. For three (Mo + P) codoped SrTiO3 configuration cases, all of the VBMs and CBMs positions shifted towards the low-energy direction, inducing narrowed band gaps of 1.73, 1.59 and 1.49 eV, respectively. These narrowed band gaps are greatly helpful for exciting electrons from the VB to the CB and broadening the optical absorption spectra edge. As seen from Fig. 5, there are IBs located near the VBs in the three codoping cases, and they appeared as non-continuous states above the VBM, which is located below the Fermi level, suggesting that the IBs are fully occupied. The partial DOS results imply that the IBs mainly originated from the hybridization of Mo 4d and O 2p states, as shown in the bottom panel of Fig. 5(a)–(c). The impurity Mo 4d states appeared far below the CBMs due to the more negative energy of the Mo 4d orbital than the Ti 3d orbital. However, for the three codoping cases, the presence of the partially occupied Mo 4d states in the CB can facilitate the formation of the CB with Mo 4d character, which is different from the Ti 3d character in the pure SrTiO3. Interestingly, within the band gap of the CBs, there is slight hybridization from the P 3p and O 2p states, indicating weak P–O coupling in the (Mo + P) codoped SrTiO3, which also leads to the CBM shifting to the low-energy direction. For the (Mo + P) codoped SrTiO3 system, the delocalized and fully occupied IBs formed by the p–d coupling can effectively narrow the band gap and induce the band downward shift to about 1.9 eV as compared with that of the pure SrTiO3. These can lead to a decrease in the photo-excitation energy; thus the absorption edge is extended to the visible light region due to the synergistic effect of the Mo and P codoping. In particular, some photo-generated electrons were excited directly from the IBs to the CB in the presence of both Mo and P dopants, which could be responsible for the fact that the new absorption peaks (365–650 nm) appeared in the above optical spectra (see Fig. 3).
To describe the hybridization in the (Mo + P) codoped SrTiO3, we plotted the charge density distribution in the (010) planes for pure SrTiO3, codoped within the near and the medium configurations and in (011) planes for the far configuration, as shown in Fig. 6. The distances between the neighbouring elements on the same surface were obtained from CASTEP outcome files, while the distances between other elements were graphically measured linear distances. For comparison, the charge density of the pure SrTiO3 is plotted in Fig. 6(a). It was found that the Ti–O bonds were in the form of covalent bonds in pure SrTiO3. Checking the inner part of the optimized geometry of the pure SrTiO3, the labeled atom positions correspond to that in Fig. 1(a), the Ti1–Ti2, Ti1–Ti3, and Ti1–Ti4 distances were 3.922, 5.546, and 6.793 Å, respectively and the Ti1–O1 bond length was 1.961 Å. For the three (Mo + P) codoped systems, the Mo atom substituted for the Ti1 atom, and the P atom replaced the Ti2, Ti3 and Ti4 atoms in the three cases, respectively. The optimized Mo–P distances in the three cases were 3.899, 5.512, 6.755 Å, respectively, showing obvious contractions as compared with those corresponding to the Ti–Ti distances in pure SrTiO3. Moreover, the Mo–O bonds in the three codoped models were 2.207, 2.017 and 2.007 Å, respectively, which are larger than that of the Ti1–O1 bond (1.961 Å) before codoping, indicating that the covalency of the Mo–O bond is weaker than that of Ti–O bond. In contrast, due to the relatively small atomic radius of the P atom, the entered P dopant generated a large lattice distortion. This could be because the P–O bond lengths in the three codoped cases were 1.682, 1.715 and 1.734 Å, respectively, which are significantly smaller as compared to those of the Ti1–O1 bonds in pure SrTiO3. Such the relatively shortened P–O bond lengths indicate that the P dopant is strongly bonded to the nearest O atom. As shown in Fig. 6(b)–(d), after (Mo + P) codoping, the Mo–O bond showed the typical covalent nature, and there was charge transfer from the nearest neighboring O atoms to the Mo dopants. Moreover, the P–O bonds in the three codoped configurations displayed a significant degree of covalency. We observed that the charge density around the P dopant was lower than that of the near O atoms, which is mainly due to the relatively large electronegativity of the O atom.
It is well known that the photoconversion efficiency of a semiconductor for water splitting to produce hydrogen is governed by the position of the band edge with respect to the redox potential. Theoretically, the relevant potential level of donor dopants is required to be more negative than the valence band potential, while the potential level of acceptor species needs to be more positive than the conduction band potential. In order to describe the oxidation and reduction capacity of the (Mo + P) codoped SrTiO3 systems, the CBM (ECB) and VBM (EVB) are calculated empirically according to the following formula:
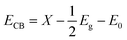 | (6) |
To estimate the water reduction performance of different (Mo + P) codoped systems, the band alignments with respect to the water redox levels of three different codoped SrTiO3 systems as well as pure SrTiO3 were calculated and plotted on one diagram, as shown in Fig. 7. For the pure SrTiO3 system, the calculated band gap is 3.22 eV, in which the VBM is located 1.17 eV below the water oxidation (H2O/O2) level and the CBM lies 0.82 eV above the water reduction (H+/H2) level, and this is consistent with the experimental results.53 For three (Mo + P) codoped SrTiO3 configurations, there were IBs in the forbidden band with band widths of 0.86, 1.05 and 0.95 eV, respectively. These fully occupied IBs can be considered as stepping-stones to bridging the VBM and the CBM, thus photo-generated electrons are directly excited from the IBs to the CB. As seen from Fig. 7, the (Mo + P) codoping can effectively narrow the band gap by introducing the delocalized energy levels, and also greatly perturb the positions of both the VBM and CBM. As for the three (Mo + P) codoped SrTiO3 configurations, the band edge positions of both the VBM and CBM straddle the water redox potential levels, which meet the thermodynamic criterion for hydrogen production in solar-light-driven water splitting.
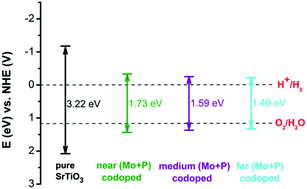 | ||
| Fig. 7 The band edge alignment of the pure and codoped SrTiO3 systems with respect to the water redox band edge position potentials. | ||
The calculated CBM and VBM potentials vs. NHE for the different systems are listed in Table 2. The calculated ECB and EVB of pure SrTiO3 are −1.17 and 2.05 eV, respectively, which possesses a strong reducing ability under UV light due to its more negative CBM than the water reduction potential (0 eV vs. NHE at pH 0). Moreover, its VBM is more positive than the water oxidation potential level (1.23 eV vs. NHE). Therefore, it has the capacity for photocatalytic water splitting. As mentioned previously in the literature, the enhanced oxidation or reduction capacity depends on the downward shift of the VBM or the upward shift of the CBM, respectively.54,55 In the cases of the three (Mo + P) codoped configurations, the CBMs shifted upward by 0.83, 0.90 and 0.95 eV, respectively, as compared to the pure SrTiO3, which implies that the photo-reduction ability is enhanced, and the absorption edges move towards the visible light region. The VBMs of the three codoped systems moved downward as compared to that of pure SrTiO3 by 0.66, 0.73 and 0.78 eV, respectively, which means that the oxidation ability is also improved. From the calculated ECB and EVB in Table 2, we can deduce that splitting water into H2 with the (Mo + P) codoped SrTiO3 photocatalyst is thermodynamically feasible. The results show that the (Mo + P) codoped SrTiO3 system possessed the more suitable band gap and band edge positions for photocatalytic water splitting.
| Model | SrTiO3 | (Mo + P)-near | (Mo + P)-medium | (Mo + P)-far |
|---|---|---|---|---|
| ECB (eV) | −1.17 | −0.34 | −0.27 | −0.22 |
| EVB (eV) | 2.05 | 1.39 | 1.32 | 1.27 |
Solar energy provides an attractive way to produce hydrogen via water splitting. When the energy of the incident light is larger than the band gap of the SrTiO3-based catalytic materials, electrons and holes are generated in the VB and CB, respectively. The photo-generated electrons (e−) and holes (h+) that migrate from the bulk of the semiconductor towards the reaction sites on the photocatalyst surface without recombination can reduce and oxidize water molecules to produce hydrogen and oxygen, respectively. A schematic diagram of the (Mo + P) codoped SrTiO3 photocatalyst for water splitting is shown in Fig. 8. Based on the above electronic band structure, the introduction of Mo and P dopants in metal oxide will generate new IBs energy levels between the VBM and CBM, resulting in the narrowed minimum light absorption energy gap of the host lattice. This will reduce the photon excitation energy from the VB to CB, thus extending its range of optical absorption to the visible-light region. Furthermore, the proximity of IBs to the VB allows for efficient electron replenishment, which possesses ability to separate photoexcited electrons from reaction holes, reducing the probability of trapping electrons from the CB, so the photocatalytic activity of the (Mo + P) codoped SrTiO3 system will be good. As schematically depicted in Fig. 8, the band edge potential of the (Mo + P) codoped SrTiO3 photocatalyst by theoretical prediction is appropriate for water-oxidation/-reduction processes.
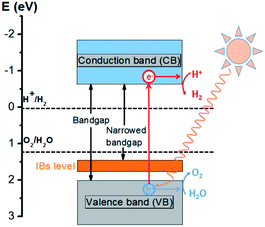 | ||
| Fig. 8 Schematic plot of the energy band alignment of the (Mo + P) codoped SrTiO3 Photocatalyst and the redox potential of the water-splitting reaction. | ||
4. Conclusion
We have explored the metal/non-metal (Mo + P) codoping effects on the electronic structure and photocatalytic performance of SrTiO3 based on first principles predictions. Theoretical results indicate that the (Mo + P) codoped SrTiO3 is feasible in O-rich environments due to low formation energy. The (Mo + P) codoping leads to a strong hybridization between Mo 4d and O 2p, which induces the fully occupied and delocalized IBs near the valence band, possessing the ability to separate photo-excited electrons from reaction holes and reducing the probability of trapping electrons from the CB. We found that both the strong Mo–O hybridization and weak P–O coupling can narrow the band gap of SrTiO3, and also improve its photocatalytic performance. The calculated optical absorption spectra of the (Mo + P) codoped SrTiO3 also verified the improved visible light absorption by donor–acceptor pair codoping. The band edge positions of the (Mo + P) codoped SrTiO3 system also satisfy the criterion for water splitting.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Key Projects of Natural Science Foundation of Colleges and Universities of Anhui Province (KJ2019A0117), Stand-up Fund for Talent Introduction of Anhui University of Science and Technology, the College Physics Teaching Team of Anhui Province (2019jxtd046), the Top Talents of Colleges and Universities of Anhui Province (GxbjZD14), and the Key Technologies R&D Program of Anhui Province of China (1604a0802122 and 17030901091).References
- S. Patial, V. Hasija, P. Raizada, P. Singh, A. A. Parwaz, A. Singh and A. M. Asiri, Tunable photocatalytic activity of SrTiO3 for water splitting: strategies and future scenario, J. Environ. Chem. Eng., 2020, 8, 103791 CrossRef CAS.
- B. L. Phoon, C. W. Lai, J. C. Juan, P. L. Show and G. T. Pan, Recent developments of strontium titanate for photo-catalytic water splitting application, Int. J. Hydrogen Energy, 2019, 44, 14316–14340 CrossRef CAS.
- K. V. Benthem, C. Elsässer and R. H. French, Bulk electronic structure of SrTiO3: experiment and theory, J. Appl. Phys., 2001, 90, 6156–6164 CrossRef.
- K. V. Sopiha, O. I. Malyi, C. Persson and P. Wu, Band gap modulation of SrTiO3 upon CO2 adsorption, Phys. Chem. Chem. Phys., 2017, 19, 16629–16637 RSC.
- K. Sivula and R. van de Korol, Semiconducting materials for photoelectrochemical energy conversion, Nat. Rev. Mater., 2016, 70, 16010 CrossRef.
- M. Rioult, H. Magnan, D. Stanescu and A. Barbier, Single crystalline hematite films for solar water splitting: Ti-doping and thickness effects, J. Phys. Chem. C, 2014, 118, 3007–3014 CrossRef CAS.
- Y. Yang, W. J. Zheng, D. J. Cheng and D. P. Cao, Designing transition metal and nitrogen-codoped SrTiO3 (001) perovskite surfaces as efficient photocatalysts for water splitting, Sustainable Energy Fuels, 2017, 1, 1968–1980 RSC.
- H. Muhammad, L. Xu, L. Zhou, Z. P. Zheng, Q. Y. Fu and W. Luo, Exceptional co-catalyst free photocatalytic activities of B and Fe co-doped SrTiO3 for CO2 conversion and H2 evolution, Nano Res., 2018, 11, 6931–6404 CrossRef.
- H. Yu, J. Wang, S. Yan, T. Yu and Z. G. Zou, Elements doping to expand the light response of SrTiO3, J. Photochem. Photobiol., A, 2014, 275, 65–71 CrossRef CAS.
- F. P. Cai, Y. B. Tang, F. Y. Chen, Y. Yan and W. D. Shi, Enhanced visible-light-driven photocatalytic degradation of tetracycline by Cr doping SrTiO3 cubic nanoparticles, RSC Adv., 2015, 5, 21290–21296 RSC.
- B. Modak and S. K. Ghosh, Insight into the enhanced photocatalytic activity of SrTiO3 in the presence of a (Ni, V/Nb/Ta/Sb) pair, Phys. Chem. Chem. Phys., 2018, 20, 20078–20087 RSC.
- L. Ye, X. Hu, X. Wang, F. L. Chen, D. Tang, D. H. Dong and K. Xie, Enhanced CO2 electrolysis with a SrTiO3 cathode through a dual doping strategy, J. Mater. Chem. A, 2019, 7, 2764–2772 RSC.
- C. Zhang, Y. Z. Jia, Y. Jing and Y. Yao, New insights into assessing the favorable codoping dopants with various co-doped cases for the band gap engineering of SrTiO3, Int. J. Hydrogen Energy, 2015, 40, 1343–1351 CrossRef CAS.
- H. W. Kang, S. N. Lim and S. B. Park, Co-doping schemes to enhance H2 evolution under visible light irradiation over SrTiO3: Ni/M (M = La or Ta) prepared by spray pyrolysis, Int. J. Hydrogen Energy, 2012, 37, 5540–5549 CrossRef CAS.
- H. W. Kang and S. B. Park, Improved performance of tri-doped photocatalyst SrTiO3: Rh/Ta/F for H2 evolution under visible light irradiation, Int. J. Hydrogen Energy, 2016, 41, 13970–13978 CrossRef CAS.
- Y. Y. Liu, W. Zhou, C. Wang, L. L. Zhou and P. Wu, Electronic structure and optical properties of SrTiO3 codoped by W/Mo on different cationic sites with C/N from hybrid functional calculations, Comput. Mater. Sci., 2018, 146, 150–157 CrossRef CAS.
- W. J. Shi, Ab initio study on band-gap narrowing in SrTiO3 with Nb–C–Nb codoping, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 1541–1545 Search PubMed.
- Y. Liu, W. Zhou and P. Wu, Electronic structure and optical properties of Ta-doped and (Ta, N)-codoped SrTiO3 from hybrid functional calculations, J. Appl. Phys., 2017, 121, 075102 CrossRef.
- B. Modak and S. K. Ghosh, Origin of enhanced visible light driven water splitting by (Rh, Sb)-SrTiO3, Phys. Chem. Chem. Phys., 2015, 17, 15274–15283 RSC.
- T. S. Jamil, H. A. Abbas, A. M. Youssief, E. S. Mansor and F. F. Hammad, The synthesis of nano-sized undoped, Bi doped and Bi, Cu co-doped SrTiO3 using two sol-gel methods to enhance the photocatalytic performance for the degradation of dibutyl phthalate under visible light, Cron. Chim., 2017, 20, 97–106 CrossRef CAS.
- W. Chen, H. Liu, X. Y. Li, S. Liu, L. Gao, L. Q. Mao, Z. Y. Fan, W. F. Shangguan, W. J. Fang and Y. S. Liu, Polymerizable complex synthesis of SrTiO3:(Cr/Ta) photocatalysts to improve photocatalytic water splitting activity under visible light, Appl. Catal., B, 2016, 192, 145–151 CrossRef CAS.
- J. L. Yan and Y. N. Zhao, DFT study on electronic structure and optical properties of N-doped, S-doped, and N/S co-doped SrTiO3, Phys. B, 2012, 55, 654–659 CAS.
- H. F. Liu, Effect of nitrogen and carbon doping on electronic properties of SrTiO3, Solid State Commun., 2012, 152, 2063–2065 CrossRef CAS.
- P. Reunchan, N. Umezawa, S. Ouyang and J. H. Ye, Mechanism of photocatalytic activities in Cr-doped SrTiO3 under visible-light irradiation: an insight from hybrid density-functional calculations, Phys. Chem. Chem. Phys., 2012, 14, 1876–1880 RSC.
- A. Yamakata, M. Kawaguchi, R. Murachi, M. Okawa and I. Kamiya, Dynamics of photogenerated charge carriers on Ni- and Ta-doped SrTiO3 photocatalysts studied by time-resolved absorption and emission spectroscopy, J. Phys. Chem. C, 2016, 120, 7997–8004 CrossRef CAS.
- Y. Yamaguchi, S. Usuki, K. Yamatoya, N. Suzuki, K. Katsumata, C. Terashima, A. Fujishima, A. Kudo and K. Nakata, Efficient photocatalytic degradation of gaseous acetaldehyde over ground Rh–Sb co-doped SrTiO3 under visible light irradiation, RSC Adv., 2018, 8, 5331–5337 RSC.
- M. M. Fadlallah, M. F. Shibl, T. J. H. Vlugt and U. Schwingenschlögl, Theoretical study on cation codoped SrTiO3 photocatalysts for water splitting, J. Mater. Chem. A, 2018, 6, 24342–24349 RSC.
- W. Du, Q. Xu, D. Q. Jin, Y. Shu, L. M. Kong and X. Y. Hu, Visible-light-induced photo-fenton process for the facile degradation of metronidazole by Fe/Si codoped TiO2, RSC Adv., 2018, 8, 40022–40034 RSC.
- Y. M. Lin, Z. Y. Jiang, C. Y. Zhu, X. Y. Hu, H. Y. Zhu, X. D. Zhang, J. Fan and S. H. Lin, The optical absorption and hydrogen production by water splitting of (Si,Fe)-codoped anatase TiO2 photocatalyst, Int. J. Hydrogen Energy, 2013, 38, 5209–5214 CrossRef CAS.
- A. EI Mragui, O. Zegaoui, I. Daou and J. Silva, Preparation, characterization, and photocatalytic activity under UV and visible light of Co, Mn, and Ni mono-doped and (P,Mo) and (P,W) co-doped TiO2 nanoparticles: a comparative study, Environ. Sci. Pollut. Res., 2019 DOI:10.1007/s11356-019-04754-6.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. I. J. Probert, K. Refson and M. C. Payne, First principles methods using CASTEP, Z. Kristallogr., 2005, 220, 567–570 CAS.
- X. Ma, Y. Wu, Y. H. Lv and Y. F. Zhu, Correlation effects on lattice relaxation and electronic structure of ZnO within the GGA + U formalism, J. Phys. Chem. C, 2013, 117, 26029–26039 CrossRef CAS.
- J. P. Li, H. T. Lu, Y. H. Li, S. H. Meng and Y. M. Zhang, First-principles generalized gradient approximation (GGA) + Ud + Up studies of electronic structures and optical properties in cubic HfO2, Solid State Commun., 2015, 211, 38–42 CrossRef CAS.
- M. Nolan and G. W. Watson, Hole localization in Al doped silica: A DFT + U description, J. Chem. Phys., 2006, 125, 144701 CrossRef.
- Z. P. Hu and H. Metiu, Choice of U for DFT + U Calculations for Titanium Oxides, J. Phys. Chem. C, 2011, 115, 5841–5845 CrossRef CAS.
- B. Modak, K. Srinivasu and S. K. Ghosh, A hybrid DFT based investigation of the photocatalytic activity of cation–anion codoped SrTiO3 for water splitting under visible light, Phys. Chem. Chem. Phys., 2014, 16, 24527–24535 RSC.
- Y. Q. Wang, C. Zhang, Y. Liu, M. X. Zhang and F. F. Min, Efficient visible-light-induced photocatalytic activity of SrTiO3 co-doped with Os and N: A GGA + U investigation, Phys. Status Solidi B, 2019, 256, 1800574 CrossRef.
- H. Lee, M. Y. Jeong, J. H. Sim, H. Yoon, S. Ryee and M. J. Han, Charge density functional plus U calculation of lacunar spinel GaM4Se8 (M = Nb, Mo, Ta, and W), Europhys. Lett., 2019, 15, 47005 CrossRef.
- J. Wang, Q. Meng, J. Huang, Q. Li and J. L. Yang, Band structure engineering of anatase TiO2 by metal-assisted P–O coupling, J. Chem. Phys., 2014, 140, 174705 CrossRef.
- J. W. Shi, S. H. Shen, Y. B. Chen, L. J. Guo and S. S. Mao, Visible light-driven photocatalysis of doped SrTiO3 tubular structure, Opt. Express, 2012, 20, A351–A359 CrossRef CAS.
- R. Niishiro, H. Kato and A. Kudo, Nickel and either tantalum or niobium-codoped TiO2 and SrTiO3 photocatalysts with visible-light response for H2 or O2 evolution from aqueous solutions, Phys. Chem. Chem. Phys., 2005, 7, 2241–2245 RSC.
- W. Chang, J. A. Bellotti, S. W. Kirchoefer and J. M. Pond, Strain tensor effects on SrTiO3 incipient ferroelectric phase transition, J. Electroceram., 2005, 77, 173–187 CAS.
- V. Jeyalakshmi, R. Mahalakshmy, K. R. Krishnamurthy and B. Viswanathan, Strontium titanates with perovskite structure as photo catalysts for reduction of CO2 by water: influence of co-doping with N, S & Fe, Catal. Today, 2018, 300, 152–159 CrossRef CAS.
- L. G. Devi and B. G. Anitha, Effective band gap engineering by the incorporation of Ce, N and S dopant ions into the SrTiO3 lattice: exploration of photocatalytic activity under UV/solar light, J. Sol-Gel Sci. Technol., 2020, 94, 50–66 CrossRef CAS.
- M. Chiodi, C. P. Cheney, P. Vilmercati, E. Cavaliere, N. Mannella, H. H. Weitering and L. Gavioli, Enhanced dopant solubility and visible-light absorption in Cr–N codoped TiO2 nanoclusters, J. Phys. Chem. C, 2012, 116, 311–318 CrossRef CAS.
- Q. Hou, C. Zhao and Z. Xu, Effect of Zr doping on the electrical and optical properties of ZnO, Chem. Phys. Lett., 2016, 658, 336–342 CrossRef CAS.
- Y. Liu, W. Zhou and P. Wu, Tuning electronic structure and optical properties of SrTiO3 by site-specific doping by Nb with N/B from hybrid functional calculations, Mater. Chem. Phys., 2017, 195, 170–175 CrossRef CAS.
- T. Ohno, T. Tsubota, Y. Nakamura and K. Sayama, Preparation of S, C cation-codoped SrTiO3 and its photocatalytic activity under visible light, Appl. Catal., A, 2005, 288, 74–79 CrossRef CAS.
- V. Mishra, A. Sati, M. K. Warshi, A. B. Phatangare, S. Dhole, V. N. Bhoraskar, H. Ghosh, A. Sagdeo, V. Mishra, R. Kumar and P. R. Sagdeo, Effect of electron irradiation on the optical properties of SrTiO3: an experimental and theoretical investigations, Mater. Res. Express, 2018, 5, 036210 CrossRef.
- B. Modak and S. K. Ghosh, Exploring the role of La codoping beyond charge compensation for enhanced hydrogen evolution by Rh–SrTiO3, J. Phys. Chem. B, 2015, 119, 11089–11098 CrossRef CAS.
- R. Ahuja, O. Eriksson and B. Johansson, Electronic and optical properties of BaTiO3 and SrTiO3, J. Appl. Phys., 2001, 90, 1854 CrossRef CAS.
- J. H. Liu, M. Y. Weng, S. B. Li, X. Chen, J. H. Cen, J. S. Jie, W. J. Xiao, J. X. Zheng and F. Pan, High throughput HSE study on the doping effect in anatase TiO2, Phys. Chem. Chem. Phys., 2020, 22, 39–53 RSC.
- Y. Schoonen and A. A. Martin, The absolute energy positions of conduction and valence bands of selected semiconducting minerals, Am. Mineral., 2000, 85, 543–556 CrossRef.
- L. Xu, Z. L. Ma, Q. Li, T. Chen, B. J. Peng, J. Zeng, Y. B. Zhang, K. W. Luo, L. L. Wang and C. J. Shuai, 2D layered SiC/C2N van der Walls type-II heterostructure: a visible-light-driven photocatalyst for water splitting, New J. Chem., 2020, 36, 15439–15445 RSC.
- S. C. Han, Y. Li and Z. Wang, PtSe2/SiH van der Walls type-II heterostructure: a high efficiency photocatalyst for water splitting, Phys. Chem. Chem. Phys., 2020, 30, 17145–17151 RSC.
| This journal is © The Royal Society of Chemistry 2020 |