Open Access Article
Open Access ArticleOne-pot reductive amination of carbonyl compounds with nitro compounds over a Ni/NiO composite†
Yusuke Kita a,
Sayaka Kaia,
Lesandre Binti Supriadi Rustada,
Keigo Kamata
a,
Sayaka Kaia,
Lesandre Binti Supriadi Rustada,
Keigo Kamata a and
Michikazu Hara*ab
a and
Michikazu Hara*ab
aLaboratory for Materials and Structures, Institute of Innovative Research, Tokyo Institute of Technology, Nagatsuta-cho, 4259, Midori-ku, Yokohama 226-8503, Japan. E-mail: hara.m.ae@m.titech.ac.jp
bAdvanced Low Carbon Technology Research and Development Program (ALCA), Japan Science and Technology Agency (JST), 4-1-8 Honcho, Kawaguchi 332-0012, Japan
First published on 2nd September 2020
Abstract
Easily prepared Ni/NiO acts as a heterogeneous catalyst for the one-pot reductive amination of carbonyl compounds with nitroarenes to afford secondary amines with H2 as a hydride source. This catalytic system does not require a special technique to avoid air-exposure, in contrast to the common heterogeneous Ni catalysts.
Amines are among the most important organic compounds for the chemical, materials, pharmaceutical and agrochemical industries.1 In particular, aromatic and heteroaromatic amines occupy a privileged position in medicinal chemistry,1c,2 as exemplified in top-selling drugs such as atorvastatin, hydrochlorothiazide, furosemide and acetaminophen.3 The general methods to prepare aryl and heteroaryl amines are the reductive amination of carbonyl compounds,4 direct alkylation of amines with alkyl halides,5 Buchwald–Hartwig amination6 and Ullman-type C–N bond formation.7 These amination systems utilize aniline derivatives which are usually prepared in advance by hydrogenation of nitroarenes.8 Direct amine synthesis using nitroarenes is attractive because it eliminates the hydrogenation step, which saves time, energy and cost. Among the amination reactions, reductive amination has been actively investigated due to its high atom economy and ease of industrial application (Scheme 1);9 however, reductive amination has frequently been accompanied by unwanted side products due to the reduction of carbonyl compounds to alcohols and/or over-alkylation of product amines. One-pot reductive amination with nitro compounds has been achieved by precious metal catalysis.10 Recently, precious metal alloy nanoparticles were demonstrated to exhibit high catalytic performance for one-pot reductive amination with nitro compounds, even under mild reaction conditions.11 In the context of economic efficiency and a ubiquitous element strategy, the replacement of precious metals with earth-abundant metals has gained much attention. Although some catalytic systems based on Fe,12 Cu,13 Co
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 and Mo
14 and Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 have been reported using H2 as a reductant, severe reaction conditions are typically required (Table S1, ESI†). It is noteworthy that the nitrogen-doped carbon supported cobalt catalysts were reported to be active for one-pot reductive amination using nitroarenes using formic acid or CO/H2O as a reductant though high temperature was required (Table S2, ESI†).16
15 have been reported using H2 as a reductant, severe reaction conditions are typically required (Table S1, ESI†). It is noteworthy that the nitrogen-doped carbon supported cobalt catalysts were reported to be active for one-pot reductive amination using nitroarenes using formic acid or CO/H2O as a reductant though high temperature was required (Table S2, ESI†).16
To develop active catalysts for one-pot reductive amination using nitro compounds, we have focused on nickel catalysts, which are known as active catalysts for many transformation reactions.17,18 The active species is typically metallic nickel, which is easily oxidized in air and becomes covered with NiO.19 Therefore, special techniques to avoid air-exposure (i.e., pre-reduction in the reaction vessel, glovebox) are required to achieve high catalytic performance for liquid phase reactions. Herein we report an easily prepared Ni/NiO composite as a heterogeneous catalyst for one-pot reductive amination using nitro compounds. This Ni catalyst can be handled under an air atmosphere, even though the supposedly active species, metallic nickel, is oxidized by air-exposure.
Ni/NiO was prepared by the partial reduction of NiO with H2 in the temperature range from 200 to 500 °C (Ni/NiO-X: X = reduction temperature). X-ray diffraction (XRD) patterns of the prepared Ni catalysts after exposure to air are summarized in Fig. 1(A). When NiO is treated in H2 at 200 °C, the dominant phase produced is still nickel oxide. Metallic nickel was formed by reduction at ≥250 °C, which is consistent with the H2-temperature programmed reduction (H2-TPR) profile of NiO in which the H2 consumption peak begins to increase at around 250 °C.20 The ratio of Ni to NiO, determined by Rietveld analysis, increased with increasing reduction temperature and no peaks attributed to metallic nickel were evident for Ni/NiO-500 (Table 1). The Brunauer–Emmett–Teller (BET) specific surface area of Ni/NiO was decreased by the reduction treatment with H2 (Table 1). The decrease of the specific surface areas is due to the aggregation of Ni particles during reduction, as evidenced by scanning electron microscopy (SEM) images of Ni/NiO-X (Fig. S3, ESI†) and crystallite diameter estimated from (111) diffraction lines using Scherrer's equation (Table S3, ESI†). Fig. 1(C) shows an SEM image of Ni/NiO-300, where the particle size was estimated to be 0.5–3 μm, and the layered structure of NiO was maintained after the reduction treatment (see also Fig. S4, ESI†).
 | ||
| Fig. 1 (A) XRD patterns for Ni/NiO-X; (a) Ni/NiO-500, (b) Ni/NiO-400, (c) Ni/NiO-350, (d) Ni/NiO-300, (e) Ni/NiO-250, and (f) Ni/NiO-200 (♦: Ni, ○: NiO), and (B) SEM images of Ni/NiO-300. | ||
| Entry | Catalyst | Specific surface areaa (m2 g−1) | Weight ratiob (%) | |
|---|---|---|---|---|
| Ni | NiO | |||
| a Specific surface areas were obtained from BET measurements.b Weight ratios were obtained by Rietveld analysis. | ||||
| 1 | Ni/NiO-200 | 82 | — | 100 |
| 2 | Ni/NiO-250 | 78 | 2 | 98 |
| 3 | Ni/NiO-300 | 41 | 43 | 57 |
| 4 | Ni/NiO-350 | 25 | 66 | 34 |
| 5 | Ni/NiO-400 | 10 | 85 | 15 |
| 6 | Ni/NiO-500 | <5 | 100 | — |
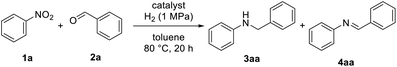
The one-pot reductive amination of benzaldehyde (2a) with nitrobenzene (1a) was evaluated with the prepared Ni catalyst using molecular hydrogen as the reductant (Table 2). In addition to the desired secondary amine 3aa, aniline, benzyl alcohol and imine intermediate 4aa were observed in the reaction mixture by gas chromatography (GC) analysis. The catalytic activity of the Ni/NiO catalyst was strongly affected by the reduction temperature during catalyst preparation with Ni/NiO-300 showing the highest activity (entry 2). The lack of activity for Ni/NiO-200 can be rationalized by insufficient reduction, as evident from the XRD pattern (entry 1). In addition, a low yield of 3aa was obtained with the catalysts prepared by reduction at elevated temperatures (entries 6–8). The lower activities of Ni/NiO-350, -400 and -500 were probably due to smaller amounts of active sites due to the reduction of the surface area and/or the aggregation of metallic Ni particles (Tables 1 and S3†). For comparison of Ni/NiO with simple supported Ni catalysts, one-pot reductive amination was conducted over Ni catalysts supported on simple metal oxides of Nb2O5, TiO2, SiO2 and ZrO2 (entries 9–12). Ni/SiO2 exhibited comparable activity to Ni/NiO (entry 9); however, significant leaching (3.9%) of the Ni species was observed in the reaction mixture, as opposed to that with Ni/NiO-300 (0.1%). Although RANEY® Ni is known to be active for reductive amination,21 3aa was obtained only in 22% yield under the present reaction conditions (entry 13). No desired product was observed using unreduced NiO and Ni(OH)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 (entries 14 and 15). In the case of the reactions with low material balances such as Ni/NiO-400, Ni/Nb2O5 and Ni/ZrO2, we observed benzyl alcohol as a main byproduct, suggesting that the hydrogenation of aldehydes is the competitive side reaction. Because of the high selectivity of Ni/NiO-300, NiO support can contribute to suppress the hydrogenation of aldehyde. Further optimization was then conducted (Table S2, ESI†). The yield of 3aa was finally increased to 92% under higher concentration conditions using 1.5 equivalents of 2a (entry 4). Reductive amination proceeded even under lower hydrogen pressure (0.5 MPa), although a longer reaction time was required (entry 5). The lower loading of Ni/NiO (15 mg) was accomplished by prolonging the reaction time, affording 3aa in 90% yield (entry 10, Table S4†).
22 (entries 14 and 15). In the case of the reactions with low material balances such as Ni/NiO-400, Ni/Nb2O5 and Ni/ZrO2, we observed benzyl alcohol as a main byproduct, suggesting that the hydrogenation of aldehydes is the competitive side reaction. Because of the high selectivity of Ni/NiO-300, NiO support can contribute to suppress the hydrogenation of aldehyde. Further optimization was then conducted (Table S2, ESI†). The yield of 3aa was finally increased to 92% under higher concentration conditions using 1.5 equivalents of 2a (entry 4). Reductive amination proceeded even under lower hydrogen pressure (0.5 MPa), although a longer reaction time was required (entry 5). The lower loading of Ni/NiO (15 mg) was accomplished by prolonging the reaction time, affording 3aa in 90% yield (entry 10, Table S4†).
| Entry | Catalyst | Conv. of 1a (%) | Yield of 3aa (%) | Yield of 4aa (%) |
|---|---|---|---|---|
| a Reaction conditions: catalyst (0.05 g), 1a (1 mmol), 2a (1 mmol), toluene (5 mL), H2 (1 MPa), 80 °C, 20 h. Conversion and yield were determined by GC analysis.b 1.2 mmol of 2a and 1 mL of toluene were used.c Run at 0.5 MPa H2 pressure for 50 h.d Methanol was used as a solvent.e Generated by the treatment of RANEY® alloy with NaOH. | ||||
| 1 | Ni/NiO-200 | 24 | — | — |
| 2 | Ni/NiO-250 | 22 | — | 4 |
| 3 | Ni/NiO-300 | >99 | 77 | 5 |
| 4b | Ni/NiO-300 | >99 | 92 | — |
| 5c | Ni/NiO-300 | >99 | 89 | — |
| 6 | Ni/NiO-350 | >99 | 62 | 2 |
| 7 | Ni/NiO-400 | 82 | 9 | 30 |
| 8 | Ni/NiO-500 | 16 | — | 6 |
| 9 | Ni/Nb2O5 | >99 | 12 | 51 |
| 10 | Ni/TiO2 | 36 | — | 27 |
| 11 | Ni/SiO2 | >99 | 73 | 17 |
| 12 | Ni/ZrO2 | >99 | 25 | 33 |
| 13d | RANEY® Nie | >99 | — | 22 |
| 14 | NiO | 23 | — | — |
| 15 | Ni(OH)2 | 20 | — | — |
The reusability of Ni/NiO-300 was examined next. The used Ni/NiO-300 could be recovered from the reaction mixture by simple filtration, washing with methanol, and drying at 90 °C. The XRD patterns and the ratio of Ni to NiO were not changed during one-pot reductive amination (Fig. S2, ESI†). The SEM images of the recovered catalyst indicates that morphological changes were negligible (Fig. S6, ESI†). The recovered Ni/NiO catalyst was reactivated by pre-treatment (150 °C, 1 h under H2 flow), by which the catalytic activity was maintained at a high level without obvious decline, even after the 3rd reuse (Fig. 2(A)).
A time-course analysis was then conducted under the optimized conditions (Fig. 2(B)). Aniline was generated by the hydrogenation of nitrobenzene, and imine 4aa was then gradually formed through the dehydrative coupling of 2a with aniline. Hydrogenation of 4aa subsequently afforded the secondary amine 3aa. To determine which step is key for the one-pot reductive amination, each step was examined using Ni/NiO-300 and Ni/SiO2, respectively. Imine formation step proceeded smoothly even without catalyst (Table S5, ESI†). For the hydrogenation of 1a, Ni/NiO and Ni/SiO2 gave the comparable results (Table S6, ESI†). For the imine hydrogenation step, we observed the higher activity of Ni/NiO than Ni/SiO2 (Fig. 3 and Table S7†). With these results, the high hydrogenating ability of Ni/NiO for imine hydrogenation is the key for the high activity on one-pot reductive amination.
 | ||
| Fig. 3 Hydrogenation of 4aa over Ni/NiO and Ni/SiO2. Reaction conditions: catalyst (0.05 g), 4aa (1 mmol), toluene (1 mL), H2 (1 MPa), 80 °C. | ||
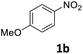
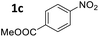
Ni/NiO-300 could be applied to the one-pot reductive amination of other substrates (Table 3). A number of functional groups, such as ether, ester, chloro and bromo groups proved to be compatible with this catalytic system. The reaction was not sensitive to the steric environment of the nitro group, but was sensitive to that of the formyl group (entries 4 and 8). Electron-donating groups retarded the imine hydrogenation step and thus required higher reaction temperatures (entries 1 and 5). On the other hand, longer reaction times were necessary for the electron-withdrawing group on nitrobenzene derivatives due to the weak nucleophilicity of the aniline intermediates (entry 2). Indeed, imine formation is delayed by electron-withdrawing group on aniline derivative (Table S5†). The electron-withdrawing group on the benzaldehyde derivative retarded reductive amination, although it could facilitate both imine formation and imine hydrogenation (entry 6). Aliphatic aldehyde was also applicable though the yield was low due to the decomposition of aldehyde (entry 9).
The surface of metallic Ni is easily oxidized in air; therefore, the surface states of Ni/NiO-300 were analysed by X-ray photoelectron spectroscopy (XPS) and the results are shown in Fig. 3. In the Ni 2p region of the spectrum for Ni/NiO-300, the main 2p3/2 peak is observed at 856.1 eV, which is assignable to Ni(OH)2.23 The catalyst was exposed to ambient conditions; therefore, hydroxylation of the surface was inevitable.24 Similarly, NiO and RANEY® Ni were also covered with Ni(OH)2 after exposure to ambient conditions (Fig. 4). Considering the lack of activity for NiO and the low activity of RANEY® Ni for one-pot reductive amination (Table 1, entries 13 and 14), the boundary region among Ni, NiO and Ni(OH)2 is considered to be crucial for high catalytic performance. The lack of a metallic Ni peak (852.6 eV)25 in the XPS spectrum suggests that Ni2+ species covers the catalyst surface. This is the reason why Ni/NiO can be handled under an air atmosphere, in contrast to common heterogeneous Ni catalysts.
 | ||
| Fig. 4 Ni 2p XPS spectra for (a) Ni/NiO-300, (b) NiO, and (c) RANEY® Ni after exposure to ambient conditions. | ||
In summary, Ni/NiO acts as a catalyst for one-pot reductive amination with nitro compounds to afford the secondary amines. No special technique (pre-reduction in the reaction vessel or glovebox) is required in the reaction setup. The reaction could proceed under milder conditions than those reported for typical catalytic systems. Ni/NiO could be reused without any significant loss of activity. This catalytic system could be applied to a variety of substrates that bear functional groups. Mechanistic studies suggested that Ni(OH)2 on metallic nickel can exhibit catalytic activity toward the present one-pot reductive amination. These results provide new insights into the development of heterogeneous Ni catalysts.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Advanced Low Carbon Technology Research and Development Program (ALCA) of the Japan Science and Technology Agency (JST) (JPMJAL1205). A part of this work was supported by the Nagoya University microstructural characterization platform as a program of the “Nanotechnology Platform” of the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).Notes and references
- (a) S. A. Lawrence, Synthesis Properties and Applications, Cambridge University Press, 2004 Search PubMed; (b) A. Ricci, Amino Group Chemistry: from Synthesis to the Life Sciences, Wiley-VCH, Weinheim, 2008 Search PubMed; (c) S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451–3479 CrossRef CAS.
- J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177–2250 CrossRef CAS.
- Top 200 Drugs of 2019, https://clincalc.com/DrugStats/Top200Drugs.aspx Search PubMed.
- E. W. Baxter and A. B. Reitz, Org. React., 2004, 59, 1–714 Search PubMed.
- R. N. Salvatore, C. H. Yoon and K. W. Jung, Tetrahedron, 2001, 57, 7785–7811 CrossRef CAS.
- (a) D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 6338–6361 CrossRef CAS; (b) J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534–1544 CrossRef CAS; (c) D. S. Surry and S. L. Buckwald, Chem. Sci., 2011, 2, 27–50 RSC.
- (a) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400–5449 CrossRef CAS; (b) D. S. Surry and S. L. Buchwald, Chem. Sci., 2010, 1, 13–31 RSC.
- J. Gui, C.-M. Pan, Y. Jin, T. Qin, J. C. Lo, B. J. Lee, S. H. Spergel, M. E. Mertzman, W. J. Pitts, T. E. La Cruz, M. A. Schmidt, N. Darvatkar, S. R. Natarajan and P. S. Baran, Science, 2015, 348, 886–891 CrossRef CAS.
- H. Alinezhad and H. Yavari, Curr. Org. Chem., 2015, 19, 1021–1049 CrossRef CAS.
- (a) M. O. Sydnew, M. Kuse and M. Isobe, Tetrahedon, 2008, 64, 6406–6414 CrossRef; (b) B. Sreedhar, P. S. Reddy and D. K. Devi, J. Org. Chem., 2009, 74, 8806–8809 CrossRef CAS; (c) Y. Yamane, X. Liu, A. Hamasaki, T. Ishida, M. Haruta, T. Yokoyama and M. Tokunaga, Org. Lett., 2009, 11, 5162–5165 CrossRef CAS; (d) M. M. Dell'Anna, P. Mastrorilli, A. Rizzuti and C. Leonelli, Appl. Catal., A, 2011, 401, 134–140 CrossRef; (e) L. Hu, X. Cao, D. Ge, H. Hong, Z. Guo, L. Chen, X. Sun, J. Tang, J. Zheng, J. Lu and H. Gu, Chem.–Eur. J., 2011, 17, 14283–14287 CrossRef CAS.
- (a) L. Li, Z. Liu, S. Cai, Y. Zhi, H. Li, H. Rong, L. Liu, L. Liu, W. He and Y. Li, Chem. Commun., 2013, 49, 6843–6845 RSC; (b) A. Cho, S. Byun and B. M. Kim, Adv. Synth. Catal., 2018, 360, 1253–1261 CrossRef CAS; (c) I. Choi, S. Chun and Y. K. Chung, J. Org. Chem., 2017, 82, 12771–12777 CrossRef CAS.
- T. Stemmler, A.-E. Surkus, M.-M. Pohl, K. Junge and M. Beller, ChemSusChem, 2014, 7, 3012–3016 CrossRef CAS.
- A. L. Nuzhdin, E. A. Artiukha, G. A. Bukhtiyarova, E. A. Derevyannikova and V. I. Bukhtiyarov, Catal. Commun., 2017, 102, 108–113 CrossRef CAS.
- (a) T. Stemmler, F. A. Westerhaus, A.-E. Surkus, M.-M. Pohl, K. Junge and M. Beller, Green Chem., 2014, 16, 4535–4540 RSC; (b) R. V. Jagadeesh, K. Murugesan, A. S. Alshammari, H. Neumann, M.-M. Pohl, J. Radnik and M. Beller, Science, 2017, 358, 326–332 CrossRef CAS; (c) L. Jiang, P. Zhou, Z. Zhang, Q. Chi and S. Jin, New J. Chem., 2017, 41, 11991–11997 RSC; (d) X. Cui, K. Liang, M. Tian, Y. Zhu, J. Ma and Z. Dong, J. Colloid Interface Sci., 2017, 501, 231–240 CrossRef CAS.
- Y. Zhang, Y. Gao, S. Yao, S. Li, H. Asakura, K. Teramura, H. Wang and D. Ma, ACS Catal., 2019, 9, 7967–7975 CrossRef CAS.
- Other reductants were also used for one-pot reductive amination over base-metal catalyst, see (a) L. Jiang, P. Zhou, Z. Zhang, S. Jin and Q. Chi, Ind. Eng. Chem. Res., 2017, 56, 12556–12565 CrossRef CAS; (b) P. Zhou, Z. Zhang, L. Jiang, C. Yu, K. Lv, J. Sun and S. Wang, Appl. Catal., B, 2017, 210, 522–532 CrossRef CAS; (c) P. Zhou and Z. Zhang, ChemSusChem, 2017, 10, 1892–1897 CrossRef CAS; (d) P. Zhou, C. Yu, L. Jiang, K. Lv and Z. Zhang, J. Catal., 2017, 352, 264–273 CrossRef CAS.
- (a) M. Yadav and R. K. Sharma, Curr. Opin. Green Sustain. Chem., 2019, 15, 47–59 CrossRef; (b) D. Wang and D. Astruc, Chem. Soc. Rev., 2017, 46, 816–854 RSC.
- J. Li, B. Wang, Y. Qin and L. Chen, Catal. Sci. Technol., 2019, 9, 3726–3734 RSC.
- (a) J. Park, E. Kang, S. U. Son, H. M. Park, M. K. Lee, J. Kim, K. W. Kim, H.-J. Noh, J.-H. Park, C. J. Bae, J.-G. Park and T. Hyeon, Adv. Mater., 2005, 17, 429–434 CrossRef CAS; (b) I. S. Lee, N. Lee, J. Park, B. H. Kim, Y.-W. Yi, T. Kim, T. K. Kim, I. H. Lee, S. R. Paik and T. Hyeon, J. Am. Chem. Soc., 2006, 128, 10658–10659 CrossRef CAS.
- T. A. Le, M. S. Kim, S. H. Lee, T. W. Kim and E. D. Park, Catal. Today, 2017, 293–294, 89–96 CrossRef CAS.
- C. F. Winans, J. Am. Chem. Soc., 1939, 61, 3566–3567 CrossRef CAS.
- W. Yang, X. Yang, C. Hou, B. Li, H. Gao, J. Lin and X. Luo, Appl. Catal., B, 2019, 259, 118020 CrossRef CAS.
- M. A. Peck and M. A. Langell, Chem. Mater., 2012, 24, 4483–4490 CrossRef CAS.
- M. B. Mahajan and P. A. Joy, Phys. Chem. Chem. Phys., 2013, 15, 20808–20812 RSC.
- A. P. Grosvenor, M. C. Biesinger, R. St, C. Smart and N. S. McIntyre, Surf. Sci., 2006, 600, 1771–1779 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06937j |
| This journal is © The Royal Society of Chemistry 2020 |