Open Access Article
Open Access ArticleSimplified waste-free process for synthesis of nanoporous compact alumina under technologically advantageous conditions
Alena Fedoročková *a,
Gabriel Sučika,
Beatrice Plešingerováa,
Ľuboš Popoviča,
Mária Kovaľaková
*a,
Gabriel Sučika,
Beatrice Plešingerováa,
Ľuboš Popoviča,
Mária Kovaľaková b and
Martin Vavrac
b and
Martin Vavrac
aFaculty of Materials, Metallurgy and Recycling, Technical University of Košice, Letná 9, 042 00 Košice, Slovakia. E-mail: alena.fedorockova@tuke.sk
bFaculty of Electrical Engineering and Informatics, Technical University of Košice, Letná 9, 042 00 Košice, Slovakia
cFaculty of Science, Pavol Jozef Šafárik University in Košice, Šrobárova 2, 041 54 Košice, Slovakia
First published on 2nd September 2020
Abstract
Precipitated ammonium aluminium carbonate hydroxide (NH4Al(OH)2CO3) is a promising precursor for preparation of nanostructured Al2O3. However, the experimental conditions, such as the low concentration of Al3+ salt solution, high temperature and/or pressure, long reaction time, and excessive amount of the (NH4)2CO3 precipitating agent, make this process expensive for large-scale production. Here, we report a simpler and cheaper route to prepare nanostructured alumina by partial neutralisation of a nearly saturated aqueous solution of Al(NO3)3 with (NH4)2CO3 as a base at pH < 4. Synthesis in the acidic region led to formation of a polynuclear aluminium cluster (Al13), which is an important “green” solution precursor for large-area preparation of Al2O3 thin films and nanoparticles. Control of the textural properties of the final alumina product during calcination of the prepared aluminium (oxy)hydroxide gel was accomplished by adding low-solubility aluminium acetate hydroxide (Al(OH)(CH3COO)2) as a seed to the Al(NO3)3 solution before neutralisation. The large Brunauer–Emmett–Teller specific surface area (376 m2 g−1) and narrow pore size distribution (2–20 nm) of the prepared compact alumina suggest that the chelating effect of the acetate ions affects the structures of the forming transition aluminas, and the evolved gases produced by decomposition of Al(OH)(CH3COO)2 and NH4NO3 as a by-product of the reaction during calcination prevent particle agglomeration. Other advantages of the proposed process are its versatility and the ability to obtain high purity materials without producing large amounts of by-products without the need for washing and energy saving by using a low processing temperature, and the possibility of recycling the generated CO2 and NH3 gases as the (NH4)2CO3 reagent.
1. Introduction
Alumina (Al2O3) is one of the most widely used materials and it has considerable potential in many diverse applications, such as heat insulation,1 as a catalyst2–6 and catalyst support,1,2,4–13 as a high-strength and wear-resistant material in ball mills,7,8,14 in grinding paste for metal polishing,8,9 as an inert biomaterial in implants,15 as a substrate in the electronic industry,8 in wastewater purification,3 and in automobile exhaust emission reduction systems and flue gas cleaning.3,4,6,11,13,16The widespread use of Al2O3 arises from its high chemical and thermal stability and high mechanical strength, which are predominantly determined by the crystal phase and its composition and structure, mainly the shape and size of the particles and/or pores. In recent years, it has been shown that the performance of alumina in many of the above-mentioned applications can be improved by producing nanoscale structures.1–3,8–11,17–23 Therefore, current strategies have shifted towards synthesis of aluminium oxide with controllable nanostructure and morphology, because altering the surface area, pore structure, and morphology at the nanoscale can potentially lead to materials with desired physical and chemical properties tailored for various applications.
Several methods have been applied to prepare nanostructured alumina, including gas-phase deposition,3,14 mechanical grinding,3 thermal decomposition,1 combustion methods,14 microemulsion synthesis,3,17 the sol–gel process,1,3,6,8,10,11,14,18,19 precipitation,3,8,14,21,22 and hydrothermal synthesis.1,3,8,10,14,21 Among these methods, precipitation is the most popular process for preparation of ultrafine alumina powders because of its simplicity, low cost, safety, and energy and time saving.3,8,14
Aluminium hydroxides and (oxo)hydroxides, which are the common precursors to generate Al2O3 by thermal treatment, are usually prepared by precipitation of an aluminium salt solution with a base such as ammonia.7,11,14,18,20 It has been demonstrated that bulk precipitation is so rapid that nucleation and growth of particles simultaneously occur, resulting in particles with irregular shapes and a wide size distribution,7,24 which limit their practical applications.
Recently, synthesis of ammonium aluminium carbonate hydroxide (AACH, NH4Al(OH)2CO3) has become a promising alternative for preparation of nanostructured Al2O3.1–3,9,14,20–23 AACH is typically prepared by liquid–liquid precipitation using different aqueous aluminium solutions (NH4Al(SO4)2, Al(NO3)3, AlCl3, and NaAlO2).3,9,14,20 It has been shown that the choice of an appropriate precipitating agent is crucial for preparation of pure AACH. The product of precipitation using NH4HCO3 is a mixture of boehmite (AlO(OH)) and AACH,14,20 whereas using (NH4)2CO3 results in formation of only the AACH phase with low crystallinity.14 Thus, ammonium carbonate is preferred to ammonium hydrogen carbonate because the latter leads to significant formation of boehmite20 and the alumina particles prepared using the (NH4)2CO3 precipitant show a much narrower distribution and a smaller average diameter than those prepared using NH4HCO3.14 However, precipitation of AACH is very pH sensitive (optimal pH range 9.92–10.28), because AACH can change to AlO(OH) or [Al(OH)4]− in concentrated alkaline solution.3 An alternative novel technique for AACH synthesis that enables control of the morphology of the particles is hydrothermal treatment. Reaction of Al(NO3)3 aqueous solution or aluminium hydroxide xerogel with a significant stoichiometric excess of ammonium carbonate solution at high temperature and vapour pressure leads to flake-like2 or rod-like9 particles.
Thermal treatment after precipitation is accompanied by phase conversion to various aluminium oxide forms. The following sequence has been commonly reported: AlO(OH) → γ-Al2O3 → δ-Al2O3 → θ-Al2O3 → α-Al2O3.8,25 The required temperature for each phase transformation depends on the synthesis method and the specific characteristics of the precursors.8 For the NH4Al(OH)2CO3 precursor, it has been found that transformation to Al2O3 does not directly proceed by thermal treatment. During the first step, low crystallinity boehmite (AlO(OH)) is generated. In the second step, boehmite transforms to aluminium oxide with different crystal structures.9
Control of the morphology and particle size distribution of the final Al2O3 powder can be accomplished by reducing the phase transformation temperature.7 Various oxides of aluminium, copper, iron, chromium, and magnesium as seeding agents are therefore introduced into the aluminium salt solution before precipitation to act as crystallisation centres to decrease the activation energy of transformation.25,26 It has been found that the introduced seed particles do not act as nuclei of the new phase, but they affect the structure of the aluminium hydroxide gel formed around them or the structure of the forming transient aluminium oxide.25
Although synthesis of nanostructured Al2O3 from Al precursors prepared by precipitation has been widely performed, the nanoparticles tended to strongly agglomerate, which caused an increase of the particle size and decreased the specific surface area.3,14,27 Agglomeration is greatly affected by the aging time of the precipitated precursor.14 Thus, alumina prepared by precipitation generally exhibits a surface area lower than 300 m2 g−1.20 Various approaches have been applied to prevent undesirable aggregation of the nanoparticles during precipitation from solution, as well as during the following drying and thermal treatment, such as addition of organic solvents, surfactants, or chelators.3,8,11 Even though these dispersants can improve the colloid stability, reduce the temperature required for sintering, enhance green densification, and control the particle morphology,8 they affect the product crystallinity and are difficult to completely remove from the final materials.10 In this context, phase transformation of ammonium aluminium carbonate hydroxide to alumina can be helpful to suppress agglomeration, because the CO2, NH3, and H2O gases released during thermal treatment play significant roles in reducing agglomeration of the final Al2O3 particles.14 However, the prepared alumina may have high impurity content owing to insufficient removal of the supernatant solution.20 Increasing the purity of alumina powder requires a large amount of washing solvent and results in loss of up to 25% of Al2O3, as well as generation of solvent waste.7 Furthermore, excessive washing of AACH (Vwashing/Vsuspension > 1.6) leads to disappearance of the AACH phase and progressive formation of the boehmite phase.20
Since the preparation of monodispersed Al2O3 in the published works was carried out with a very low starting concentration of the Al3+ solution (≤0.13 M),1–3,14,21 low productivity can be a significant problem if such monodispersed particles are to be used as an industrial product, despite their ideally controlled properties. In addition, taking into account the reaction conditions used to prepare AACH, such as relatively high temperature (≥50 °C)1–3,9,20 and pressure,2,9,21 long reaction time (≥24 h),1–3,9 significant excess of (NH4)2CO3 as a precipitant (molar ratio of (NH4)2CO3 to Al3+ ≥4),2,9,20,21 subsequent separation of the precipitate by filtration1,3,14,20,21 or centrifugation,3 and its washing, this procedure is costly for mass-produced products. Therefore, in contrast to published works, the present work focuses on reaction conditions that are interesting from a technological point of view for large-scale production.
The aim of this work is to design simpler and cheaper preparation route from a nearly saturated aqueous solution of Al(NO3)3 in the acid region, leading to formation of especially stable polynuclear [Al13O4(OH)24(H2O)12]7+ complex cations,28 which are the dominant species found in solution at pH < 4.10 This aluminium cluster (Al13) with the Keggin structure, which consists of a central tetrahedral AlO4 unit surrounded by 12 AlO6 octahedra, is an important “green” solution precursor for large-area preparation of Al2O3 thin films and nanoparticles.10,27
This work focused on
• Preparation of aluminium clusters (Al13) by partially neutralising a nearly saturated aqueous solution of Al(NO3)3 with (NH4)2CO3 as a base at pH < 4 by the sol–gel method.
• Simplifying the process by eliminating washing and filtration, and energy saving by using a low processing temperature (22 °C).
• The effect of low solubility aluminium hydroxide acetate (Al(OH)(CH3COO)2) as a nucleating agent on the morphology, specific surface area, and pore size distribution (PSD) of the final alumina products prepared by thermal decomposition of synthesised alumina gels.
2. Experimental
2.1 Material synthesis
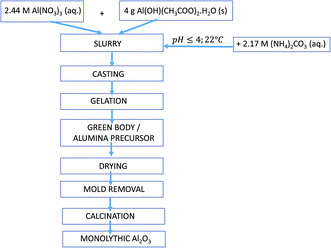
Aluminium nitrate nonahydrate (Al(NO3)3·9H2O, ACS reagent grade, ≥98%, Merck, KGaA, Darmstadt, Germany), ammonium carbonate ((NH4)2CO3, ACS reagent grade, ≥30% NH3 basis, Merck), aluminium hydroxide acetate hydrate (Al(OH)(CH3COO)2·H2O, purity ≥28% Al2O3, Merck), and deionised water were used as the starting chemicals without further purification.Synthesis of the alumina precursors was performed in a stirred reactor equipped with a mechanical stirrer. Typically, the exact amount (2–4 g) of Al(OH)(CH3COO)2·H2O powder was dispersed in 5 mL of 2.44 M Al(NO3)3 aqueous solution with pH = 0.2 and stirred with a constant agitation speed of 500 rpm until a homogeneous suspension formed. Subsequently, the calculated amount of 2.17 M (NH4)2CO3 (pH = 8.5) was added drop-wise from a burette (1 mL min−1) to the suspension with constant stirring and pH monitoring. The amount of added (NH4)2CO3 solution was selected so that after mixing the resulting pH was ≤ 4. After 1 h stirring, the white colloid solution or gel was transferred into a teflon mould (3 cm × 3 cm) and dried at 22 °C under reduced humidity (Concept OV 1000 dehumidifier). The prepared alumina precursors were then calcined at different temperatures (220–1100 °C) for 2 h with a heating rate of 3.75 °C min−1. The proposed procedure is schematically shown in Fig. 1.
2.2 Characterisation
The size of the particles was determined with a particle size analyser (Mastersizer 2000S, Malvern, UK) in deionised water by ultrasonic treatment. The thermal gravimetric analysis and differential thermal analysis (TG-DTA) measurements were performed with a NETZCH STA 449F3 Jupiter analyser with a SiC furnace under a N2 atmosphere at a heating rate of 10 °C min−1. The powder X-ray diffraction (XRD) patterns were obtained with an X-ray powder diffractometer (Rigaku MiniFlex 600) with Cu-Kα radiation (with a Ni filter) operated at 40 kV and 15 mA. Diffraction was performed from 2θ = 5° to 90° at a rate of 2° min−1. The nature of the bonding in the samples was determined by infrared (IR) spectroscopy and 27Al NMR spectroscopy. Fourier transform IR (FT-IR) spectroscopy at a resolution of 4 cm−1 was performed with a Nicolet 6700 FT-IR spectrometer (Thermo Scientific) using a Smart orbit device. The 27Al NMR spectra were recorded with a solid-state NMR spectrometer (Varian 400 MHz, Palo Alto, CA, USA) at a Larmor frequency of 104.18 MHz using a probe head with a 4 mm rotor at a magic angle spinning rate of 12 kHz. 3100 scans were accumulated with a recycle delay of 5 s with the rf pulse length of 0.235 s corresponding to the flip angle of 9° for the central transition in solids. The spectra were processed with Varian Vnmrj 3.2 and Mestrelab Mnova 12.0 software. A 1 mol dm−3 aqueous [Al(H2O)6]3+ solution (primary standard) was used as the reference for the chemical shifts. The textural properties of the prepared samples were measured through the nitrogen adsorption/desorption isotherms (NOVA 1000e, Quantachrome Instruments, USA). Prior to the measurements, the samples were outgassed at different temperatures (110–290 °C) for 20 h. The specific surface area was determined by the multipoint (SA,M) Brunauer–Emmett–Teller (BET) method using the adsorption data in the relative pressure range 0.05 ≤ p/p0 ≤ 0.35, and the t-plot and αS-plot methods were used to evaluate the microporosity. The PSDs were derived from the adsorption/desorption branches of the isotherms using the Barrett–Joyner–Halenda (BJH) model. The pore volume (Vp) was calculated from the adsorbed volume of gas at different relative pressures (p/p0), and the results were evaluated on the basis of density functional theory (DFT). The pore size distribution was calculated by NLDFT adsorption model (non-local DFT) under conditions of adsorbate of N2 77 K and adsorbent of zeolite/silica for the cylindrical-spherical pores. Scanning Electron Microscopy (SEM) observations were performed on a TESCAN Mira 3 using 30 kV accelerating voltage at a working distance of 8.94 mm.3. Results and discussion
3.1 Effect of the reaction conditions on alumina precursor formation
Polynuclear [Al13O4(OH)24(H2O)12]7+ complex cations (known as Keggin ε-Al13 ions) were synthesised by partial neutralisation of Al3+ solution with a base at OH−/Al3+ molar ratios from 0.5 to 2.5, corresponding to pH values less than 4.10,24,27 The results of mechanochemical synthesis of polymeric ε-Al13 ions in the concentrated state between [Al(H2O)6]Cl3 and (NH4)2CO3 have shown that the maximum concentration of ε-Al13 species (85%) is achieved at a molar ratio of CO32−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ = 2.55
Al3+ = 2.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.24 In this study, two different molar ratios of (NH4)2CO3 to Al(NO3)3 within the surrounding range (nCO32−
2.24 In this study, two different molar ratios of (NH4)2CO3 to Al(NO3)3 within the surrounding range (nCO32−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nAl3+ = 2.25
nAl3+ = 2.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2.75
2 and 2.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were used to prepare Al13 clusters as a solution precursor of Al2O3. To control the textural properties of the final Al2O3 product, various amounts of low solubility aluminium hydroxide acetate hydrate (Al(OH)(CH3COO)2·H2O) were added to the Al(NO3)3 solution before base addition. It was hypothesised that chelation of aluminium ions with acetate ions could affect the stability of precursor colloid solution,8 the structure of the formed aluminium hydroxide gel, and thus the textural characteristics of the final Al2O3. In addition, Al(OH)(CH3COO)2OH·H2O particles as crystallisation centres could contribute to lowering the transformation temperature of the prepared aluminium gel to various forms of Al2O3.
2) were used to prepare Al13 clusters as a solution precursor of Al2O3. To control the textural properties of the final Al2O3 product, various amounts of low solubility aluminium hydroxide acetate hydrate (Al(OH)(CH3COO)2·H2O) were added to the Al(NO3)3 solution before base addition. It was hypothesised that chelation of aluminium ions with acetate ions could affect the stability of precursor colloid solution,8 the structure of the formed aluminium hydroxide gel, and thus the textural characteristics of the final Al2O3. In addition, Al(OH)(CH3COO)2OH·H2O particles as crystallisation centres could contribute to lowering the transformation temperature of the prepared aluminium gel to various forms of Al2O3.
Particle size analysis of the Al(OH)(CH3COO)2·H2O powder showed that 90% of the particles had mean size d(90) ≤ 18.8 μm. The experimental conditions are given in Table 1.
| Sample | Selected (NH4)2CO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al(NO3)3 molar ratio Al(NO3)3 molar ratio |
Initial suspension Al(NO3)3(aq) + Al(OH)(CH3COO)2(s) (AA) | + Base (NH4)2CO3(aq) | Final mixture/prepared alumina precursor | |||||
|---|---|---|---|---|---|---|---|---|---|
| nAl(NO3)3 [mol] | mAA [g] | pHinitial | V(NH4)2CO3 [dm3] | pHfinal | Alumina precursor consistency | ||||
| After 1 h stirring | After 72 h drying | Upon exposure to air | |||||||
| a All of the experiments were performed at T = 22 °C and agitation speed of 500 rpm using cAl(NO3)3 = 2.44 mol dm−3, VAl(NO3)3 = 0.005 dm3, and c(NH4)2CO3 = 2.17 mol dm−3. | |||||||||
| 1 | 2.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.0122 | 2 | 0.67 | 0.0063 | 3.73 | Liquid | Compact solid with a smooth glossy surface | “Cotton wool” surface |
| 2 | 2.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.0122 | 4 | 0.95 | 0.0063 | 3.94 | Liquid | Compact solid with a smooth glossy surface | Compact solid with a smooth glossy surface |
| 3 | 2.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.0122 | 2 | 0.67 | 0.0078 | 4.09 | Gel | Compact solid with a rough surface | Diffluent surface |
| 4 | 2.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.0122 | 4 | 0.95 | 0.0078 | 4.18 | Thick foam | Compact solid with a rough surface | Compact solid with a rough surface |
Taking into account the properties of the prepared samples during synthesis and after drying (Table 1), in relation to handling under technological conditions, only sample 2 was considered to be a suitable alumina precursor for further study. The reason for its selection was the longer gelation time (up to 48 h) than the other samples. This provides longer time for handling the precursor, as well as its stable compact form, which does not undergo surface dehydration when exposed to air. Rapid dehydration of the surface of the Keggin-type aluminium clusters is attributed to the loss of less tightly bound water molecules that contract the crystals and disrupt the crystal surface,29 as confirmed by formation of “cotton wool” on the surface of sample 3 within 1 week.
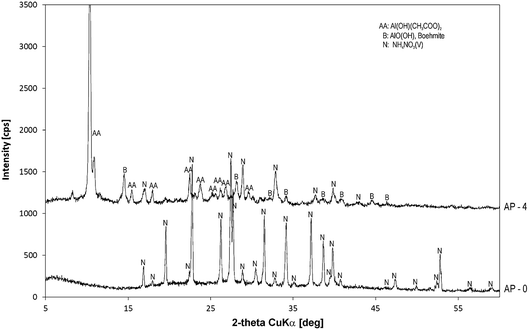
The XRD pattern of the selected alumina precursor (sample 2 in Table 1) obtained at a molar ratio of CO32− to Al3+ = 2.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with 4 g of aluminium hydroxide acetate as a nucleation additive is shown in Fig. 2. For easier identification of the formed (oxy)hydroxide phases, the peaks of aluminium hydroxide acetate (AA) and ammonium nitrate (N) formed as a by-product of the reaction are also assigned in the XRD pattern. The effect of aluminium hydroxide acetate on the type of aluminium hydroxide oxide can be seen by comparison with the diffractogram of the sample prepared under the same reaction conditions but without addition of aluminium hydroxide acetate (AP-0).
2 with 4 g of aluminium hydroxide acetate as a nucleation additive is shown in Fig. 2. For easier identification of the formed (oxy)hydroxide phases, the peaks of aluminium hydroxide acetate (AA) and ammonium nitrate (N) formed as a by-product of the reaction are also assigned in the XRD pattern. The effect of aluminium hydroxide acetate on the type of aluminium hydroxide oxide can be seen by comparison with the diffractogram of the sample prepared under the same reaction conditions but without addition of aluminium hydroxide acetate (AP-0).
The results of XRD analysis revealed that the selected alumina precursor was composed of a mixture of crystalline NH4NO3 (ICDD 01-083-0520), Al(OH)(CH3COO)2 (ICDD 00-004-0113), and boehmite (ICDD 01-083-1506). The broad diffraction peaks of the boehmite phase (γ-AlO(OH)) indicated that it had low crystallinity, which is related to the high concentration of Al3+ and hence to the high nucleation rate, resulting in formation of small particles.20 From Fig. 2, for the sample without addition of Al(OH)(CH3COO)2, the boehmite phase (despite its higher proportion in the mixture) did not form, suggesting that aluminium hydroxide acetate had a significant effect on boehmite formation.
3.2 Thermal transformation of the prepared alumina precursor to Al2O3
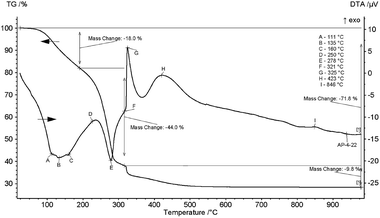
Upon heating, boehmite sequentially transforms to γ-alumina, δ-alumina, θ-alumina, and α-alumina.30 Determination of the suitable calcination temperature for preparation of active alumina with a nanoporous structure was performed by TG–DTA. The TG–DTA curves obtained from decomposition of the prepared alumina precursor are shown in Fig. 3.The thermogravimetric curve showed that weight loss occurred in several steps, corresponding to the reactions associated with NH4NO3 decomposition and transformation of Al(OH)(CH3COO)2·H2O and γ-AlO(OH) to Al2O3.
The gradual weight loss up to 200 °C is related to release of part of the –OH and acetate groups (18%), while the sharp weight loss above 200 °C (44%) is correlated with simultaneous removal of residual acetate groups and decomposition of NH4NO3.31,32 The significant exothermic peak centred around 325 °C corresponds to combustion of released acetate groups (as “fuel”) with NH4NO3 as an oxidising agent. The broad exothermic peak at 423 °C is ascribed to transformation of boehmite to γ-Al2O3,20 and the slight exothermic peak at around 846 °C is attributed to gradual formation of the θ-Al2O3 crystalline structure, which is in good agreement with XRD analysis of boehmite annealed at temperatures up to 1000 °C.30 The total weight loss at 1000 °C was 71.8%, which is in agreement with the theoretical value of 74.7% (Table 2), and the individual stages of decomposition are in accordance with the published TG–DTA data of the individual components.20,31,32
| Reactants | Additive | Prepared alumina precursor | |||||
|---|---|---|---|---|---|---|---|
| Products | Additive | ||||||
| Stoichiometric molar ratio | Al(NO3)3 | (NH4)2CO3 | Al(OH)(CH3COO)2 | → | AlO(OH) | NH4NO3 | Al(OH)(CH3COO)2 |
| 2 mol | 3 mol | 2 mol | 6 mol | ||||
| Used molar ratio | 2 mol | 2.25 mol | — | → | 2 mol | 5 mol | — |
| Input amount ni [mol] | 0.0122 | 0.0137 | 0.022 | Output amount ni [mol] | 0.0122 | 0.0274 | 0.022 |
| Input mass mi = niMi [g] | 2.599 | 1.315 | 4 | Output mass mi = niMi [g] | 0.732 | 2.192 | 4 |
| Mass before calcination [g] | 6.924 | ||||||
| Theoretical mass loss of individual component wi [%] | 15 | 100 | 71.67 | ||||
| Mass after calcination [g] | 0.622 | 0 | 1.133 | ||||
| 1.755 | |||||||
| Theoretical mass loss of the alumina precursor wi [%] | 74.7 | ||||||
The prepared alumina precursor was calcined at 220–1100 °C, and the nitrogen adsorption–desorption isotherms were immediately measured after calcination to evaluate the textural properties. Calcination at 220 °C was performed to remove the NH4NO3 by-product from the precursor. Transformation of the boehmite (γ-AlO(OH)) formed in the mixture with Al(OH)(CH3COO)2 into various forms of Al2O3 was observed at temperatures above 220 °C.
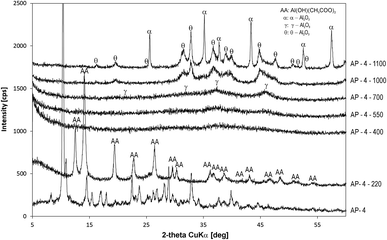
By heat treatment, aluminium (oxy)hydroxides pass through a series of phase transitions during which the hydroxyl groups are removed. Because of the different degrees of dehydroxylation, these intermediates (so-called transition aluminas) exhibit structural differences resulting from the variability of the aluminium coordination numbers, which is reflected in poorly resolved X-ray diffractograms.30 This phenomenon is also evident from the diffractograms (Fig. 4) of the prepared alumina precursor (AP-4, Fig. 2) calcined in the temperature range 400–700 °C, which show formation of gel-type X-ray amorphous phases, which were identified by 27Al NMR. In the range 700–1000 °C, γ-Al2O3 (ICDD 00-010-0425) transformed to θ-Al2O3 (ICDD 01-035-0121), and above 1100 °C θ-Al2O3 recrystallised to α-Al2O3 (corundum, ICDD 01-089-3072).
The nature of bonding in the prepared alumina precursor and its calcined products obtained at different calcination temperatures was characterised by FT-IR analysis (Fig. 5). The IR spectra of the precursor and its individual accompanying admixtures, such as aluminium acetate hydroxide and ammonium nitrate, are shown in the left part of Fig. 5. The IR spectrum of the prepared sample confirmed the presence of all of the functional groups from NH4NO3 (ν(N–H) = 3228, 3061 cm−1; δ(NO3) = 1409, 1295 cm−1) and Al(OH)(CH3COO)2·H2O (ν(O–H) = 3554, 3487, 3292 cm−1; ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) = 1582 cm−1; δ(CH3) = 1464, 1423 cm−1; ν(C–C) = 1041, 987 cm−1; ν(Al–O) = 675, 625, 482, 428 cm−1).
O) = 1582 cm−1; δ(CH3) = 1464, 1423 cm−1; ν(C–C) = 1041, 987 cm−1; ν(Al–O) = 675, 625, 482, 428 cm−1).
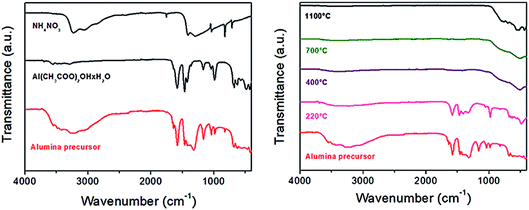 | ||
| Fig. 5 FT-IR spectra of the prepared alumina precursor compared with the spectra of its individual components (left side) and calcined products prepared at different temperatures (right side). | ||
The IR spectra of the prepared precursor and its calcined products are compared in the right side of Fig. 5. The IR spectrum of the sample calcined at 220 °C only contained absorption bands originating from aluminium hydroxide acetate (ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) = 1581 cm−1; δ(CH3) = 1471, 1408, 1331 cm−1; ν(C–C) = 980 cm−1; ν(Al–O) = 486 cm−1), while the bands assigned to ammonium nitrate disappeared, indicating its complete decomposition, which is consistent with the results of TG–DTA analysis (Fig. 3). The IR spectra of the samples calcined at higher temperatures (400, 700, and 1100 °C) only contained the ν(Al–O) absorption band, confirming the presence of aluminium oxide. Moreover, the different numbers and positions of the ν(Al–O) absorption bands indicated different modifications of the final products due to phase transformation from γ-Al2O3 through θ−Al2O3 to α−Al2O3, which are related to the different coordination of Al3+ (hexa-coordinated AlO6, penta-coordinated AlO5 and tetra-coordinated AlO4),8,9,33–36 and is in accordance with the results of X-ray analysis (Fig. 4). The samples calcined at 400 and 700 °C both showed one band at 509 cm−1. The sample calcined at 1100 °C showed absorption bands at 702, 633, 559, 521, and 436 cm−1.
O) = 1581 cm−1; δ(CH3) = 1471, 1408, 1331 cm−1; ν(C–C) = 980 cm−1; ν(Al–O) = 486 cm−1), while the bands assigned to ammonium nitrate disappeared, indicating its complete decomposition, which is consistent with the results of TG–DTA analysis (Fig. 3). The IR spectra of the samples calcined at higher temperatures (400, 700, and 1100 °C) only contained the ν(Al–O) absorption band, confirming the presence of aluminium oxide. Moreover, the different numbers and positions of the ν(Al–O) absorption bands indicated different modifications of the final products due to phase transformation from γ-Al2O3 through θ−Al2O3 to α−Al2O3, which are related to the different coordination of Al3+ (hexa-coordinated AlO6, penta-coordinated AlO5 and tetra-coordinated AlO4),8,9,33–36 and is in accordance with the results of X-ray analysis (Fig. 4). The samples calcined at 400 and 700 °C both showed one band at 509 cm−1. The sample calcined at 1100 °C showed absorption bands at 702, 633, 559, 521, and 436 cm−1.
Because transformation between the distinct intermediate phases is a gradual process, the exact temperatures to obtain the individual phases cannot be determined solely by XRD experiments.13 A reliable tool that allows unambiguous identification of these phases is 27Al solid-state NMR.13,30 Clarification of the structures of the prepared Al2O3 precursor and its heat-treated products was performed by 27Al NMR measurements.
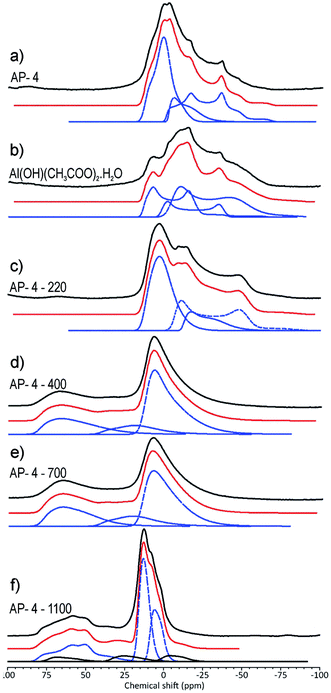
The 27Al NMR spectra of transition aluminas show signals characteristic of tetra-coordinated AlO4, hexa-coordinated AlO6 and penta-coordinated AlO5.30 Simulations of the spectra provide the NMR parameters: the isotropic chemical shift δiso, quadrupole coupling constant CQ, and asymmetry parameter ηQ. The Al coordination is reflected in isotropic chemical shift values.13,30 The quadrupole coupling constant CQ (in MHz) reflects distortion from a perfect octahedron or tetrahedron, i.e., a large CQ value means distorted Al sites. Simulation of the 27Al NMR spectra (Fig. 6a–f) using the QuadFit program37 are given in Table 3.
| Sample | Aluminium site | δiso [ppm] | CQ [MHz] | ηQ | Δ [Hz] | Occupancy [%] |
|---|---|---|---|---|---|---|
| Prepared alumina precursor | Al6 (1) | 13.2 | 4.1 ± 0.7 | 1.0 ± 0.1 | 500 | 48.1 |
| Al6 (2) | 2.9 | 7.1 ± 0.2 | 0.4 ± 0.1 | 250 | 37.2 | |
| Al6 (3) | 0.65 | 4.9 ± 1.6 | 0.2 ± 0.05 | 100 | 14.7 | |
| Aluminium hydroxide acetate hydrate | Al6 (1) | 11.0 | 7.5 ± 0.1 | 0.1 ± 0.05 | 400 | 17.7 |
| Al6 (2) | 19.0 | 6.6 ± 0.2 | 0.2 ± 0.15 | 300 | 33.1 | |
| Al6 (3) | 5.5 | 8.1 ± 1.3 | 0.1 ± 0.05 | 800 | 49.2 | |
| Boehmite 220 °C | Al6 (1) | 13.35 | 4.5 ± 1.6 | 0.65 ± 0.1 | 600 | 50.5 |
| Al6 (2) | 3.8 | 8.1 ± 0.6 | 0.1 ± 0.1 | 450 | 34.9 | |
| Al6 (3) | −8.7 | 5.8 ± 1.8 | 0.2 ± 0.05 | 250 | 14.6 | |
| γ-Al2O3 400 °C | Al4 | 83.0 | 6.8 ± 2.6 | 0.4 ± 0.1 | 700 | 24.4 |
| Al5 | 41.0 | 6.2 ± 2.0 | 0.4 ± 0.1 | 800 | 12.6 | |
| Al6 | 15.3 | 5.6 ± 3.3 | 0.4 ± 0.1 | 700 | 63.0 | |
| γ-Al2O3 700 °C | Al4 | 81.0 | 6.4 ± 2.1 | 0.4 ± 0.1 | 700 | 27.3 |
| Al5 | 42.0 | 6.2 ± 2.0 | 0.7 ± 0.1 | 800 | 14.6 | |
| Al6 | 16.9 | 5.5 ± 2.8 | 0.3 ± 0.1 | 700 | 58.1 | |
| α- and θ-Al2O3 1100 °C | Al4 | 81.2 | 6.5 ± 2.0 | 0.65 ± 0.1 | 600 | 25.0 (θ) |
| Al6 (1) | 16.6 | 2.9 ± 0.9 | 0.0 | 500 | 38.8 (α) | |
| Al6 (2) | 9.7 | 3.2 ± 0.6 | 0.0 | 450 | 20.7 (θ) | |
| Al4 | 78.0 | 5.1 ± 1.5 | 0.25 ± 0.1 | 800 | 4.2 | |
| Al5 | 42.0 | 6.0 ± 1.5 | 0.4 ± 0.1 | 500 | 6.5 | |
| Al6 | 4.0 | 4.5 ± 0.8 | 0.3 | 700 | 4.8 |
The spectrum of the prepared Al2O3 precursor (Fig. 6a) showed features between 25 and −75 ppm. The isotropic chemical shift values were in the range typical of hexa-coordinated aluminium (AlO6). The first line with a δiso value of 13.2 ppm can be assigned to boehmite, although its CQ value was larger than that reported by Chandran et al.30 This can be explained by the larger disorder in the prepared precursor owing to the presence of aluminium acetate, which probably gives rise to the other two lines in the spectrum with relatively large CQ values. The 27Al NMR spectrum of pure aluminium acetate (Fig. 6b) consisting of signals in the range typical of hexa-coordinated aluminium was tentatively simulated using three lines with relatively large CQ and low ηQ values (Table 3). The difference between the NMR parameters obtained for aluminium acetate in the prepared Al2O3 precursor and those of the pure chemical can be attributed to the obvious difference between the 27Al environments in the pure compound and prepared material. Calcination of the prepared alumina precursor at 220 °C (Fig. 6c) resulted in a slightly different shape of the NMR spectrum, in which there was still evidence for the presence of aluminium acetate. Calcination at 400 °C (Fig. 6d) was expected to completely remove aluminium acetate from the sample and induce a phase transition to γ-alumina.
γ-Alumina crystallises in the spinel structure, where Al3+ ions occupy tetrahedral and octahedral interstitial sites,12 with some degree of crystallographic distortion related to the presence of penta-coordinated Al(V) species.13,30,38,39 The simulations of the spectra measured after calcination at 400 and 700 °C (Fig. 6d and e) confirmed signals with isotropic chemical shift values characteristic of tetra-, hexa- and penta-coordinated aluminium.30 However, the respective CQ values were larger than the reported values, which indicates a large deviation from spherical symmetry, probably caused by the presence of aluminium acetate in the course of sample preparation. Calcination at 1100 °C (Fig. 6f) resulted in phase transitions to θ- and α-alumina. The CQ and η values (Table 3) were in agreement with those reported in the literature.30
The textural properties of the prepared alumina precursor and its calcined products obtained at different temperatures were determined by N2 adsorption–desorption measurements (Fig. 7), and the results are summarised in Table 4.
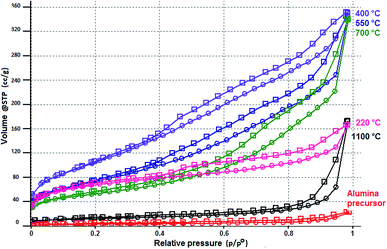 | ||
| Fig. 7 N2 adsorption–desorption isotherms of the prepared alumina precursor and its calcined products obtained at different temperatures. | ||
| Sample | Specific surface area SA,M Prel ∈ (0.05–0.35) [m2 g−1] | Evaluation of the porosity by the t-plot method[m2 g−1] | Micropore volume evaluated by the αS-plot method [cm3 g−1] | Vtot determined from desorption [cm3 g−1] | Average pore diameter [nm] | |||
|---|---|---|---|---|---|---|---|---|
| SA,micro | SA,exta | d < 2 nm | d < 20 nm | d < 135 nm | Prel ∈ (0–0.99) | |||
| a SA;ext - mezo-, macropores and external surface. | ||||||||
| Alumina precursor | 9 | 0 | 9 | 0 | 0.006 | 0.02 | 0.035 | 15 |
| 220 °C | 224 | 107 | 114 | 0.072 | 0.108 | 0.19 | 0.26 | 4.5 |
| 400 °C | 376 | 55 | 320 | 0.102 | 0.162 | 0.48 | 0.63 | 5.6 |
| 550 °C | 267 | 0 | 267 | 0.041 | 0.116 | 0.38 | 0.53 | 8 |
| 700 °C | 184 | 0 | 185 | 0.005 | 0.088 | 0.35 | 0.52 | 11 |
| 1100 °C | 41 | 0 | 41 | 0 | 0.020 | 0.08 | 0.26 | 26 |
The shapes and relative positions of the curves in Fig. 7, as well as the results in Table 4, indicate the changes that occurred during thermal treatment of the prepared precursor.
From the values in Table 4, the prepared alumina precursor had a very low specific surface area (SA = 9 m2 g−1). However, its subsequent calcination at 220 °C (associated with removal of NH4NO3) led to development of micro- (<2 nm) and mesopores (2–50 nm), which was accompanied by a significant increase in the surface area (224 m2 g−1). The presence of mesopores is indicated by a characteristic hysteresis loop that closes at a relative pressure of ∼0.4. Heating to 400 °C caused a further increase in the specific surface area (376 m2 g−1), while the proportion of micropores decreased. The considerable increase in SA up to 400 °C can be attributed to gaps left by the gas molecules released by both decomposition of NH4NO3 and Al(OH)(CH3COO)2 and dehydroxylation of boehmite (AlO(OH)) to γ-Al2O3 (see Fig. 3). Calcination above 550 °C was associated with a gradual decrease of the specific surface area because of recrystallisation and sintering of alumina nanoparticles.
The PSDs of the prepared alumina precursor and its calcined products obtained at different temperatures determined from the adsorption/desorption curves (dV/d) by the BJH method are shown in Fig. 8, and the volume histograms determined by DFT40 are shown in Fig. 9.
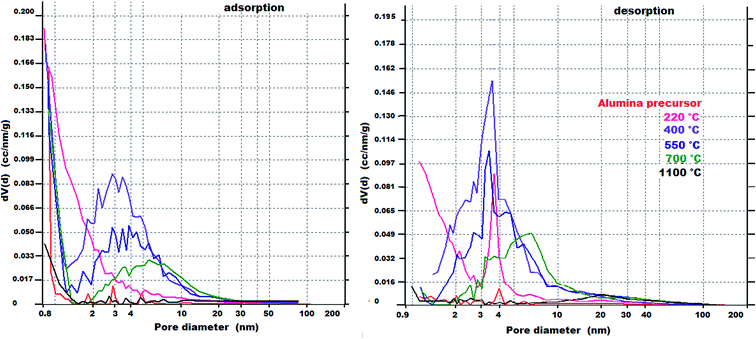 | ||
| Fig. 8 PSDs of the prepared alumina precursor and its calcined products obtained at different temperatures determined by BET analysis using the BJH model. | ||
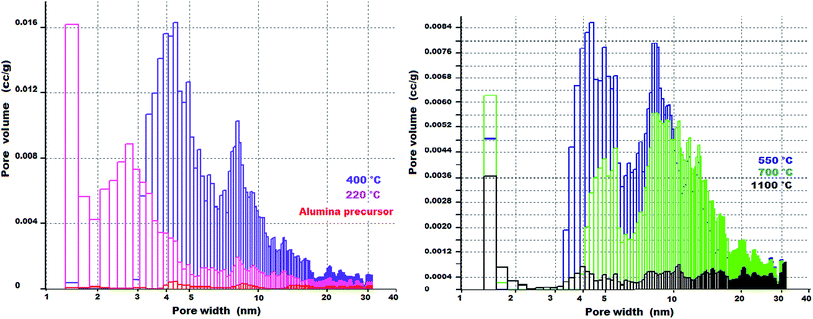 | ||
| Fig. 9 Pore volume histograms of the prepared alumina precursor and its calcined products obtained at different temperatures determined by DFT. | ||
From Fig. 8 and 9, although the precursor before calcination had a low specific surface area (9 m2 g−1), it contained micropores with sizes less than 1 nm. Calcination at 220 °C led to development of micropores. For calcination at 400 °C, mesopores with a narrow bimodal distribution (maxima at 3 and 10 nm) accounted for the significant increase in the pore volume.
At temperatures above 400 °C, the micropores disappeared and the mesopores became larger, leading to a decrease in the pore volume, which is related to agglomeration of Al2O3 crystallites. The complete disappearance of the microporosity at higher temperature (>400 °C) is probably associated with loss of the intracrystalline porosity owing to sintering, which is a typical phenomenon, especially when sintering small particles.20 Nevertheless, it is important to emphasise that the γ-Al2O3 synthesised in this work shows comparable parameters of open porosity than those synthesised by thermal decomposition of ammonium aluminium carbonate hydroxide (AACH) at comparable calcination temperatures – Table 5.
| Method of preparation of alumina precursor | Alumina source | Base | pH | Semiproduct | Calcination temperature and time | Pore characteristics of Al2O3 | Lit. | ||
|---|---|---|---|---|---|---|---|---|---|
| Surface area (BET) [m2 g−1] | Total pore volume [cm3 g−1] | Average pore diameter [nm] | |||||||
| a Values obtained at slow heating rate (5 °C min−1). | |||||||||
| Precipitation | 0.11 M Na[Al(OH)4](aq) | (NH4)2CO3 (aq) | 10 | NH4Al(OH)2CO3 | 400 °C/3 h | 618.68a | 0.54a | 4.43a | 3 |
| 500 °C/3 h | 552.77a | 0.55a | 4.71a | ||||||
| 0.13 M Al(NO3)3·9H2O(et) | (NH4)2CO3 (aq) | 7.5 | AlO(OH)·nH2O | 600 °C/5 h | 260.8 | 1.80 | 20.6 | 1 | |
| 8.5 | AlO(OH)·nH2O + NH4AlO(OH)HCO3 | 276.6 | 2.08 | 6.9; 39.7 | |||||
| 9.5 | NH4AlO(OH)HCO3 | 290 | 2.41 | 7.3; 49.5 | |||||
| 1.2 M Al(NO3)3·9H2O(aq) | (NH4)2CO3 (aq) | 7–8 | NH4Al(OH)2CO3 | 500 °C/4 h | 407 | 1.17 | 2.7–29.3 | 20 | |
| 0.4 M AlCl3·6H2O(aq) | NH4HCO3 (aq) | >7 | NH4Al(OH)2CO3 | 600 °C/5 h | 325 | 0.73 | 5.5 | 21 | |
| Hydrothermal synthesis | Al(OH)3 xerogel (70 wt% Al2O3) | NH4HCO3 (aq) | 10.5 | NH4Al(OH)2CO3 | 350 °C/2 h | 441 | 0.67 | 6.8 | 9 |
| 500 °C/2 h | 246 | 0.7 | 10.7 | ||||||
| 0.4 M AlCl3·6H2O(aq) | (NH4)2CO3 (aq) | >7 | NH4Al(OH)2CO3 | 600 °C/2 h | 300 | 0.44 | 4.2 | 21 | |
| Solid-state | AlCl3·6H2O(s) | (NH4)2CO3 (s) | NH4Al(OH)2CO3 | 600 °C/2 h | 298 | 0.99 | 9.7 | 21 | |
| Sol–gel | 2.44 M Al(NO3)3·9H2O(aq) + Al(OH)(CH3COO)2(s) | (NH4)2CO3 (aq) | 3.8 | AlO(OH) | 400 °C/2 h | 376 | 0.63 | 5.6 | This work |
| 550 °C/2 h | 267 | 0.53 | 8.0 | ||||||
It can be assumed that the textural characteristics of the nanoporous Al2O3 (Table 4) are probably influenced by the addition of Al(OH)(CH3COO)2 which as a component of the prepared alumina precursor acts as a textural modifier. This assumption is based on the fact, that the acetate anions (OAc−) formed by partial dissociation of Al(OH)(CH3COO)2 (ref. 41) initially combine with Al3+ cations in [Al13O4(OH)24(H2O)12]7+ complex cluster into a binuclear mixed-hydroxo species [Al2(OH)2OAc)]3+, in which acetate ion acting as a bridge between the apices of two dihydroxo-bridged Al(III) octahedra.42–44 Chelation of aluminium ions with acetate ions could, like the citric acid11 or phosphoric acid,45 affect the stability of the colloidal solution8 and thus also the structure of the aluminium hydroxide gel formed around the undissociated part of Al(OH)(CH3COO)2 and/or the structure of the final Al2O3 product. With increasing pH, the concentration of [Al2(OH)2OAc)]3+ complex cluster cations increases, and at pH = 4 (i.e. at reaction conditions used in this work) strong predominance of the Al(III)- acetate complexes was confirmed.42 The addition of OH− will reduced the surface positive charge of polymeric Al-micelle thereby the hydroxyl bridges in polymeric Al are converted into oxygen bridges.46
The acetate ions are weakly chelating agent, so chelates formed between aluminium and acetate ions have a low stability constant. Since chelates of the lower stability constants provide higher supersaturation in the nucleation stage as well, they usually provide smaller particles with a narrow size distribution.7,47
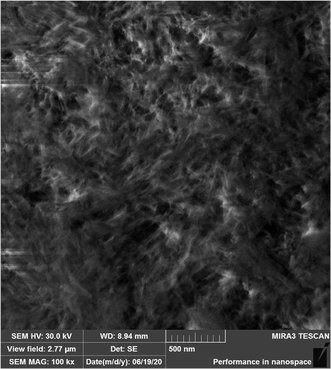
This assumption is confirmed by the SEM image (Fig. 10) of the alumina monolith prepared by calcination at 400 °C for 2 hours at 100k× magnification.
The Fig. 10 shows a fiber-like structure morphology composed from thin nanofibers of about 100 nm in length with the diameter of about 5 nm leading to the formation of a highly porous material. The fibrous structure may be related to the –OH groups in the (010) and (100) facets of γ-AlO(OH) precursor, which could act as the adsorption site for anions.47,48 The adsorption would decrease the surface energy and limit the aggregation of the γ-AlO(OH) nanoparticles on these facets, which would result in the oriented aggregation of γ-AlO(OH) along the (001) direction.48 The adsorption ability of the acid anions is related to the charge/size ratio, and the anions with the larger charge/size ratio would be adsorbed more easily on the particle surface of γ-AlO(OH). Since acetate ions have a relatively high charge-to-size ratio (6.289) their strong adsorption on the particle surfaces of γ-AlO(OH) leads to the formation of nanorods that grew along the (001) direction.48 From the Fig. 10 it can be seen that the corresponding shape retained γ-Al2O3 was obtained after calcination.
It is important to emphasize the fact that the nanoporous alumina monolith was prepared without the use of surfactants, while according to the literature the monolithic alumina aerogels with hierarchically porous structure were prepared by sol–gel5 or emulsion-gel casting49 using aluminum isopropoxide with organic surfactant (Pluronic P 1235, Sodium Dodecyl Sulfate (SDS)49). High price of aluminium alkoxides as alumina sources and surfactants cause that these are less attractive for large-scale production.1 Thus, ammonium carbonate and aluminium nitrate which were used in this work are the reagents that are inexpensive, easily available and widely used in the chemical industry.1
In addition, the conclusions of previously published works on the preparation of NH4Al(OH)2CO3 (AACH) as a precursor of nanostructured Al2O3 showed that boehmite (AlO(OH)) is an intermediate in the synthesis of AACH1 and its excessive washing (due to its reactive nature in deionized H2O)20 as well as in its transformation to Al2O3.3 In the context of these findings, the formation of boehmite prepared under the reaction conditions in this work (pH < 4; 22 °C) is an advantage in comparison to the preparation of AACH at pH > 7 and T ≥ 50 °C in terms of prevention of particle aggregation, which is confirmed by the following published statements:
(a) Surface property of boehmite may mitigate the hard aggregation of particles during calcination because the interface hydrophobicity, determining by the contact angles (θ) of AACH, AlO(OH) and Al2O3 using water, increased in the order of AACH (θ = 30.82°) > boehmite (θ = 33.79°) > Al2O3/200 °C (θ = 53.45°).3
(b) High temperature and pH values close to the boehmite point of zero charge PZC (pH = 8.2) favours strong aggregation.20
4. Conclusions
We have reported a simplified procedure for preparation of nanostructured compact alumina under technologically advantageous conditions.(1) Alumina precursors for synthesis of nanoporous Al2O3 by thermal treatment were prepared by partial neutralisation of a nearly saturated aqueous solution of Al(NO3)3 with (NH4)2CO3 as a base at pH < 4. Synthesis in the acidic region led to formation of polynuclear aluminium clusters (Al13), which are important “green” solution precursors for preparation of active Al2O3 thin films and nanoparticles.
(2) Control of the textural properties of the final alumina during calcination of the prepared aluminium (oxy)hydroxide gels was accomplished by adding low solubility aluminium hydroxide acetate hydrate (Al(OH)(CH3COO)2·H2O) as a nucleating agent to the Al(NO3)3 solution before neutralisation. It was found that Al(OH)(CH3COO)2·H2O affects the structure of the aluminium hydrated form formed around the nucleation centres and thus the structure of the forming intermediate aluminium oxide forms. It was confirmed that while addition of Al(OH)(CH3COO)2·H2O resulted in formation of the boehmite phase (γ-AlO(OH)), boehmite did not formed without the nucleating agent. This suggests that addition of aluminium hydroxide acetate as a nucleating agent has a significant effect on formation of boehmite.
(3) Considering the gelation time required for handling the precursor, as well as its stable compact form that is not surface dehydrated upon exposure to air, the molar ratio of (NH4)2CO3 to Al(NO3)3 = 2.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with addition of 4 g of Al(OH)(CH3COO)2·H2O (per 0.0122 moles of Al(NO3)3(aq)) were optimal.
2 with addition of 4 g of Al(OH)(CH3COO)2·H2O (per 0.0122 moles of Al(NO3)3(aq)) were optimal.
(4) The large BET specific surface area (376 m2 g−1) and relatively narrow pore distribution (2–20 nm) of the compact Al2O3 synthesised at 400 °C are likely to be because of the chelating effect of the acetate ions and the gases produced by decomposition of Al(OH)(CH3COO)2·H2O and the NH4NO3 by-product during calcination, which prevent agglomeration of Al2O3 particles. The heat released by decomposition of NH4NO3 can also contribute to preserving the textural properties during transformation of boehmite to transition aluminas by lowering the transformation temperature.
(5) Other advantages of the proposed process are its versatility, the ability to obtain high purity materials without large amounts of by-products without the need for washing, energy saving by using a low processing temperature, and the possibility to recycle the generated CO2 and NH3 gases to form the (NH4)2CO3 reagent. It is also possible to use NaAlO2 waste solution from dissolution of aluminium hydroxide or bauxite.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic and the Slovak Academy of Sciences under contract VEGA 1/0176/19.References
- C. Liu, J. Li, K. Liew, J. Zhu and M. R. Bin Nordin, RSC Adv., 2012, 2, 8352–8358 RSC.
- J. Wang, K. Shang, Y. Guo and W. C. Li, Microporous Mesoporous Mater., 2013, 181, 141–145 CrossRef CAS.
- G. Wu, G. Liu, X. Li, Z. Peng, Q. Zhou and T. Qi, RSC Adv., 2019, 9, 5628–5638 RSC.
- R. Takahashi, A. Onishi, F. Sato and M. Kuramoto, J. Ceram. Soc. Jpn., 2017, 125, 742–746 CrossRef CAS.
- K. Zhang, Z. Fu, T. Nakayama, T. Suzuki, H. Suematsu and K. Niihara, Mater. Res. Bull., 2011, 46, 2155–2162 CrossRef CAS.
- T. F. Baumann, A. E. Gash, S. C. Chinn, A. M. Sawvel, R. S. Maxwell and J. H. Satcher Jr, Chem. Mater., 2005, 17, 395–401 CrossRef CAS.
- J. Y. Park, S. G. Oh, U. Paik and S. K. Moon, Mater. Lett., 2002, 56, 429–434 CrossRef CAS.
- R. López-Juárez, N. Razo-Perez, T. Pérez-Juache, O. Hernandez-Cristobal and S. Y. Reyes-López, Results Phys., 2018, 11, 1075–1079 CrossRef.
- G. C. Li, Y. Q. Liu, L. L. Guan, X. F. Hu and C. G. Liu, Mater. Res. Bull., 2012, 47, 1073–1079 CrossRef CAS.
- T. E. Bell, J. M. Gonzáles-Carballo, R. P. Tooze and L. Torrente-Murciano, RSC Adv., 2017, 7, 22369–22377 RSC.
- K. Ullmann, P. Ádám and K. Sinkó, J. Non-Cryst. Solids, 2018, 499, 394–400 CrossRef CAS.
- L. Samain, A. Jaworski, M. Edén, D. M. Ladd, D. K. Seo, F. J. Garcia-Garcia and U. Häussermann, J. Solid State Chem., 2014, 217, 1–8 CrossRef CAS.
- A. R. Ferreira, E. Küçükbenli, A. A. Leitão and S. De Gironcoli, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84(23), 235119 CrossRef.
- D. C. Shin, S. S. Park, J. H. Kim, S. S. Hong, J. M. Park, S. H. Lee, D. S. Kim and G. D. Lee, J. Ind. Eng. Chem., 2014, 20, 1269–1275 CrossRef CAS.
- R. Rogojan, E. Andronescu, C. Ghitulicā and B. S. Vasile, U.P.B. Sci. Bull., 2011, 73, 67 CAS.
- A. Cheraitia, A. Ayral, A. Julbe, V. Rouessac and H. Satha, J. Porous Mater., 2010, 17, 259–263 CrossRef CAS.
- P. Pentyala, M. Shahid, S. Ramamirtham and M. G. Basavaraj, Colloids Surf., A, 2018, 544, 172–178 CrossRef CAS.
- H. Kong, J. K. Lee and S. J. Lee, J. Ceram. Process. Res., 2017, 18, 726–730 Search PubMed.
- K. Roy, C. Jatejarungwong and P. Potiyaraj, J. Appl. Polym. Sci., 2018, 135, 46248–46256 CrossRef.
- L. Lafficher, M. Digne, F. Salvatori, M. Boualleg, D. Colson and F. Puel, Powder Technol., 2017, 320, 565–573 CrossRef.
- V. A. Matveev and D. V. Mairov, Russ. J. Inorg. Chem., 2019, 64(4), 438–444 CrossRef CAS.
- P. Wang, Z. D. Zhao, L. W. Bi, Y. X. Chen, L. Zhang and L. T. Sun, Mater. Res. Innovations, 2012, 16(2), 121–125 CrossRef CAS.
- X. Hu, Y. Liu, Z. Tang, G. Li, R. Zhao and C. Liu, Mater. Res. Bull., 2012, 47(12), 4271–4277 CrossRef CAS.
- P. Billik and B. Horváth, Inorg. Chem. Commun., 2008, 11, 1125–1127 CrossRef CAS.
- G. R. Karagedov, Chem. Sustainable Dev., 2011, 19, 339–345 Search PubMed.
- G. R. Karagedov, S. S. Kosolobov, A. V. Latyshev, N. Z. Lyakhov and A. L. Myz, Chem. Sustainable Dev., 2013, 21, 603–608 Search PubMed.
- W. Wang, W. Liu, I. Y. Chang, L. A. Wills, L. N. Zakharov, S. W. Boettcher, P. H. Y. Cheong, C. Fang and D. A. Keszler, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(46), 18397–18401, DOI:10.1073/pnas.1315396110.
- M. A. Hubbe, J. R. Metts, D. Hermosilla, M. A. Blanco, L. Yerushalmi, F. Haghighat, P. Lindholm-Lehto, Z. Khodaparast, M. Kamali and A. Elliott, BioResources, 2016, 11(3), 7953–8091 Search PubMed.
- S. Abeysinghe, D. K. Unruh and T. Z. Forbes, Cryst. Growth Des., 2012, 12(4), 2044–2051 CrossRef CAS.
- C. V. Chandran, C. E. A. Kirschhock, S. Radhakrishnan, F. Taulelle, J. A. Martens and E. Breynaert, Chem. Soc. Rev., 2019, 48(1), 134–156, 10.1039/c8cs00321a.
- T. Sato, S. Ikoma and F. Ozawa, Thermochim. Acta, 1984, 75, 129–137 CrossRef CAS.
- M. Olszak-Humienik, Thermochim. Acta, 2001, 378, 107–112 CrossRef CAS.
- A. Aghaeinejad-Meybodi, A. Ebadi, S. Shafiei, A. Khataee and A. D. Kiadehi, Sep. Purif. Technol., 2019, 211, 551–563 CrossRef CAS.
- Z. Zidi, M. Ltifi, Z. Ben Ayadi and L. El Mir, J. Australas. Ceram. Soc., 2019, 7(4), 524–535 CrossRef.
- S. Ali, Y. Abbas, Z. Zuhra and I. S. Butler, Nanoscale Adv., 2019, 1, 213–218 RSC.
- H. Koopi and F. Buazar, Ceram. Int., 2018, 44(8), 8940–8945 CrossRef CAS.
- T. F. Kemp and M. E. Smith, Solid State Nucl. Magn. Reson., 2009, 35(4), 243–252 CrossRef CAS.
- T. Shirai, H. Watanabe, M. Fuji and M. Takahashi, J. Mater. Sci., 2009, 9, 23–31 Search PubMed.
- Y. Li, J. Su and R. Li, Microporous Mesoporous Mater., 2017, 243, 9–15 CrossRef CAS.
- J. Landers, G. Y. Gor and V. Neimark, Colloids Surf., A, 2013, 437, 3–32 CrossRef CAS.
- R. Laucournet, C. Pagnoux, T. Chartier and J. F. Baumard, J. Am. Ceram. Soc., 2000, 83(11), 2661–2667 CrossRef CAS.
- P. Persson, M. Karlsson and L. O. Öhman, Geochim. Cosmochim. Acta, 1998, 62(23/24), 3657–3668 CrossRef CAS.
- A. R. Barron, Dalton Trans., 2014, 43, 8127–8143 RSC.
- X. Wang, Ch. Li, Z. Shi, M. Zhi and Z. Hong, RSC Adv., 2018, 8, 8011–8020 RSC.
- Y. Bang, S. J. Han, J. Yoo, J. H. Choi, J. K. Lee, J. H. Song, J. Lee and I. K. Song, Appl. Catal., B, 2014, 148–149, 269–280 CrossRef CAS.
- S. Bi, C. Wang, Q. Cao and C. Zhang, Coord. Chem. Rev., 2004, 248, 441–455 CrossRef CAS.
- T. Sugimoto, Monodispersed Particles, Elsevier Science B.V., Amsterdam, 2001, pp. 273–275 Search PubMed.
- L. Zhang, X. Jiao, D. Chen and M. Jiao, Eur. J. Inorg. Chem., 2011, 2011(34), 5258–5264 CrossRef CAS.
- N. Nayak, N. Vitorino, J. R. Frade, A. V. Kovalenski, V. D. Alves, J. G. Grespo and C. A. M. Portugal, Mater. Des., 2018, 157, 119–129 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |