Open Access Article
Open Access ArticleThe application of “plug-in molecules” method in novel strobilurin fungicides screening†
Xuelian Liu‡
 ,
Dongyan Yang‡
,
Dongyan Yang‡ ,
Fahong Yin,
Jia-Qi Li
,
Fahong Yin,
Jia-Qi Li ,
Yumei Xiao,
Bin Fu and
Zhaohai Qin
,
Yumei Xiao,
Bin Fu and
Zhaohai Qin *
*
College of Sciences, China Agricultural University, Beijing 100193, China. E-mail: qinzhaohai@263.net
First published on 24th November 2020
Abstract
Based on the “plug-in molecular” method, a series of novel strobilurin derivatives with aryloxypyridinyl-1-ethanone oxime side chains were designed, synthesized, and screened. The biological activity experiment showed that they had an excellent fungicidal effect on plant pathogens, especially Sclerotinia sclerotiorum. Compounds 5-01 and 5-09 had significant fungicidal activity and broad fungicidal spectrum. The structure–activity relationship indicates that the cis configuration, increasing the number of pharmacophores, substitution of the 2 position of the pyridine ring, and the introduction of chlorine atom on the benzene ring were not conducive to the fungicidal activity of such compounds. The model of 3D-QSAR indicated the introduction of large electropositive groups at the 4 position of the benzene ring and the introduction of small electronegative groups at the 2 position of the benzene ring were beneficial to the fungicidal activity, and compounds 6 were designed. Compared with azoxystrobin, compound 6-02 had a more effective fungicidal effect against Sclerotinia sclerotiorum (Lib.) de Bary. Cytotoxicity test and transmission electron microscopy showed that the modification of strobilurins fungicide by the “plug-in molecular” method would not affect its toxicity and mechanism. The “plug-in molecular” method is an efficient method for screening highly active compounds, which has important guiding significance for creating new pesticide molecules.
Introduction
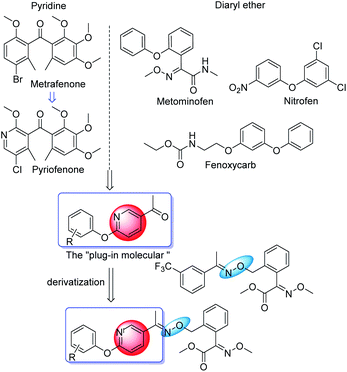
The “plug-in molecular” method is a new pesticide molecular design method proposed by Qin et al.1 It is mainly to obtain high-frequency active groups through screening and design them as easily derived “molecular plug-in” and then through simple chemical reactions to derive various highly active compounds. Based on the excellent biological activity of diaryl ether and pyridine, a series of aryloxypyridine compounds (compounds 3) has been designed and synthesized as “molecular plug-in” for deriving highly active compounds.Diaryl ether compounds are a regular class of organic compounds with a wide range of biological activities such as insecticidal,2 bactericidal,3 and herbicidal activities. The most famous of these is the diphenyl ether herbicide such as fomesafen.4,5 Diphenyl ether herbicides,6 a protoporphyrinogen oxidase (PPO) inhibitor,7 have the advantages of high efficiency, broad-spectrum and safety to crops and the environment, as well as occupy an extremely significant position in the herbicide market. Compared with herbicidal activity, people paid less attention to the fungicidal and insecticidal activities of these compounds. However, due to its unique physical and chemical properties and biological activity, diaryl ethers have been widely used as active groups in pesticide creation.8–11 For example, the introduction of diphenyl ether structure in the alcohol part of pyrethroid solved the problem of poor light stability.12 Moreover, the introduction of diphenyl ether structure in benzoylurea insecticides reduced the toxicity to 50% of the original.13 At present, the diaryl ether structure has been applied to various pesticide varieties such as pyrethroid insecticides, carbamate insecticides and triazole fungicides, and has been important biologically active groups in creating new pesticides.
With the development of heterocyclic compounds, replacing benzene with pyridine has become an important research direction.14,15 Compared with benzene, pyridine has a lower hydrophobic constant,16 and the compounds where the benzene ring was replaced by a pyridine ring, as well as had a higher biological activity or higher stability or higher selectivity or lower toxicity. For example, pyriofenone, a metrafenone analog, has excellent fungicidal activity, especially for Botrytis cinerea. Pyriprole, a fipronil analog, still has good insecticidal activity. Bicyclopyrone has a significant herbicidal activity after the introduction of a pyridine structure.17
Strobilurins are a type of broad-spectrum fungicide, which has excellent fungicidal effects on almost all fungal diseases.18,19 Compared with other strobilurins, trifloxystrobin has the advantages of fast penetration, good systemic absorption, rapid distribution, resistance to rain washout, and long shelf life.20–23 To obtain highly active fungicides, a series of new compounds were synthesized by introducing the core structure of trifloxystrobin to the derivatize of the “plug-in molecular” of aryloxypyridine (Scheme 1). Biological activity experiments showed that these compounds had excellent fungicidal activity.
Results and discussion
Chemistry
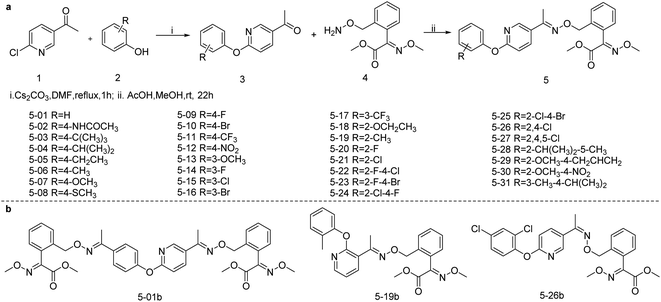
The synthetic route of targeted compounds 5 from commercially available starting materials is outlined in Scheme 2. As reported in the literature,24,25 precursor 4 was prepared from methyl (E)-2-(2-(bromomethyl)phenyl)-2-(methoxyimine)acetate and N-hydroxyphthalimide by nucleophilic substitution and hydrazidation. The condensation reaction of compounds 3 and 4 formed a carbon–nitrogen double bond; however, due to the steric hindrance, the products were mainly in trans configuration. Therefore, the targeted compounds 5 in this article were all in trans arrangement unless otherwise specified.To explore the influence of configuration on the biological activity of compounds 5-26, the cis-configuration compound 5-26b was isolated and purified from the reaction system of compound 5-26. The 1H NMR spectra of compounds 5-26 (E configuration) and 5-26b (Z configuration) displayed were not different, whereas the 13C NMR spectra showed a slight difference (Fig. S1†). In addition, the influence of the number of pharmacophores and the position of substituents on the pyridine ring with regards to the biological activity of the targeted compounds were studied, and the control drugs 5-01b and 5-19b were synthesized (Scheme 2b and S1†).
Structure–activity relationship (SAR)
A series of compounds were designed and synthesized by introducing the pharmacophore of strobilurins to the derivatize of the “molecular plug-in” of aryloxypyridine by the “plug-in molecular” method, and their in vitro antifungal activities are shown in Tables 1 and 2. Targeted compounds 5 showed medium fungicidal activities against seven phytopathogens, except Colletotrichum orbiculare and Rhizoctonia solani, which was consistent with the lead compound trifloxystrobin. Most of these compounds had an excellent fungicidal activity to Sclerotinia sclerotiorum, and the inhibition rate was more than 90% at 50 μg mL−1. Compounds 5-01, 5-06, 5-09 and 5-14 had a broad fungicidal spectrum and showed good fungicidal activity against Rhizoctonia solani, Phytophthora infestans (Mont.) de Bary (Phytophthora infestans), Pyricularia grisea, Botrytis cinerea, and Pythium aphanidermatum. The fungicidal activity of compound 5-01b was significantly lower than that of the other 4-substituted compounds, indicating that increasing the number of methoxyacrylate pharmacophores was detrimental to the fungicidal activity of these compounds (Fig. 1A). No significant difference of fungicidal activity between compounds 5-19 and 5-19b was observed, which indicated that the position of the substituent on the pyridine ring had no significant effect on the activity of these compounds (Fig. 1B). Compared with the trans-configuration compound 5-26, the cis-configuration compound 5-26b had lower fungicidal activity, especially against Sclerotinia sclerotiorum. For Sclerotinia sclerotiorum, the inhibition rate of compound 5-26 could reach 92%, and the inhibition rate of compound 5-26b was only 56% (Fig. 1C).| Compd | Mycelium growth inhibitory rate (%) at 50 μg mL−1 | Compd | Mycelium growth inhibitory rate (%) at 50 μg mL−1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | BC | PI | PA | RS | PG | CO | SS | BC | PI | PA | RS | PG | CO | ||
| a SS = Sclerotinia scleotiorum; BC = Botrytis cinerea; PI = Phytophthora infestans; PA = Pythium aphanidermatum; RS = Rhizoctonia solani; PG = Pyricularia grisea; CO = Colletotrichum orbiculare. | |||||||||||||||
| 5-01 | 94.18 | 62.64 | 54.81 | 63.07 | 40.63 | 55.94 | 34.70 | 5-19 | 95.77 | 56.90 | 50.03 | 49.44 | 33.85 | 43.85 | 27.33 |
| 5-02 | 74.67 | 46.38 | 58.06 | 64.02 | 36.67 | 65.63 | 42.65 | 5-20 | 85.33 | 47.83 | 51.13 | 45.12 | 44.17 | 48.44 | 33.82 |
| 5-03 | 84.66 | 42.07 | 40.46 | 45.00 | 30.21 | 36.90 | 22.06 | 5-21 | 78.67 | 56.52 | 54.84 | 49.24 | 36.67 | 56.25 | 44.12 |
| 5-04 | 87.83 | 42.53 | 42.05 | 45.83 | 31.25 | 41.71 | 22.59 | 5-22 | 92.06 | 45.98 | 48.43 | 48.89 | 35.42 | 40.64 | 20.48 |
| 5-05 | 94.18 | 48.28 | 44.18 | 52.33 | 37.50 | 41.18 | 24.17 | 5-23 | 88.36 | 47.70 | 48.96 | 45.28 | 35.42 | 39.04 | 22.06 |
| 5-06 | 96.00 | 60.72 | 61.61 | 45.12 | 43.33 | 42.19 | 33.82 | 5-24 | 53.33 | 63.77 | 54.84 | 48.93 | 38.33 | 51.56 | 42.65 |
| 5-07 | 89.42 | 56.32 | 49.50 | 53.89 | 35.94 | 48.66 | 27.86 | 5-25 | 89.95 | 40.23 | 45.77 | 43.33 | 33.85 | 41.71 | 19.96 |
| 5-08 | 91.53 | 47.70 | 44.18 | 45.56 | 33.85 | 47.59 | 27.33 | 5-26 | 92.06 | 54.02 | 43.65 | 42.78 | 35.42 | 37.43 | 19.96 |
| 5-09 | 95.24 | 63.79 | 48.96 | 59.73 | 35.94 | 54.45 | 27.33 | 5-27 | 95.24 | 37.93 | 39.39 | 43.89 | 31.77 | 42.25 | 27.33 |
| 5-10 | 69.33 | 46.38 | 56.45 | 46.65 | 33.33 | 46.88 | 33.82 | 5-28 | 52.00 | 36.23 | 48.39 | 46.65 | 29.50 | 50.00 | 37.35 |
| 5-11 | 93.12 | 44.83 | 44.71 | 48.33 | 36.46 | 45.45 | 26.80 | 5-29 | 57.33 | 34.78 | 50.00 | 48.02 | 36.17 | 50.00 | 48.53 |
| 5-12 | 80.95 | 51.15 | 46.84 | 46.11 | 31.25 | 41.18 | 28.38 | 5-30 | 86.67 | 43.48 | 45.16 | 39.02 | 31.17 | 39.06 | 29.41 |
| 5-13 | 96.00 | 49.28 | 46.77 | 48.17 | 30.67 | 45.31 | 22.06 | 5-31 | 93.65 | 44.31 | 39.39 | 45.28 | 31.25 | 39.57 | 20.06 |
| 5-14 | 92.93 | 60.07 | 50.00 | 50.76 | 35.00 | 58.75 | 41.18 | 5-01b | 51.85 | 30.46 | 36.20 | 39.45 | 26.04 | 39.57 | 31.02 |
| 5-15 | 96.83 | 48.28 | 45.24 | 50.00 | 31.77 | 42.25 | 24.70 | 5-19b | 82.03 | 35.78 | 41.95 | 47.89 | 32.57 | 43.89 | 20.05 |
| 5-16 | 94.67 | 44.93 | 46.77 | 43.60 | 25.00 | 43.75 | 20.59 | 5-26b | 56.08 | 47.70 | 42.05 | 45.00 | 27.60 | 35.29 | 20.48 |
| 5-17 | 93.33 | 42.03 | 46.77 | 42.68 | 28.33 | 40.63 | 26.47 | Trifloxystrobin | 100.00 | 70.12 | 52.68 | 63.33 | 40.63 | 62.78 | 24.17 |
| 5-18 | 46.67 | 56.52 | 54.84 | 54.88 | 39.50 | 55.31 | 49.12 | ||||||||
| Compd | EC50 (μg mL−1) | 95% FL (μg mL−1) | pEC50 | Predicted pEC50 | Compd | EC50 (μg mL−1) | 95% FL (μg mL−1) | pEC50 | Predicted pEC50 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CoMFA | CoMSIA | CoMFA | CoMSIA | ||||||||
| a Not determined. | |||||||||||
| 5-01 | 3.25 | 1.23–8.89 | 5.125 | 5.335 | 5.371 | 5-17 | 2.28 | 1.29–3.87 | 5.342 | 5.318 | 5.346 |
| 5-02 | 30.65 | 14.35–55.32 | 4.204 | 4.274 | 4.369 | 5-18 | 56.97 | 38.89–91.20 | 3.923 | 3.862 | 3.833 |
| 5-03 | 1.35 | 0.61–2.56 | 5.560 | 5.531 | 5.551 | 5-19 | 0.90 | 0.58–1.32 | 5.697 | 4.814 | 4.993 |
| 5-04 | 2.11 | 0.82–4.90 | 5.353 | 5.423 | 5.612 | 5-20 | 1.43 | 0.78–2.38 | 5.499 | 5.342 | 5.318 |
| 5-05 | 1.06 | 0.56–1.75 | 5.639 | 5.702 | 5.660 | 5-21 | 4.91 | 2.25–8.50 | 4.979 | 4.972 | 5.294 |
| 5-06 | 0.47 | 0.18–0.92 | 5.979 | 5.788 | 5.546 | 5-22 | 2.05 | 1.32–3.09 | 5.375 | 5.557 | 5.416 |
| 5-07 | 2.20 | 1.23–3.82 | 5.324 | 5.323 | 5.339 | 5-23 | 1.18 | 0.28–3.07 | 5.653 | 5.599 | 5.391 |
| 5-08 | 1.37 | 0.75–2.27 | 5.544 | 5.516 | 5.462 | 5-24 | 32.65 | 11.32–54.68 | 4.173 | 4.244 | 4.343 |
| 5-09 | 1.44 | 0.75–2.48 | 5.496 | 5.405 | 5.441 | 5-25 | 8.32 | 4.57–18.90 | 4.818 | 4.849 | 4.887 |
| 5-10 | 1.08 | 0.11–3.11 | 5.676 | 5.662 | 5.414 | 5-26 | 1.33 | 0.36–3.35 | 5.577 | 5.224 | 5.365 |
| 5-11 | 2.17 | 0.95–4.60 | 5.364 | 5.790 | 5.591 | 5-27 | 3.60 | 1.66–8.55 | 5.174 | 5.123 | 5.289 |
| 5-12 | 0.81 | 0.42–1.21 | 5.771 | 5.709 | 5.765 | 5-28 | 84.58 | 37.32–170.71 | 3.763 | 3.859 | 3.996 |
| 5-13 | 4.14 | 1.38–7.59 | 5.049 | 5.056 | 5.063 | 5-29 | 15.62 | 7.14–33.30 | 4.508 | 4.588 | 4.568 |
| 5-14 | 0.87 | 0.14–3.47 | 5.715 | 5.691 | 5.644 | 5-30 | 0.85 | 0.40–1.51 | 5.750 | 5.806 | 5.717 |
| 5-15 | 17.21 | 6.52–36.06 | 4.434 | 5.277 | 5.284 | 5-31 | 11.42 | 5.81–31.89 | 4.632 | 5.564 | 5.754 |
| 5-16 | 2.63 | 1.34–8.70 | 5.290 | 5.300 | 5.300 | Trifloxystrobin | 0.14 | 0.06–0.27 | —a | — | — |
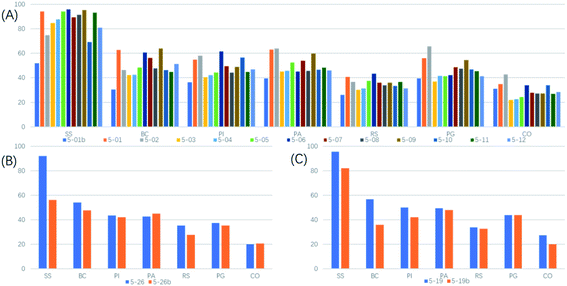 | ||
| Fig. 1 The effects of the number of pharmacophores (A), the configuration of the compound (B) and the position of the substituent on the pyridine ring (C) on seven pathogenic fungi were compared. | ||
To further study the activities of the targeted compounds against Sclerotinia sclerotiorum, the EC50 values of all the targeted compounds were tested. The number of substituents had a significant influence on the activity of the compounds. The reaction of bi-substituted compounds was worse than that of monosubstituted compounds, and the compounds containing two-electron substituents had the worst combination such as 5-28, 5-29, and 5-31. For monosubstituted compounds, the position of the substituent had no significant effect on the activity. Introducing a large substituent at the 3-position of the benzene ring instead of the 2-position was beneficial to the fungicidal activity of the compound. For the four substituted compounds, the steric hindrance and electrostatic effect of the substituents had no significant results on the reaction of the compounds. Compared with other substituents, the introduction of a chlorine atom into the benzene ring was not beneficial to the activity of the compounds. Compound 5-06 had the best fungicidal activity against Sclerotinia sclerotiorum with the EC50 values of 0.47 μg mL−1, which was comparable to trifloxystrobin.
Quantitative structure–activity relationship (QSAR) analyses
To optimize the structure of the compounds and obtain compounds with higher fungicidal activity, the 3D-QSAR protocol of Sybyl 7.3 was used to establish the model. The model parameters for CoMFA and CoMSIA are shown in Table S1.† The cross-validation q2 values of CoMFA and CoMSIA were 0.552 and 0.548, respectively. RMSE residual errors were reported to be 0.168 (CoMFA) and 0.236 (CoMSIA). q2 > 0.5 and RESE < 0.5 indicated that the built QSAR models were acceptable.26 The non-cross-validation r2 values were 0.888 (CoMFA) and 0.791 (CoMSIA), indicating that the model had a good predictive ability.26 The pEC50 values predicted by CoMFA and CoMSIA model for all 31 compounds are presented in Table 2. The predicted and experimental values of the two models had a good linear relationship. These proved that it was feasible to use the models to predict the fungicidal effect of virtual compounds (Fig. S3†).The two models indicated that steric and electrostatic fields were the primary factors in fungicidal ability. Fig. 2 shows the molecules aligned to the iso-surface of 3D-QSAR models on the electrostatic potential grid and van der Waals grid. For the electrostatic field, the low electron density favorable regions are shown in blue, and the high electron density regions are shown in red. Steric map indicating areas where steric bulk is predicted to increase (green) or decrease (yellow) activity.25 Compound 5-06 with the highest activity was selected to explain the contour map. The introduction of a big steric substituent at the 3-position and 4-position (green) and a small steric substituent at the 2-position (yellow) of the benzene ring was conducive to fungicidal activity (Fig. 2A and C). In the electrostatic field, electropositive substituents were introduced at the 3-position and 4-position (blue) of the benzene ring, whereas electronegative substituents were introduced at the 2-position (red) of the benzene ring, which was beneficial to antifungal activity (Fig. 2B and D). Therefore, compounds 5-18 that introduced the ethoxy group (large electropositive group) at the 2 position of the benzene ring had very inferior fungicidal activity. Compounds 5-03, 5-05, 5-06 and 5-08, which introduced large electropositive groups into the 4-positions of the benzene ring, had excellent fungicidal activity.
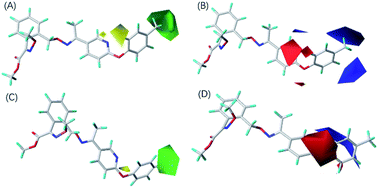 | ||
| Fig. 2 CoMFA and CoMSIA contour maps of compound ZNQ-17069 (A) CoMFA steric field (B) CoMFA electrostatic field (C) CoMSIA steric field (D) CoMSIA electrostatic field. | ||
Based on the analysis results of the above structure–activity relationship, some model compounds were designed. Their potential activities were predicted with the built QSAR models, two compounds (compounds 6-01 and 6-02) with better activity were selected for synthesis (Scheme 3 and S2†). The predicted data and experiment data of compounds 6 are shown in Table 3. Among them, compound 6-02 had a more excellent fungicidal effect on Sclerotinia sclerotiorum than compound 5-06. The EC50 value of compound 6-02 was 0.08 μg mL−1, which was ten times lower than the commercial drug azoxystrobin (EC50 = 0.79 μg mL−1). Not only that, compounds 6-01 and 6-02 had a broad fungicidal spectrum and had a good inhibitory effect on seven plant pathogens at 50 μg mL−1 (Table 4).
| Compd | Mycelium growth inhibitory rate (%) at 50 μg mL−1 | ||||||
|---|---|---|---|---|---|---|---|
| SS | BC | PI | PA | RS | PG | CO | |
| a SS = Sclerotinia scleotiorum; BC = Botrytis cinerea; PI = Phytophthora infestans; PA = Pythium aphanidermatum; RS = Rhizoctonia solani; PG = Pyricularia grisea; CO = Colletotrichum orbiculare. | |||||||
| 5-06 | 96.00 | 60.72 | 61.61 | 45.12 | 43.33 | 42.19 | 33.82 |
| 6-01 | 76.00 | 42.03 | 56.45 | 47.71 | 39.50 | 48.44 | 43.68 |
| 6-02 | 97.35 | 55.17 | 68.43 | 50.83 | 34.90 | 68.44 | 29.44 |
| Azoxystrobin | 97.88 | 81.61 | 58.53 | 61.67 | 41.15 | 64.39 | 38.92 |
In addition, to screen the activities of the compounds, five compounds were selected for precise virulence measurements for six phyto-fungi, and their EC50 values are shown in Table 5. These compounds had good antifungal activity on Botrytis cinerea, Phytophthora infestans, Pythium aphanidermatum and Pyricularia grisea, especially on Pyricularia grisea. Among them, the EC50 value of compound 5-01 against the four phytopathogens was generally <20 μg mL−1. This indicated that compound 5-01 had a broader and better fungicidal spectrum than others. The EC50 value of compound 5-09 against Botrytis cinerea was 6.59 μg mL−1, which was better than that of commercial agents. The EC50 values of compounds 5-01, 5-06 and 6-02 against Phytophthora infestans were 17.29, 21.49 and 12.60 μg mL−1, which were better than trifloxystrobin. The EC50 values of compounds 5-01 and 5-09 against Pythium aphanidermatum were 18.14 and 20.30 μg mL−1, respectively, which were better than azoxystrobin. The EC50 values of compounds 5-06 and 6-02 against Pyricularia grisea were 8.42 and 6.86 μg mL−1, which were comparable to those of control drugs.
| Compd | BC | PI | PA | RS | PG | CO |
|---|---|---|---|---|---|---|
| a BC = Botrytis cinerea; PI = Phytophthora infestans; PA = Pythium aphanidermatum; RS = Rhizoctonia solani; PG = Pyricularia grisea; CO = Colletotrichum orbiculare. | ||||||
| 5-01 | 14.16 | 17.29 | 18.14 | >200 | 10.10 | >200 |
| 5-06 | 20.57 | 21.49 | 131.90 | >200 | 8.42 | >200 |
| 5-09 | 6.59 | 72.75 | 20.30 | >200 | 23.14 | >200 |
| 5-14 | 23.61 | 63.29 | 63.13 | >200 | 15.28 | >200 |
| 6-02 | 29.66 | 12.60 | 43.92 | >200 | 6.86 | >200 |
| Azoxystrobin | 12.26 | 9.11 | 21.37 | 97.78 | 4.20 | 130.55 |
| Trifloxystrobin | 13.38 | 17.07 | 7.34 | >200 | 3.35 | >200 |
To further verify the potential of the targeted compound as a commercial drug, we first assessed the potential cytotoxicity of 5-01 and 6-02 through CCK-8 assays. Hela cells were exposed to 5-01 or 6-02 for 24 h. Consistent with strobilurins fungicides, compounds 5-01 and 6-02 showed low cytotoxicity. At a dose of 100 μg mL−1, compound 5-01 reduced cell viability to 52% of the control group. However, compound 6-02 showed significantly less cytotoxicity, i.e., 100 μg mL−1 dose showed ∼92% cell viability (Fig. S4†). Moreover, the results of transmission electron microscopy showed that the mitochondria of Sclerotinia sclerotiorum mycelium were damaged when exposed to compound 5-01 at a dose of 50 μg mL−1 (Fig. S5†). These results indicate that the compounds were expected to become potential fungicides, and the modification of strobilurins fungicide by the “plug-in molecular” method improves the activity of the compound without affecting its toxicity and mechanism.
Conclusions
A series of new compounds were synthesized by introducing the strobilurins pharmacophore to the derivatize of the “plug-in molecular” of aryloxypyridine, and their antifungal activity experiments showed that they had excellent control effects on plant pathogens, especially on Sclerotinia sclerotiorum. The results of control experiments showed that the trans configuration of the compounds had superior fungicidal effects. Furthermore, the introduction of more pharmacophores in the compound or substitution at the 2-position of the pyridine ring rather than the 6-position were all not conducive to their fungicidal activities. The 3D-QSAR models indicated that large electropositive groups were introduced into the 3 position and 4 position of the benzene rings, as well as a small electronegative group that was introduced into the 2 position of the benzene rings was beneficial to the fungicidal activity of the compounds. Based on this, the structures of high activity compounds were obtained. The result of the experiment indicated that predicted compounds 6-01 did show excellent fungicidal activity, and its fungicidal activity on Sclerotinia sclerotiorum was even ten times that of azoxystrobin while maintaining low cytotoxicity. Compounds 5-01 and 5-09 showed better fungicidal activity than the commercial drugs on some plant pathogens, highlighting that they might be potential fungicidal candidates. In general, it is feasible to obtain highly active compounds through the “plug-in molecular” method, which has important guiding significance for creating new pesticide molecules.Experimental
Instrumentation and materials
1H NMR and 13C NMR spectra were obtained at 300 MHz using a Bruker Avance DPX300 spectrometer in CDCl3 or DMSO-d6 solution with tetramethylsilane as the internal standard. Chemical shift values (δ) are given in parts per million. High-resolution mass spectrometry data were obtained with an Accurate-Mass-Q-TOF MS 6520 system equipped with an electrospray ionization (ESI) source. Melting points were determined with a Cole-Parmer microscope melting point apparatus and were uncorrected. All reagents and solvents were commercially available and used directly without further purification. (E)-Methyl 2-(2-(bromomethyl)phenyl)-2-(methoxyimino)acetate was obtained from Jiangsu Freychem Co. Intermediates 3 and 4 was synthesized according to literature reports. Experimental details are shown in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21877125).Notes and references
- C. Che, D. Yang, C. Wan, J. Wang, X. Liu, F. Zhao and Z. Qin, Chin. J. Pestic. Sci., 2017, 19, 533–542 Search PubMed.
- R. J. Brown, D. M.-T. Chan, M. H. Howard, D. J. Daniel, D. A. Clark and T. P. Selby, Arthropodicidal and fungicidal cyclic amides [triazolones] and their preparation, use, and compositions, WO9823155A1, 1998.
- M. Suzuki, T. Nagatomi, N. Sakamoto, K. Tsushima and K. Umeda, Preparation of pyridine derivatives as insecticides and acaricides, JP08208551A, 1996.
- L. F. Cieslik, R. A. Vidal and M. M. Trezzi, Acta Sci., Agron., 2014, 36, 329–334 CrossRef CAS.
- Z. Huang, H. Cui, C. Wang, T. Wu, C. Zhang, H. Huang and S. Wei, Pestic. Biochem. Physiol., 2020, 165, 104560 CrossRef CAS.
- S. O. Duke, J. M. Becerril, T. D. Sherman, J. Lydon and H. Matsumoto, Pestic. Sci., 1990, 30(4), 367–378 CrossRef CAS.
- O. Han, O. Kim, C. Kim, R.-D. Park and J.-O. Gah, Bull. Korean Chem. Soc., 1995, 16, 1013–1014 CAS.
- G. Cheng, S. P. Muench, Y. Zhou, G. A. Afanador, E. J. Mui, A. Fomovska, B. S. Lai, S. T. Prigge, S. Woods, C. W. Roberts, M. R. Hickman, P. J. Lee, S. E. Leed, J. M. Auschwitz, D. W. Rice and R. McLeod, Bioorg. Med. Chem. Lett., 2013, 23, 2035–2043 CrossRef CAS.
- J. S. Freundlich, J. W. Anderson, D. Sarantakis, H.-M. Shieh, M. Yu, J.-C. Valderramos, E. Lucumi, M. Kuo, W. R. Jacobs Jr, D. A. Fidock, G. A. Schiehser, D. P. Jacobus and J. C. Sacchettini, Bioorg. Med. Chem. Lett., 2005, 15, 5247–5252 CrossRef CAS.
- J. J. Kennedy-Smith, N. Arora, J. R. Billedeau, J. Fretland, J. Q. Hang, G. M. Heilek, S. F. Harris, D. Hirschfeld, H. Javanbakht, Y. Li, W. Liang, R. Roetz, M. Smith, G. Su, J. M. Suh, A. G. Villasenor, J. Wu, D. Yasuda, K. Klumpp and Z. K. Sweeney, RSC Med. Chem., 2010, 1, 79–83 RSC.
- J. Zhang, Z. Kang, J. Yang, M. Li and C. Liu, Nongyao, 2011, 50, 313–319 CAS.
- K. Fujimoto, N. Itaya, Y. Okuno, T. Kadota and T. Yamaguchi, Agric. Biol. Chem., 1973, 37, 2681–2682 CrossRef CAS.
- D. M. Suckling, L. Kuijpers and D. J. Rogers, Proc. N. Z. Weed Pest Control Conf., 1985, 38, 45–49 Search PubMed.
- C. Mu and Z. Qin, Xiandai Nongyao, 2003, 2, 4–10 CAS.
- C. Mu and Z. Qin, Xiandai Nongyao, 2003, 2, 1–6 CAS.
- W. Zhao, J. Wang, D. Yuan, T. Luo and Z. Li, Nongyao, 2002, 41, 8–11 CAS.
- J. Yang, R. Dai, Y. Liu, Q. Wu and C. Liu, Nongyao, 2011, 50, 625–629 CAS , 648.
- T. S. Thind, C. Mohan, P. Raj and J. K. Arora, Indian Phytopathol., 2004, 57, 104–106 CAS.
- Y.-m. Xiao, Y.-h. Wu, J.-p. Liu, Y.-f. Li, N. Li and Z.-h. Qin, Guangpuxue Yu Guangpu Fenxi, 2008, 28, 2370–2374 CAS.
- J. Chen, B. Loo and C. Ray, J. Agric. Food Chem., 2008, 56, 1829–1837 CrossRef CAS.
- R. B. K. Farber, K. M. Chin and N. Leadbitter, Pest Manage. Sci., 2002, 58, 261–267 CrossRef CAS.
- M. Reuveni, Can. J. Plant Pathol., 2001, 23, 52–59 CrossRef CAS.
- A. Santomauro, G. Tauro, C. Dongiovanni, C. Giampaolo, A. Abbatecola, M. Miazzi, H. Hajjeh and F. Faretra, Pflanzenschutz-Nachr. Bayer (Engl. Ed.), 2003, 56, 373–386 CAS.
- R. Ross, D. V. Nguyen, E. M. Szapacs, F. D. Smith and S. H. Shaber, US6348627B1, 2002.
- D. Yang, C. Wan, M. He, C. Che, Y. Xiao, B. Fu and Z. Qin, RSC Med. Chem., 2017, 8, 1007–1014 RSC.
- A. Junaid, F. P. L. Lim, L. H. Chuah and A. V. Dolzhenko, RSC Adv., 2020, 10, 12135–12144 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06263d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |