Open Access Article
Open Access ArticleSynthesis of selenated isochromenones by AgNO3-catalyzed three-component reaction of alkynylaryl esters, selenium powder and ArB(OH)2†
Guo-Qing Jin,
Wen-Xia Gao ,
Yun-Bing Zhou*,
Miao-Chang Liu
,
Yun-Bing Zhou*,
Miao-Chang Liu * and
Hua-Yue Wu
* and
Hua-Yue Wu
College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325035, People's Republic of China. E-mail: zyb@wzu.edu.cn; mcl@wzu.edu.cn
First published on 19th August 2020
Abstract
Reported is the AgNO3-catalyzed three-component reaction of alkynylaryl esters, selenium powder and ArB(OH)2, providing a facile entry to selenated isochromenones. This work highlights the use of selenium powder as a selenium reagent in the synthesis of selenated isochromenones for the first time.
Organoselenium compounds are of increasing importance as synthetic targets, largely owing to their applications in drugs,1 agriculture chemistry,2 catalysis,3 synthetic intermediates4 and materials.5 In light of their importance, the incorporation of an organoselenium group into organic heterocyclic skeletons has drawn growing attention in organic synthesis. In this context, the installation of a selenium moiety onto isochromenones which exhibit a range of biological activities would be meaningful, because the resultant selenated isochromenones can serve as potential lead candidates that may bring about a vast improvement in enhanced physical and biological properties. Despite the attractive properties provided by the introduction of a selenium moiety, the development of the methods for the synthesis of selenated isochromenones have been less reported. One of the most commonly used strategies for the preparation of selenated isochromenones relied on the intramolecular electrophilic addition of PhSeCl/RFCl to alkynylaryl esters.6 Nevertheless, selenenyl chlorides used as selenium reagents are usually unstable, costly and not easily available, eroding their overall appeal (Scheme 1a). Another access to selenated isochromenones via FeCl3-promoted electrophilic cyclization of alkynylaryl esters with ArSeSeAr was reported by Zeni's group (Scheme 1b).7 Recently, Du's group disclosed an efficient approach to in situ generate selenenyl chlorides from the reaction between diselenides and PhICl2, which enabled electrophilic intramolecular of alkynes to deliver selenated isochromenones (Scheme 1c).8 As far as we know, there have been no reports on the use of selenium powder as selenium reagent in the synthesis of selenated isochromenones.
The utilization of element selenium as selenium source in the synthesis of organoselenium compounds is undoubtedly attractive due to its easy availability and stability. As part of our ongoing interests in the construction of C–Se bond,9 we report the preparation of selenated isochromenones via radical cascade cyclization of 2-alkynylaryl esters, selenium powder and arylboronic acids (Scheme 1d). Our synthetic strategy provides an efficient method for the installation of selenium moiety onto the isochromenones scaffold. In addition, this methodology can construct a pyrone ring, two C–Se bonds and a C–O bond in a single step from cheap and accessible raw materials.
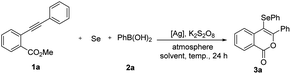
Our investigation began by selecting methyl 2-(phenylethynyl)benzoate (1a), selenium powder, and PhB(OH)2 as model substrates for optimization of reaction conditions (Table 1). Accordingly, when the three-component reaction was performed in dioxane at 120 °C under the air atmosphere in the presence of the catalytic system containing AgNO2 and K2S2O8, we observed the formation of the selenated product 3a in 89% yields (entry 1). The survey of silver salts revealed that the employment of AgNO3 as a catalyst afforded the best result (entry 2), while other silver salts such as AgSbF6 and Ag2SO4 also catalyzed the transformation albeit in inferior yields (entries 3 and 4). Further investigations of solvents including CH3CN, toluene, THF, CH3OH and DMF led to no improvements in the yields of the desired product (entries 5–9). It was found that the reaction temperature had a significant impact on the reaction outcome (entries 10–12). In addition, decreasing the loading of catalyst to 15 mol% had a negative effect on the yield of the desired product (entry 13). The reaction under the O2 atmosphere gave a similar yield to that under the air atmosphere (entry 14). The use of N2 atmosphere resulted in the formation of 3a in low yield with about 50% of 1a being recovered (entry 15). In the absence of an Ag catalyst, the reaction didn't take place (entry 16). Therefore, the conditions employed in entry 2 proved to be optimal.
| Entry | Catalyst | Solvent | Temp (°C) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), selenium powder (0.6 mmol), 2a (0.6 mmol), catalyst (0.06 mmol), K2S2O8 (0.45 mmol), solvent (2.0 mL), under air atmosphere, isolated yield.b AgNO3 (15 mol%).c Under the O2 atmosphere.d Under the N2 atmosphere. | ||||
| 1 | AgNO2 | Dioxane | 120 | 89 |
| 2 | AgNO3 | Dioxane | 120 | 92 |
| 3 | AgSbF6 | Dioxane | 120 | 46 |
| 4 | Ag2SO4 | Dioxane | 120 | 22 |
| 5 | AgNO3 | CH3CN | 120 | 23 |
| 6 | AgNO3 | Toluene | 120 | 49 |
| 7 | AgNO3 | THF | 120 | 65 |
| 8 | AgNO3 | CH3OH | 120 | 0 |
| 9 | AgNO3 | DMF | 120 | 30 |
| 10 | AgNO3 | Dioxane | 130 | 90 |
| 11 | AgNO3 | Dioxane | 110 | 85 |
| 12 | AgNO3 | Dioxane | 100 | 77 |
| 13b | AgNO3 | Dioxane | 120 | 71 |
| 14c | AgNO3 | Dioxane | 120 | 91 |
| 15d | AgNO3 | Dioxane | 120 | 46 |
| 16 | — | Dioxane | 120 | 0 |
With the optimal reaction conditions in hand, we turned our attention to exploring the generality of our method (Table 2). We started our investigations with organoboronic acids. The reaction was efficient for various arylboronic acids carrying a series of substituents on the phenyl ring. For example, the groups such as halogens, alkyls and trifluoromethyl at the para position of ring A were well tolerated and gave the expected products (3ba–3ha) in 36–92% yields. Arylboronic acids bearing at the meta- and ortho-position also yielded the desired products (3ia–3ma) efficiently. Note that shifting the substituents from the para position to the ortho position led to a reduced yield probably due to the increased steric hindrance (3la vs. 3fa; 3ma vs. 3ca). (2,4,6-trimethylphenyl)boronic acid was a suitable substrate for this transformation despite its steric repulsion. Additionally, arylboronic acid bearing a fused ring or a heterocyclic ring was also compatible, affording the corresponding products in 46% (3oa) and 53% (3pa) yields respectively. Next, the scope with respect to 2-alkynylaryl esters was assessed by reacting with 2a and selenium powder. Moderate to good yields were obtained (3ab–3ad) using substrates containing electron-withdrawing and electron-rich substituents on ring B (1b–1d). Ring B was also a fused ring (1e) and resulted in a good yield. The substituents such as chloro or methoxy group on the ring C (1f and 1g) were compatible with the reaction conditions. The three-component tandem cyclization reaction could be scaled up to 10 mmol scale as demonstrated with the synthesis of product 45.
| a Reaction conditions: 1 (0.3 mmol), selenium powder (0.6 mmol), 2 (0.6 mmol), AgNO3 (0.06 mmol), K2S2O8 (0.45 mmol), dioxane (2.0 mL), 120 °C, under the air atmosphere, isolated yields. |
|---|
 |
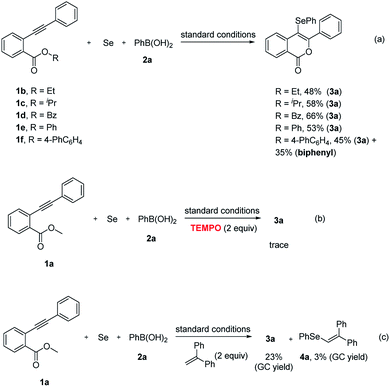
To further investigate the substituents on oxygen atom on the reaction efficiency, the methyl group in 1a was replaced by other substituents. It was found that the replacement of the methyl group by other groups such as ethyl, isopropyl, benzyl or phenyl respectively led to inferior yields (Scheme 2a). When the substituent on oxygen atom was changed into 4-PhC6H4, the reaction delivered a byproduct biphenyl in 35% yield aside from the desired product 3a. The presence of TEMPO could completely inhibit this reaction, supporting a radical way (Scheme 2b). The addition of ethene-1,1-diyldibenzene to the model reaction resulted in 23% yield of product 3a, as well as 3% yield of product 4a that was detected by GC-MS, suggesting that the reaction involved a PhSe radical intermediate (Scheme 2c).
Based on our experimental observations and previous studies,9,10 a plausible mechanism for the three-component reaction is proposed in Scheme 3. At the beginning, phenylboronic acid produces a phenyl radical (I) in the presence of AgNO3. The trapping of the phenyl radical by selenium powder provides a selenium-centred radical, followed by radical addition with 1a to generate intermediate III. The intermediate III undergoes intramolecular radical cyclization to afford the final product 3a as well as methyl radical which goes through H-abstraction to give methane.
Conclusions
In summary, we have disclosed an AgNO3-catalyzed radical cyclization of alkynylaryl esters, selenium powder and ArB(OH)2. The methodology displays good efficiency towards a variety of arylboronic acids and 2-alkynylaryl esters, and allows facile access to various selenated isochromenones. The three-component reactions are flexibly scalable and proceed via a radical way. Such a radical strategy in which selenium powder serves as the selenium reagent should contribute to constructing other valuable selenium-containing heterocycle skeletons.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (21901187 and 21672164).Notes and references
- (a) G. Mugesh, W. Mont and H. Sies, Chem. Rev., 2001, 101, 2125–2180 CrossRef CAS PubMed; (b) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255–6286 CrossRef CAS PubMed; (c) S. T. Manjare, Y. Kim and D. G. Churchill, Acc. Chem. Res., 2014, 47, 2985–2998 CrossRef CAS PubMed; (d) T. G. Back and Z. Moussa, J. Am. Chem. Soc., 2003, 125, 13455–13460 CrossRef CAS PubMed.
- (a) H. Cao, Y. Yang, X. Chen, J. Liu, C. Chen, S. Yuan and L. Yua, Chin. Chem. Lett., 2020, 31, 1887–1889 CrossRef CAS; (b) X. Mao, P. Li, T. Lia, M. Zhao, C. Chen, J. Liu, Z. Wang and L. Yu, Chin. Chem. Lett., 2020 DOI:10.1016/j.cclet.2020.06.033.
- (a) L. Shao, Y. Li, J.-M. Lu and X. Jiang, Org. Chem. Front., 2019, 6, 2999–3041 RSC; (b) L. Liao, R. Guo and X. Zhao, Angew. Chem., Int. Ed., 2017, 56, 3201–3205 CrossRef CAS PubMed; (c) R. Guo, J. Huang and X. Zhao, ACS Catal., 2018, 8, 926–930 CrossRef CAS; (d) X. Liu, Y. Liang, J. Ji, J. Luo and X. Zhao, J. Am. Chem. Soc., 2018, 140, 4782–5478 CrossRef CAS PubMed; (e) X.-J. Zhou, H.-Y. Liu, Z.-Y. Mo, X.-L. Ma, Y. Chen, H.-T. Tang, Y.-M. Pan and Y.-L. Xu, Chem.–Asian J., 2020, 15, 1536–1539 CrossRef CAS PubMed; (f) H. Cao, R. Qian and L. Yu, Catal. Sci. Technol., 2020, 10, 3113–3121 RSC; (g) C. Chen, Y. Cao, X. Wu, Y. Cai, J. Liu, L. Xu, K. Ding and L. Yu, Chin. Chem. Lett., 2020, 31, 1078–1082 CrossRef CAS.
- (a) J. H. Rigby, U. S. M. Maharoof and M. E. Mateo, J. Am. Chem. Soc., 2000, 122, 6624–6628 CrossRef CAS; (b) H. Azuma, S. Tamagaki and K. Ogino, J. Org. Chem., 2000, 65, 3538–3541 CrossRef CAS PubMed; (c) E. M. Treadwell, J. D. Neighbors and D. F. Wiemer, Org. Lett., 2002, 4, 3639–3642 CrossRef CAS PubMed; (d) K. B. Sharpless and R. F. Lauer, J. Am. Chem. Soc., 1973, 95, 2697–2699 CrossRef CAS; (e) T. Hori and K. B. Sharpless, J. Org. Chem., 1978, 43, 1689–1697 CrossRef CAS; (f) W. Dumont, P. Bayet and A. Krief, Angew. Chem., 1974, 86, 857 (Angew. Chem., Int. Ed., 1974, 13, 804–806) CrossRef CAS; (g) J. Remion, W. Dumont and A. Krief, Tetrahedron Lett., 1976, 17, 1385–1388 CrossRef; (h) D. Van Ende, W. Dumount and A. Krief, Angew. Chem., 1975, 87, 742 (Angew. Chem., Int. Ed., 1975, 14, 700–702) CrossRef; (i) D. Van Ende and A. Krief, Tetrahedron Lett., 1976, 17, 457–460 CrossRef; (j) M. Gruttadauri, C. Aprile, S. Riela and R. Noto, Tetrahedron Lett., 2001, 42, 2213–2215 CrossRef; (k) T. S. Chisholm, S. S. Kulkarni, K. R. Hossain, F. Cornelius, R. J. Clarke and R. J. Payne, J. Am. Chem. Soc., 2020, 142, 1090–1100 CrossRef CAS PubMed; (l) M. Liu, Y. Li, L. Yu, Q. Xu and X. Jiang, Sci. China: Chem., 2018, 61, 294–299 CrossRef CAS.
- (a) H. Xu, W. Cao and X. Zhang, Acc. Chem. Res., 2013, 46, 1647–1658 CrossRef CAS PubMed; (b) S.-Y. Jang, I.-B. Kim, M. Kang, Z. Fei, E. Jung, T. McCarthy-Ward, J. Shaw, D.-H. Lim, Y.-J. Kim, S. Mathur, M. Heeney and D.-Y. Kim, Adv. Sci., 2019, 6, 1900245–1900251 CrossRef PubMed; (c) L. Yu, H. Cao, X. Zhang, Y. Chen and L. Yu, Sustainable Energy Fuels, 2020, 4, 730–736 RSC.
- (a) Q. Glenadel, E. Ismalaj and T. Billard, Org. Lett., 2018, 20, 56–59 CrossRef CAS PubMed; (b) L. C. Wilkins, B. A. R. Ggnther, M. Walther, J. R. Lawson, T. Wirth and R. L. Melen, Angew. Chem., Int. Ed., 2016, 55, 11292–11295 CrossRef CAS PubMed; (c) E. C. Y. Woon, A. Dhami, M. F. Mahonb and M. D. Threadgill, Tetrahedron, 2006, 62, 4829–4837 CrossRef CAS; (d) T. Yao and R. C. Larock, J. Org. Chem., 2003, 68, 5936–5942 CrossRef CAS PubMed.
- A. Sperança, B. Godoi, S. Pinton, D. F. Back, P. H. Menezes and G. Zeni, J. Org. Chem., 2011, 76, 6789–6797 CrossRef PubMed.
- L. Xing, Y. Zhang, B. Li and Y. Du, Org. Lett., 2019, 21, 3620–3624 CrossRef CAS PubMed.
- (a) T. Leng, G. Wu, Y.-B. Zhou, W.-X. Gao, J. Ding, X. Huang, M. Liu and H. Wu, Adv. Synth. Catal., 2018, 360, 4336–4340 CrossRef CAS; (b) Y.-F. Yang, C.-Y. Li, T. Leng, X.-B. Huang, W.-X. Gao, Y.-B. Zhou, M.-C. Liu and H.-Y. Wu, Adv. Synth. Catal., 2020, 362, 2168–2172 CrossRef; (c) C. An, C.-Y. Li, X.-B. Huang, W.-X. Gao, Y.-B. Zhou, M.-C. Liu and H.-Y. Wu, Org. Lett., 2019, 21, 6710–6714 CrossRef CAS PubMed.
- (a) W. Liu, Y.-Q. Hu, X.-Y. Hong, G.-X. Li, X.-B. Huang, W.-X. Gao, M.-C. Liu, Y. Xia, Y.-B. Zhou and H.-Y. Wu, Chem. Commun., 2018, 54, 14148–14151 RSC; (b) H. Cao, M. Liu, R. Qian, X. Zhang and L. Yu, Appl. Organomet. Chem., 2019, 33, e4599 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06016j |
| This journal is © The Royal Society of Chemistry 2020 |