Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible light-induced photocatalytic C–H ethoxycarbonylmethylation of imidazoheterocycles with ethyl diazoacetate†
Suvam Bhattacharjee,
Sudip Laru,
Sadhanendu Samanta ,
Mukta Singsardar and
Alakananda Hajra
,
Mukta Singsardar and
Alakananda Hajra *
*
Department of Chemistry, Visva-Bharati (A Central University), Santiniketan, 731235, West Bengal, India. E-mail: alakananda.hajra@visva-bharati.ac.in; Web: http://www.visvabharati.ac.in/AlakanandaHajra.html
First published on 28th July 2020
Abstract
A visible light-mediated regioselective C3-ethoxycarbonylmethylation of imidazopyridines with ethyl diazoacetate (EDA) was achieved under mild reaction conditions. In contrast to the carbene precursors from α-diazoester a first C3-ethoxycarbonylmethylation of imidazopyridines via a radical intermediate has been established. The present methodology provides a concise route to access pharmacologically useful esters with wide functional group tolerance in high yields.
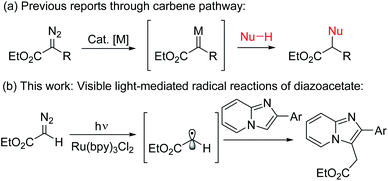
Ester groups are a most powerful and versatile synthetic building block in natural products as well as in organic synthesis since they can be derivatized to diverse functional groups such as carbonyl, hydroxymethyl, and amide, etc.1 Beside the traditional methods of using xanthates for ethoxycarbonylmethylation,2 the introduction of diazoesters represents a very important strategy for the synthesis of acetate derivatives.3 Generally, thermal,4a photoinduced,4b or metal catalyzed4c reactions of diazo compounds produce reactive carbene precursors which further undergo an addition reaction, insertion reaction, cyclopropanation, Wolff rearrangement, etc. (Scheme 1a).4 In contrast to the carbene precursors from α-diazoester, a new methodology is always highly desirable especially with a significantly different mode of activation under a distinct mechanism.5 In this context, a new strategy for installation of alkyl radicals from diazo compounds under photoredox-catalysis is very important.6 Recently, visible light-mediated photoredox catalyzed direct C–H bond functionalization reactions have come to the forefront in synthetic organic chemistry7 where Ru(II)-polypyridyl complexes have been extensively used as photoredox-catalysts due to their unique photo-physical properties.8
 | ||
| Scheme 1 Visible light-mediated ethoxycarbonylmethylation. (a) Previous reports through carbene pathway. (b) This work: visible-light mediated radical reaction of diazoacetate with imidazopyridine. | ||
Imidazo[1,2-a]pyridines, an important class of nitrogen-based privileged structural motifs widely found in a variety of natural products and alkaloids.9 In pharmaceutical chemistry, it possesses large number of biological activities like antitumor, antibacterial, antifungal, antipyretic, analgesic, antiinflammatory, etc.10a It is also important in material sciences due to its unique excited state intramolecular proton transfer phenomena (ESIPT).10b In particular, ethoxycarbonylmethylated imidazopyridines are the core structure of several marketed drugs such as alpidem, zolpidem, saripidem, etc.11 Therefore, practical methodologies for the synthesis of alkylated ester substituted imidazoheterocycles are highly desirable and will be of great interest to the synthetic chemists.11 However, to the best of our knowledge there is no such method for the direct photo-initiated ethoxycarbonylmethylation reaction of imidazoheterocycles with diazo compounds through distinct radical pathway.11–13 As part of our ongoing research on photoredox-catalyzed C–H functionalization reactions,13 herein we describe an environmentally benign visible light-promoted ethoxycarbonylmethylation for the synthesis of alkyl substituted imidazopyridines by the coupling between imidazopyridines and ethyl diazoacetate using Ru(bpy)3Cl2 as photoredox-catalyst under argon atmosphere at room temperature (Scheme 1b).
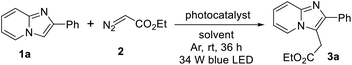
To begin, the reaction was carried out using 2-phenylimidazo[1,2-a]pyridine (1a) and ethyl diazoacetate (EDA) (2) (2.0 equiv.) in presence of 0.2 mol% of Ru(bpy)3Cl2 as a photoredox-catalyst under the irradiation of 34 W blue LED lamp in methanol at room temperature. To our delight, 49% yield of the ethyl 2-(2-phenylimidazo[1,2-a]pyridin-3-yl)acetate (3a) was obtained after 36 h under argon atmosphere. However, no desired product was formed in aerobic conditions (Table 1, entry 1). Next, we screened the effect of different solvents such as EtOH, DCM, MeCN, and toluene but these were not suitable like MeOH (Table 1, entries 2–5). Interestingly, the binary solvent mixture (MeOH and H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was found to be effective for the formation of alkylated product in 76% yield (Table 1, entry 6). The yield of the reaction was increased using a binary mixture of MeOH
1) was found to be effective for the formation of alkylated product in 76% yield (Table 1, entry 6). The yield of the reaction was increased using a binary mixture of MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2
H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 1, entry 7). However, only a trace amount of product was detected with the mixture of (1
1) (Table 1, entry 7). However, only a trace amount of product was detected with the mixture of (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) MeOH
2) MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O and no product was observed in H2O (Table 1, entries 8 and 9). Instead of MeOH
H2O and no product was observed in H2O (Table 1, entries 8 and 9). Instead of MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2
H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by using EtOH
1) by using EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2
H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) desired product (3a) was obtained in 68% yield after 36 h (Table 1 entry 10). The reaction did not proceed at all in presence of other classical photoredox-catalysts like Ir(ppy)3, rose bengal, eosin Y, eosin B, and rhodamine B (Table 1, entries 11–15). Moreover, no product was formed in absence of photoredox-catalyst as well as light source (Table 1, entries 16 and 17) which confirm that photo-catalyst and light source are essential for this transformation. We also performed the reaction in thermal condition at 60 °C (Table 1, entry 18). The desired product (3a) was not formed which indicated that the reaction probably go through the charge transfer/EDA-type complex not via carbene pathway. The yield of the reaction decreased when the amount of ethyl diazoacetate was either lowered or increased (Table 1, entry 19). In addition, 56% yield of the desired product was obtained under the irradiation of 20 W blue LED and no alkylated product was observed using 30 W white LED (Table 1, entry 20). From this experiment, we confirmed that the probable reaction pathway via charge-transfer/EDA-type complex absorbs wave length only from the emitted blue LED light not from the emitted 30 W white LED. Finally, optimized yield (89%) was achieved using 0.2 mol% Ru(bpy)3Cl2 and 2.0 equiv. ethyl diazoacetate in 2.0 mL mixture of MeOH
1) desired product (3a) was obtained in 68% yield after 36 h (Table 1 entry 10). The reaction did not proceed at all in presence of other classical photoredox-catalysts like Ir(ppy)3, rose bengal, eosin Y, eosin B, and rhodamine B (Table 1, entries 11–15). Moreover, no product was formed in absence of photoredox-catalyst as well as light source (Table 1, entries 16 and 17) which confirm that photo-catalyst and light source are essential for this transformation. We also performed the reaction in thermal condition at 60 °C (Table 1, entry 18). The desired product (3a) was not formed which indicated that the reaction probably go through the charge transfer/EDA-type complex not via carbene pathway. The yield of the reaction decreased when the amount of ethyl diazoacetate was either lowered or increased (Table 1, entry 19). In addition, 56% yield of the desired product was obtained under the irradiation of 20 W blue LED and no alkylated product was observed using 30 W white LED (Table 1, entry 20). From this experiment, we confirmed that the probable reaction pathway via charge-transfer/EDA-type complex absorbs wave length only from the emitted blue LED light not from the emitted 30 W white LED. Finally, optimized yield (89%) was achieved using 0.2 mol% Ru(bpy)3Cl2 and 2.0 equiv. ethyl diazoacetate in 2.0 mL mixture of MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2
H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with the irradiation of 34 W blue LED for 36 h at room temperature under argon atmosphere (Table 1, entry 7).
1) with the irradiation of 34 W blue LED for 36 h at room temperature under argon atmosphere (Table 1, entry 7).
| Entry | Photocatalyst (0.2 mol%) | Solvent | Yield (%) |
|---|---|---|---|
| a Reaction conditions: all reactions were carried out with 0.25 mmol of 1a, 0.5 mmol of 2, and 0.2 mol% of photocatalyst in 2.0 mL of solvent for 36 h at room temperature under argon atmosphere and irradiation with 34 W blue LED. NR = no reaction.b In aerobic condition.c Absence of Ru(bpy)3Cl2.d Without light source at rt.e Reaction at 60 °C without light scource.f 1.5 equiv. EDA.g 2.5 equiv. EDA.h Irradiation with 20 W blue LED.i 30 W white LED. | |||
| 1 | Ru(bpy)3Cl2 | MeOH | 49, NRb |
| 2 | Ru(bpy)3Cl2 | EtOH | 21 |
| 3 | Ru(bpy)3Cl2 | DCM | 43 |
| 4 | Ru(bpy)3Cl2 | MeCN | 47 |
| 5 | Ru(bpy)3Cl2 | Toluene | NR |
| 6 | Ru(bpy)3Cl2 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
76 |
| 7 | Ru(bpy)3Cl2 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
89 |
| 8 | Ru(bpy)3Cl2 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Trace |
| 9 | Ru(bpy)3Cl2 | H2O | NR |
| 10 | Ru(bpy)3Cl2 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
68 |
| 11 | Ir(ppy)3 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Trace |
| 12 | Rose bengal | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NR |
| 13 | Eosin Y | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NR |
| 14 | Eosin B | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NR |
| 15 | Rhodamine B | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NR |
| 16 | — | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NRc |
| 17 | Ru(bpy)3Cl2 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NRd |
| 18 | Ru(bpy)3Cl2 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
NRe |
| 19 | Ru(bpy)3Cl2 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
45f, 84g |
| 20 | Ru(bpy)3Cl2 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
56h, NRi |
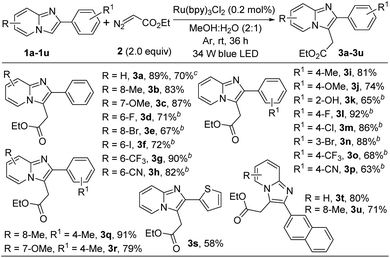
With the optimized reaction conditions in hand we explored the substrate scope of this methodology with various substituted imidazopyridines. At first we checked the effect of various substituents on the pyridine ring of imidazopyridine derivatives and the results are summarized in Scheme 2. Imidazopyridine containing various electron-donating groups (8-Me and 7-OMe) efficiently reacted with ethyl diazoacetate to provide the desired products in good yields (3b and 3c). It is important to note that substrates bearing electron-withdrawing groups did not afford alkylated products under the optimized reaction condition. However, addition of 10 mol% N,N-dimethyl-m-toluidine as a redox-active additive gave a satisfactory yield of the desired products (see ESI†). Therefore in modified reaction conditions, halogens containing imidazopyridines like –F, –Br, –I and strong electron-withdrawing group such as –CF3, –CN containing substrates were successfully afforded the desired products in 67–90% yields (3d–3h). Next, we examined the substrate scope with various substitutions in phenyl part of imidazo[1,2-a]pyridine. Imidazopyridine with electron-releasing (4-Me, and 4-OMe), halogens (4-F, 4-Cl, and 3-Br) and strong electron-withdrawing group (4-CF3, and 4-CN) containing substrates produced the desired products with good to excellent yields (3i–3p). 2-Hydroxy substituted imidazo[1,2-a]pyridine was also worked well in this transformation (3k). In addition, disubstituted imdazopyridines with different substituents at both the phenyl and pyridine ring underwent the reaction very smoothly (3q, and 3r). Heteroaryl and naphthyl substituted imidazopyridines produced the desired products without any difficulties (3s–3u). However, unsubstituted imidazo[1,2-a]pyridine did not produce the desired product in this protocol. To highlight the applicability of the present protocol a gram-scale reaction was carried out taking 1a in 6.0 mmol scale under normal laboratory setup. The yield of ethyl 2-(2-phenylimidazo[1,2-a]pyridin-3-yl)acetate (3a) was slightly diminished to 70% after 36 h along with the recovery of excess starting material.
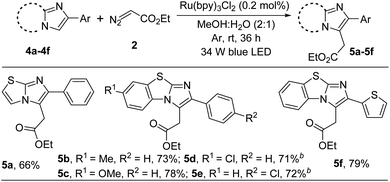
Next, to show the generality of this present methodology, we investigated the reaction taking other imidazoheterocycles like imidazo[2,1-b]thiazole and benzo[d]imidazo[2,1-b]thiazoles (Scheme 3). 6-Phenylimidazo-[2,1-b]thiazole reacted smoothly to afford 5a in 66% yield. Benzo[d]imidazo[2,1-b]thiazole containing –Me, –OMe, and –Cl substituted derivatives were well tolerable under the present reaction conditions (5b–5e). The structure of ethyl 2-(7-methoxy-2-phenylbenzo[d]imidazo[2,1-b]thiazol-3-yl)acetate (5c) was confirmed by X-ray crystallography.14 In addition, thiophene substituted benzo[d]imidazo[2,1-b]thiazole furnished the corresponding alkylated product in good yield (5f).
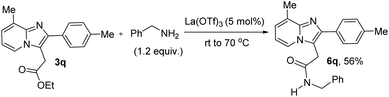
After that, we have directly modified the synthesized ethoxycarbonylmethylated product (3q) to amide derivative (6q) in 56% yield using benzylamine in the presence of La(OTf)3 as a catalyst under ambient air at rt to 70 °C as shown in Fig. 1.
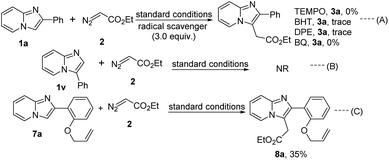
A few control experiments were performed to understand the mechanistic insights of the present protocol (Scheme 4). The alkylation reaction did not proceed in the presence of 3.0 equiv. radical scavengers like 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), 2,6-di-tert-butyl-4-methyl phenol (BHT), 1,1-diphenylethylene (DPE), and p-benzoquinone (BQ) which suggest the reaction probably proceeds through radical pathway (Scheme 4, eqn (A)). Moreover, 3-phenylimidazo[1,2-a]pyridine (1v) did not produce the C-2 alkylated product that signifies the regioselectivity of the present protocol (Scheme 4, eqn (B)). Taking 2-(2-(vinyloxy)phenyl)imidazo[1,2-a]pyridine we also performed this reaction with ethyl diazoacetate under this standard reaction conditions. Desired C-3 alkylated product (8a) was formed in 35% yield (Scheme 4, eqn (C)). But cyclopropanation product was not formed in the alkene part of the allyl group via carbene pathway. From this experimental result strongly imply that the present strategy only proceeds through radical mechanistic pathway.
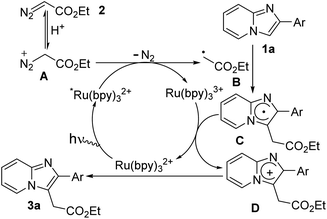
Based on the above mechanistic studies and previous literature reports,7,8,13 a possible radical mechanism was proposed for the formation of ethyl 2-(2-phenylimidazo[1,2-a]pyridin-3-yl)acetate (3a) as presented in Scheme 5. Initially, photo-catalyst Ru(bpy)32+ is transformed to its excited-state *Ru(bpy)32+ under blue LED irradiation. In the presence of MeOH/H2O, diazo compounds are in equilibrium with their protonated form (A). Then cationic diazoester involves single-electron transfer (SET) process with excited photocatalyst *Ru(bpy)32+ leading to the formation of alkyl radical intermediate (B) and Ru(bpy)33+.6b,c Next, alkyl radical intermediate (B) effectively reacts with imidazopyridine (1a) to produce imidazole radical intermediate (C) which is successively transformed to imidazolium radical cation intermediate (D) along with the generation of ground state photocatalyst. Finally, C-3 alkylated imidazopyridine (3a) is obtained through deprotonation of intermediate D. It is worthy to mention that Meggers et al.7h did not observe quenching of *Ru(bpy)32+ whereas Gryko et al.6c did observe the quenching of *Ru(bpy)32+ by ethyl diazoacetate that was accelerated in the presence of protic sources.
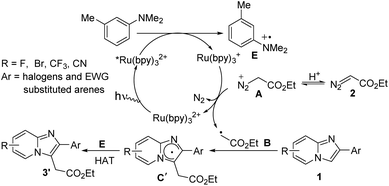
In case of halogen or electron-withdrawing substituted imidazoheterocycles a possible mechanism for the radical alkylation reaction is illustrated in Scheme 6.6b,c Reductive quenching of *Ru(bpy)32+ by SET oxidation of aniline derivatives affords radical cation E and Ru(bpy)3+. Then reduced photocatalyst Ru(bpy)3+ comes back to ground state with the generation of radical intermediate B via the elimination of N2. Subsequently, radical intermediate B reacts with imidazopyridine 1 to produce radical intermediate C′. Finally, the desired product 3′ is produced through the hydrogen atom transfer (HAT) process between radical cation E and radical intermediate C′.
Conclusions
In summary, we have developed an efficient photoredox-catalyzed ethoxycarbonylmethylation of imidazoheterocycles with ethyl diazoester as an alkylating reagent under the irradiation of blue LED at room temperature. A wide variety of functionalized imidazoheterocycles were well tolerated under the mild reaction conditions. A gram-scale synthesis was also performed to demonstrate the potential synthetic application of this new method. In contrast to the carbene precursors from α-diazoester a first C3-ethoxycarbonylmethylation of imidazopyridines via radical intermediate has been established. We believe the present protocol to be of significant applicability for the diversification of heterocyclic moieties in a multitude of medicinal applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. H. acknowledges the financial support from CSIR, New Delhi (Grant no. 02(0307)/17/EMR-II). S. B. and S. L. thanks CSIR, S. S. and M. S. thanks UGC-New Delhi for their fellowship.Notes and references
- (a) R. Brown, J. Quirk and P. Kirkpatrick, Nat. Rev. Drug Discovery, 2003, 2, 177 CrossRef CAS PubMed; (b) E. Raviña, The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs, Wiley, NewYork, 2011, pp. 254-272 Search PubMed.
- (a) Q. Huang and S. Z. Zard, Org. Lett., 2018, 20, 1413 CrossRef CAS PubMed; (b) S. Wang, X. Huang, Z. Ge, X. Wang and R. Li, RSC Adv., 2016, 6, 63532 RSC; (c) A. Kaga, X. Wu, J. Y. J. Lim, H. Hayashi, Y. Lu, E. K. L. Yeow and S. Chiba, Beilstein J. Org. Chem., 2018, 14, 3047 CrossRef CAS PubMed.
- (a) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Chem. Rev., 2015, 115, 9981 CrossRef CAS PubMed; (b) X. Zhao, Y. Zhang and J. Wang, Chem. Commun., 2012, 48, 10162 RSC; (c) N. R. Candeias, R. Paterna and P. M. P. Gois, Chem. Rev., 2016, 116, 2937 CrossRef CAS PubMed; (d) I. D. Jurberg and H. M. L. Davies, Chem. Sci., 2018, 9, 5112 RSC.
- (a) M. Peña-López and M. Beller, Angew. Chem., Int. Ed., 2017, 56, 46 CrossRef PubMed; (b) Ł. W. Ciszewski, K. Rybicka-Jasińska and D. Gryko, Org. Biomol. Chem., 2019, 17, 432 RSC; (c) Y. Xia, D. Qiu and J. Wang, Chem. Rev., 2017, 117, 13810 CrossRef CAS PubMed; (d) F. He and R. M. Koenigs, Chem. Commun., 2019, 55, 4881 RSC; (e) Q.-Q. Cheng, Y. Deng, M. Lankelma and M. P. Doyle, Chem. Soc. Rev., 2017, 46, 5425 RSC; (f) Q. Huang, H. Dong, B. Li, W. Hu and Y. Wang, Tetrahedron, 2020, 76, 130998 CrossRef CAS.
- (a) C. Grotenhuis, N. Heuvel, J. I. Vlugt and B. Bruin, Angew. Chem., Int. Ed., 2018, 57, 140 CrossRef PubMed; (b) J. Zhao, P. Li, Y. Xu, Y. Shi and F. Li, Org. Lett., 2019, 21, 9386 CrossRef CAS PubMed; (c) H. -X. Wang, Y. Richard, Q. Wan, C. -Y. Zhou and C. -M. Che, Angew. Chem., Int. Ed., 2020, 59, 1845 CrossRef CAS PubMed.
- (a) P. Li, J. Zhao, L. Shi, J. Wang, X. Shi and F. Li, Nat. Commun., 2018, 9, 1972 CrossRef PubMed; (b) K. Rybicka-Jasińska, Ł. W. Ciszewski and D. Gryko, Adv. Synth. Catal., 2016, 358, 1671 CrossRef; (c) Ł. W. Ciszewski, J. Durka and D. Gryko, Org. Lett., 2019, 21, 7028 CrossRef PubMed.
- (a) M. H. Shaw, J. Twilton and D. W. C. MacMillan, J. Org. Chem., 2016, 81, 6898 CrossRef CAS PubMed; (b) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075 CrossRef CAS PubMed; (c) L. Marzo, S. K. Pagire, O. Reiser and B. König, Angew. Chem., Int. Ed., 2018, 57, 10034 CrossRef CAS PubMed; (d) J. R. Chen, X. Q. Hu, L. Q. Lu and W. J. Xiao, Acc. Chem. Res., 2016, 49, 1911 CrossRef CAS PubMed; (e) D. P. Tiwari, S. Dabral, J. Wen, J. Wiesenthal, S. Terhorst and C. Bolm, Org. Lett., 2017, 19, 4295 CrossRef CAS PubMed; (f) K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035 CrossRef CAS PubMed; (g) J. C. Tellis, C. B. Kelly, D. N. Primer, M. Jouffroy, N. R. Patel and G. A. Molander, Acc. Chem. Res., 2016, 49, 1429 CrossRef CAS PubMed; (h) X. Huang, R. D. Webster, K. Harms and E. Meggers, J. Am. Chem. Soc., 2016, 138, 12636 CrossRef CAS PubMed; (i) P. Li, J. Zhao, L. Shi, J. Wang, X. Shi and F. Li, Nat. Commun., 2018, 9, 1 CrossRef PubMed; (j) P. H. Given and M. E. Peover, J. Chem. Soc., 1960, 385 RSC; (k) S. H. Cadle, P. R. Tice and J. Q. Chambers, J. Phys. Chem., 1967, 71, 3517 CrossRef CAS; (l) A. M. del Hoyo, A. G. Herraiz and M. G. Suero, Angew. Chem., Int. Ed., 2017, 56, 1610 CrossRef CAS PubMed; (m) H. E. Bonfield, T. Knauber, F. Lévesque, E. G. Moschetta, F. Susanne and L. J. Edwards, Nat. Commun., 2020, 11, 804 CrossRef CAS PubMed.
- (a) J. W. Tucker and C. R. J. Stephenson, J. Org. Chem., 2012, 77, 1617 CrossRef CAS PubMed; (b) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (c) J.-Y. Shao, Z.-L. Gong and Y.-W. Zhong, Dalton Trans., 2018, 47, 23 RSC.
- (a) C. Enguehard-Gueiffier and A. Gueiffier, Mini-Rev. Med. Chem., 2007, 7, 888 CrossRef CAS PubMed; (b) A. T. Baviskar, S. M. Amrutkar, N. Trivedi, V. Chaudhary, A. Nayak, S. K. Guchhait, U. C. Banerjee, P. V. Bharatam and C. N. Kundu, ACS Med. Chem. Lett., 2015, 6, 481 CrossRef CAS PubMed.
- (a) A. K. Bagdi, S. Santra, K. Monir and A. Hajra, Chem. Commun., 2015, 51, 1555 RSC; (b) V. S. Padalkar and S. Seki, Chem. Soc. Rev., 2016, 45, 169 RSC.
- (a) N. Chernyak and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2743 CrossRef CAS PubMed; (b) Q. Chang, Z. Liu, P. Liu, L. Yu and P. Sun, J. Org. Chem., 2017, 82, 5391 CrossRef CAS PubMed; (c) D. K. Nair, S. M. Mobin and I. N. N. Namboothiri, Org. Lett., 2012, 14, 4580 CrossRef CAS PubMed; (d) H. Kim, M. Byeon, E. Jeong, Y. Baek, S. J. Jeong, K. Um, S. H. Han, G. U. Han, G. H. Ko, C. Maeng, J. Y. Son, D. Kim, S. H. Kim, K. Lee and P. H. Lee, Adv. Synth. Catal., 2019, 361, 2094 CrossRef CAS.
- (a) K. Pericherla, P. Kaswan, K. Pandey and A. Kumar, Synthesis, 2015, 47, 887 CrossRef CAS; (b) C. Ravi and S. Adimurthy, Chem. Rec., 2017, 17, 1019 CrossRef CAS PubMed; (c) H. Chen, H. Yi, Z. Tang, C. Bian, H. Zhang and A. Lei, Adv. Synth. Catal., 2018, 360, 3220 CrossRef CAS; (d) P. Sun, D. Yang, W. Wei, M. Jiang, Z. Wang, L. Zhang, H. Zhang, Z. Zhang, Y. Wang and H. Wang, Green Chem., 2017, 19, 4785 RSC; (e) C. Wang, S. Lei, H. Cao, S. Qiu, J. Liu, H. Deng and C. Yan, J. Org. Chem., 2015, 80, 12725 CrossRef CAS PubMed; (f) Y. J. Guo, S. Lu, L. L. Tian, E. L. Huang, X. Q. Hao, X. Zhu, T. Shao and M. P. Song, J. Org. Chem., 2018, 83, 338 CrossRef CAS PubMed; (g) P. R. Nitha, M. M. Joseph, G. Gopalan, K. K. Maiti, K. V. Radhakrishnan and P. Das, Org. Biomol. Chem., 2018, 16, 6430 RSC; (h) Ł. Kielesiński, M. Tasior and D. T. Gryko, Org. Chem. Front., 2015, 2, 21 RSC; (i) X. M. Zhao, E. L. Huang, Y. S. Zhu, J. Li, B. Song, X. Zhu and X. Q. Hao, Org. Biomol. Chem., 2019, 17, 4869 RSC.
- (a) A. K. Bagdi and A. Hajra, Chem. Rec., 2016, 16, 1868 CrossRef CAS PubMed; (b) S. Mitra, M. Ghosh, S. Mishra and A. Hajra, J. Org. Chem., 2015, 80, 8275 CrossRef CAS PubMed; (c) M. Singsardar, S. Mondal, S. Laru and A. Hajra, Org. Lett., 2019, 21, 5606 CrossRef CAS PubMed; (d) G. Kibriya, A. K. Bagdi and A. Hajra, J. Org. Chem., 2018, 83, 10619 CrossRef CAS PubMed; (e) G. Kibriya, S. Samanta, S. Jana, S. Mondal and A. Hajra, J. Org. Chem., 2017, 82, 13722 CrossRef CAS PubMed; (f) A. K. Bagdi and A. Hajra, Org. Biomol. Chem., 2020, 18, 2611 RSC.
- Further information can be found in the CIF file. This crystal was deposited in the Cambridge Crystallographic Data Centre and assigned as CCDC 1976157†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1976157. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra05795a |
| This journal is © The Royal Society of Chemistry 2020 |