Open Access Article
Open Access ArticleApplication status and technical analysis of chitosan-based medical dressings: a review
Shanguo
Zhang†
ab,
Jianyong
Li†
ab,
Jianfeng
Li
ab,
Na
Du
 *c,
Donghai
Li
d,
Fangyi
Li
*c,
Donghai
Li
d,
Fangyi
Li
 ab and
Jia
Man
*ab
ab and
Jia
Man
*ab
aSchool of Mechanical Engineering, Key Laboratory of High Efficiency and Clean Mechanical Manufacture (Ministry of Education) Shandong University, Jinan 250061, China. E-mail: mj@sdu.edu.cn
bNational Demonstration Center for Experimental Mechanical Engineering Education (Shandong University), Jinan 250061, China
cDepartment of Geriatrics, Second Affiliated Hospital of Shandong University, Jinan 250033, China. E-mail: duna0715@163.com
dAdvanced Medical Research Institute, Shandong University, Jinan 250012, China
First published on 16th September 2020
Abstract
Chitosan has wide applications in the field of medical dressings due to its good biomedical properties. This review provides the application status and technical analysis of chitosan medical dressings. First, we introduce the source and chemical structure of chitosan. Then, we investigate the mechanism of chitosan showing different medical properties. We also show the application of supramolecular chitosan-based hydrogels in the dressing field and the formulation optimization and the preparation technology of chitosan dressings for fabricating chitosan-based dressings with various morphologies and medical functions. After that, we introduce the research process of the modification method of chitosan dressings including single modification, blending modification, crosslinking modification, etc. Finally, based on the study of the medical effects of chitosan dressings, we analyze the existing problems in the preparation process and propose corresponding solutions from the aspects of the morphology, clinical feedback effect, and future development trends. This paper can provide a reference for further studies of skin tissue engineering and the development of new chitosan medical dressings.
1. Introduction
The primary function of the skin is to serve as a protective barrier against the environment. The loss of the integrity of large portions of the skin as a result of injury or illness may lead to severe disability or even death. Medical wound dressing can protect the wound from bacterial infection and accelerate wound healing.1–5 In 1962, Winter found that a wound healed faster in a humid environment than in dry conditions.6 He proposed a new wet therapy – “sterile, moist and closed.” According to this theory, the ideal dressing should be able to keep the wound interface moist and allow gas exchange, be a microbial barrier, and also be non-toxic, non-allergenic, non-adhesion, and easy to remove.7,8 At present, a large number of research teams are committed to the production of a kind of medical dressing that meets the above characteristics through modified or composite polymer materials.Chitosan is the only basic polysaccharide in nature and contains numerous amino, hydroxyl, and other active functional groups, which attributes it with the characteristics of cationic polymerization, metal chelation, multi-functional reactivity.9,10 The raw materials of chitosan can get from shrimp and crab, which have a comprehensive resource. Thus, chitosan is widely used in the wound dressing field.
Traditional dressings like gauze or cotton wool as passive products are used to cover the wound and the healing process mainly depends on the capacity of the tissue regeneration. Besides, traditional dressings are easy adherence to the wound tissue in the process of wound healing, leading to secondary damage.11 Chitosan has good biomedical properties and can promote the regeneration, repair, and healing of wound tissue. In addition, chitosan itself as the powder can be fabricated as dressings in a variety of shapes such as membranes, gels, and sponges. Compared with traditional dressings, chitosan wound dressings are focused to keep the wound from dehydration and promote healing rather than just to cover it.12,13
Wound healing is a dynamic process that could be divided into four stages including hemostasis phase, inflammatory phase, proliferative phase, and maturation phase.14 Chitosan-based wound dressings can play a positive effect on the different stages of wound healing. (I) Hemostasis phase: the process of clotting and hemostasis occurs immediately after an injury. The functions of platelets are to clot blood, stop bleeding, and repair damaged blood vessels.15 Chitosan has the capacities of plasma sorption, erythrocytes coagulation, platelet aggregation, and platelet activation. Red blood cells are bound together by chitosan macromolecule chains, leading to agglutination, which can promote the hemostatic process.16,17 (II) Inflammatory phase: in this stage, immune cells will destroy bacteria, remove debris, and release growth factors and proteins that can promote tissue repair, causing the skin symptoms of swelling, fever, pain, and redness.18 During the state transformation of the immune cell, chitosan can enhance the release of pro-inflammatory cytokines.19,20 The anti-inflammatory effect of chitosan is achieved by regulating tumor necrosis factor-α to reduce the production of nitric oxide.21 (III) Proliferative phase: the granulation tissue will proliferate rapidly in this stage. Beside, epithelial cells begin to grow and proliferate until they cover the entire wound surface.22 Lysozymes gradually depolymerize chitosan via hydrolysis to release N-acetyl-D-glucosamine, which stimulates fibroblast proliferation and collagen deposition and remodeling.23 (IV) Maturation phase: the new tissue slowly gains strength and flexibility by the action of enzymes and stress, which will result in the formation of scar tissue.24 Chitosan contains a component of N-acetyl glucosamine, which plays an important role in reducing scar formation.25
To improve the wound-healing effect of chitosan-based dressing, scholars have treated chitosan with the method of cross-linking. According to the types of driving forces for cross-linking, the method can be divided into two major categories: covalent cross-linking and noncovalent cross-linking. Covalent cross-linking of the chitosan macromolecules is formed by non-reversible covalent bonds, which can be formed between chitosan macromolecular chains themselves or between chitosan macromolecular chains and molecular of cross-linkers such as glutaraldehyde and acrylic acid.26 For example, pendant amine groups of chitosan macromolecule can interact with the aldehydic group of the glutaraldehyde to form stable imine bonds, leading to the fabricated chitosan-based membrane with good mechanical strength.27,28 Chitosan supramolecular hydrogel wound dressing can be obtained by noncovalent interactions such as metal–ligand, host–guest recognition, and electrostatic interaction.29 For example, the facile complexation of metal ions with carboxylic, amino, and hydroxyl groups of carboxymethyl chitosan chains can form supramolecular hydrogels. The supramolecular structure endows the chitosan-based hydrogel with excellent elasticity and antibacterial property.30 Also, some groups (e.g. carboxylic, amino) in chitosan macromolecules could interact with other special groups or mental iron through covalent/noncovalent cross-linking. Generally, the chitosan membrane or hydrogel dressings fabricated by the strong covalent crosslinking have a better mechanical property than that of noncovalent crosslinking.
The modification of chitosan and the blending with other polymer materials both have an essential effect on the bacteriostatic and hemostatic property of chitosan-based dressing. The mechanism of medical performance appears more complicated with many factors. Thus, it is necessary to study the mechanism of the chitosan compound after the combination process to ensure the safety of long-term and large-scale use of it clinically.
In the process of modification or composite with other materials, the research of mechanism acts as a fundamental part of the chitosan-based dressing. This review focuses on the mechanism of the medicinal properties, formulation optimization, preparation technology, and the modification method of chitosan dressing. According to the trend of the development of chitosan-based dressing, we summarized the research directions that need to be explored.
2. General properties of chitosan
Chitosan is the deacetylated product of chitin to at least 50% of the free amine form. Chitin has abundant sources, unique structure, and excellent biomedical properties. It can not only be used as raw materials for the synthesis of various materials but can be made into gels, fiber, film or microsphere, etc., which has a wide range of applications. As a result, the study of chitin has attracted more and more attention of scholars.2.1 The source and extraction of chitosan/chitin
As a natural biomacromolecule material, chitin extensively exists in the shells of lower animals, especially arthropods, as well as the cell walls of lower plants, bacteria, and algae. For example, the content of chitin in shrimp/crab shell, mollusks (such as squid, snail, and oyster), and fungi (such as basidiomycete and alga) is 15–25%, 3–26%, less than 45%, respectively.Because of the low price and high content of chitin, shrimp/crab shells are currently used as the main raw material for obtaining chitin. Approximately 1 tonne of chitin can be produced from 7 tonne of crab shells and 2 tonne can be processed from 7 tonne of shrimp shells.31 Chitin is an important component of insect cuticle, which has less inorganic substance than shrimp/crab shell, leading to an easy demineralization treatment. Besides, insects account for more than 90% of the number of animals on the earth, thus, species-rich insects have gradually become a new raw material for chitin development.32,33
In raw materials, chitin is found as a constituent of a complex network with proteins onto which calcium carbonate deposits to form the rigid shell.34 Thus, chitin isolation from raw materials mainly requires the removal of proteins and inorganic calcium carbonate.35 The acid–alkali method is often used for the extraction of chitin from raw materials. It includes the steps of degreasing, deproteinization, demineralization, and decoloration, in which the process of deproteinization and demineralization can be exchanged. The treatment with organic solvents such as diethyl ether could remove lipids from the raw material, leaving insoluble proteins, calcium carbonate, and chitin. Demineralization is generally performed by acid treatment using HCl, HNO3, H2SO4, CH3COOH, and HCOOH, among which the preferential reagent is dilute hydrochloric acid.36 The water-insoluble calcium carbonate can react with dilute hydrochloric acid to form water-soluble calcium chloride. The common method of deproteinization is alkali liquor treatment. NaOH is the preferential reagent and it is applied at the concentration ranging from 0.125 to 5.0 M, at varying temperature (up to 160 °C) and treatment duration (from few minutes up to a few days).35 Using strong oxidants such as potassium permanganate or hydrogen peroxide for bleaching treatment in the decolorization process. Although the traditional acid–alkali method has been widely used in the extraction of chitin, the waste acid and alkali liquor produced in the production process not only has a negative impact on the environment but carries on the follow-up treatment, which increases the production cost. The steps of deproteinization and demineralization can also be achieved by the fermentation process. Although microbial fermentation has a long production cycle, it is environmentally friendly and the cost of microbial culture is relatively low, thus it is increasingly being applied.
Both acids and alkalis can be used to deacetylate chitin. However, glycosidic bonds are very susceptible to the acid; therefore, the method of alkali deacetylation is used more frequently in the production of chitosan.37
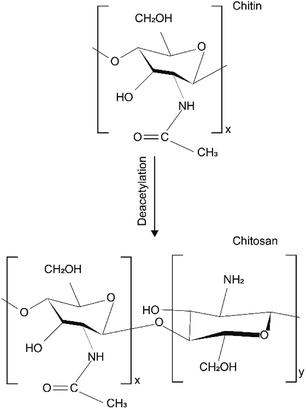
2.2 Chemical structure of chitosan/chitin
Chemical structure of chitin made up of 1–4 linked 2-acetamido-2-deoxy-β-D-glucopyranose. After the deacetylation of the chitin, chitosan is a copolymer of N-acetyl-D-glucose amine and D-glucose amine as shown in Fig. 1.38 The different chemical structures attribute to the different physical and chemical properties.The solid morphology of chitosan is maintained mainly through two hydrogen bonds, one intrachain and one interchain.39 The macromolecular chain of chitosan obtained by the deacetylation of chitin contains amino groups, so it has higher reactivity. The presence of amino groups provides the possibility for many chemical reactions, such as quaternization, alkylation, and metal chelation.40 The acetaminophen on chitin can also complete chemical reaction under certain conditions, but its chemical reaction activity is not as strong as amino groups on chitosan. The sequence of amino groups and acetylamino groups in chitosan molecules is an important parameter that affects the physical properties of chitosan. Amino makes the charge state of chitosan susceptible to the influence of the pH value. When the pH value of the chitosan dissolution system is less than 6, the amino group is positively charged by protonation, which makes the chitosan become water-soluble cationic polysaccharide. When pH value is greater than 6, deprotonation of the groups will lead chitosan macromolecules to lose charge and insoluble in water.41 Therefore, chitosan is often dissolved in dilute acids.
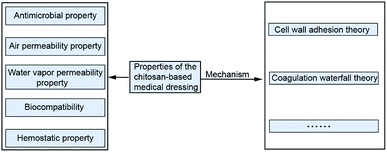
2.3 Properties and the corresponding mechanism of the chitosan-based medical dressing
Chitosan as a kind of biocompatible and antimicrobial biomaterial is generally selected to prepare wound dressings, which have the suitable properties of air permeability and water vapor transmission for wound healing.14 Exploring the mechanism of the above medical characteristics plays a vital role in the broad application of chitosan dressing. Fig. 2 shows properties and the mechanism of the chitosan-based medical dressing.To sum up, the medicinal properties of chitosan medicine is related to many factors, and it is necessary to clarify these rules to improve its healing effect. The theories of hemostatic and antibacterial properties of chitosan were comprehensive and interrelated, but the mechanism of the biocompatibility was still unclear. The changes in cell adhesion factor and cytokines determined the rejection reaction between the material and the human body, which have been proved by the biological evaluation of molecules. The next stage of the research method on the biocompatibility of chitosan can be proposed from the aspects of adhesion factor and cytokines.
2.4 Chitosan-based supramolecular medical dressings
Supramolecular hydrogels are formed in an aqueous solution by the interaction of two or more types of molecules through non-covalent bonds (e.g., stacking, oxygen bonding, hydrophobic interaction, coordination of metal ions, and host–guest interaction).71,72 Compared with traditional polymer hydrogel, supramolecular hydrogels have good reversibility. Because of the lower activation energy of non-covalent bonds, the supramolecular hydrogels can respond to various stimuli of the external environment. It is mainly showed that the hydrogen bonding interaction is sensitive to the temperature and the electrostatic interaction is sensitive to the pH value, etc.73 Besides, supramolecular medical dressings can keep the wound microenvironment moist, prevent the invasion of external bacteria from the wound, remove harmful reactive oxygen, and promote wound healing. Thus, supramolecular hydrogels have been used in the fields of medical dressings, which could be capable of dissolution upon the application of a stimulus.74 However, the supramolecular hydrogels are mechanically weak due to the low strength of non-covalent bonds.75Due to the special macromolecular structure of chitosan, it is easy to form supramolecular hydrogels driven by the non-covalent bond. The supramolecular chitosan-based hydrogel is usually prepared by integrating chitosan with other materials, which mainly include other macromolecular material such as polyvinyl alcohol, supermolecular material such as the peptide, and inorganic materials such as graphene oxide.
Diels–Alder cycloaddition could be used to fabricate chitosan supramolecular hydrogels with the advantages of fast rate, high yield, being free of byproducts and organic solutions in the reaction process.77,78 Zhang et al.79 used hydroxypropyl β-cyclodextrin as a green cross-linker to form a chitosan-based supramolecular hydrogel by the Diels–Alder reaction in aqueous solution. The process was completed in two steps. One is that the synthesis of furfural functionalized chitosan and maleimide functionalized hydroxypropyl β-cyclodextrin. Another is that the two synthesized complementary precursors were mixed in aqueous media to form supramolecular hydrogels through the Diels–Alder reaction. With the increase of the degree of furfural substitute on chitosan, the hydrogel strength increased readily, which could ascribe to the more furfural groups as reaction sites on the chitosan macromolecular chain. In addition, the supramolecular hydrogel has the drug continuously released property. The supramolecular polymer hydrogel composed of poly(vinyl alcohol) (PVA) and chitosan carbon dots could be prepared using the freezing/thawing method, which was formed by the hydrogen bonds between PVA chains and chitosan carbon dots.80
Chakraborty et al.84 adapted the “supramolecular polymer-covalent polymer” combination approach to fabricate supermolecular hydrogels with good mechanical properties and stability. Fmoc-RGD was chosen as the supramolecular polymer component and chitosan as the covalent polymer component. The preparation of the Fmoc-RGD supermolecular hydrogel was simple that it could be formed by dissolving the Fmoc-RGD powder in ultrapure water at ambient temperature. To prepare the Fmoc-RGD/chitosan composite hydrogels, the chitosan acetic acid solution was mixed with the Fmoc-RGD solution in water. The mixed solution was incubated in ambient conditions, resulting in the gelation process.
Chitosan has the propensity to form H-bonding with the C terminal free carboxylic acid groups of Fmoc-RGD, resulting in non-covalent functionalization of the peptide.85 Apart from H-bonding, electrostatic interactions also play a crucial role in non-covalent attachment of Fmoc-RGD and chitosan.
Graphene oxide has excellent medical properties such as the ability to promote angiogenesis and relatively high biocompatibility, which makes it one of the most favorable materials for the application of wound healing.26,88 The complex interaction will be formed between the hydroxyl and amino groups of cationic chitosan and hydroxyls and epoxide groups of negatively charged graphene oxide. Han et al. compound chitosan with the graphene oxide to prepare the supermolecular hydrogel, in which the graphene oxide nanosheets work as cross-linkers. The compound supermolecular hydrogel owned the thermal-reversible property by adjusting the concentration of chitosan.
In conclusion, the mechanical properties of chitosan-based supramolecular hydrogels can be greatly improved by adjusting the parameters to strengthen the non-covalent bonds in the experiment. However, the application of chitosan-based supramolecular hydrogels in medical dressings is not comprehensive and most of the researches are still in the preliminary stage. At present, researches on the supramolecular hydrogels based on chitosan only focus on the formulation that can be formed by chitosan and different materials, and the corresponding physical and chemical properties. Further medical exploration of the chitosan-based supramolecular hydrogels on wound healing is still in a state of scarcity. It is no doubt that chitosan-based supramolecular hydrogel plays an important role in the medical dressings field due to its excellent physical and medical properties. The research should focus on the medical properties and the formulation of the chitosan-based supramolecular hydrogels simultaneously.
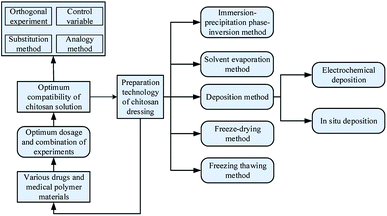
3. Formulation optimization and preparation technology of chitosan-based dressing
The formulation for chitosan dressing is composed of chitosan, other polymer materials with medical effects, or drugs. The process of formulation optimization refers to adjust parameters of different components such as concentration and quantity to make the chitosan-based dressings with excellent medicinal properties. To make each component in dressings work efficiently, different preparation technologies were used to fabricate chitosan dressing. Fig. 3 is a research framework for the formulation optimization and preparation process of chitosan dressing. In this section, the formulation optimization methods of different chitosan dressing are reviewed with the preparation technology as the mainline.3.1 Immersion precipitation phase inversion method
An immersion precipitation phase inversion method contains at least three kinds of substances, polymers, solvents, and non-solvents. Its basic process is to add non-solvent to the polymer–solvent system. In the polymer solution, the solvent spreads into the solidification bath, and the non-solvent in the solidification bath will also spread to the polymer solution. With the continuous development of this process, the system occurs phase separation. Finally, polymer sedimentation curing to form a chitosan film with different morphology and properties.89 This method is widely used in the preparation of porous and high permeability dressing membranes because of its diverse membrane morphology and excellent mechanical property. The technological process is shown in Fig. 4.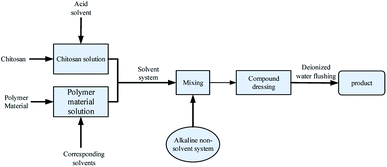 | ||
| Fig. 4 Preparation process of chitosan-based dressing by immersion precipitation phase inversion method. | ||
Reasonable solvent/non-solvent formulation and polymer selection can significantly improve the permeability and scalability of the chitosan-based dressing. Mi et al.90 used acetic acid aqueous solution as the solvent, NaOH (2 wt%)–Na2CO3 (0.05 wt%) solution as non-solvent and chitosan as a polymer to prepare chitosan dressing. The size of the microporous structure inside the membrane increased with the increase of the evaporation time, leading to the abilities of excellent oxygen permeability, controlled evaporative water loss, and promoted fluid drainage. Liang et al.91 added cellulose acetate with excellent water permeability to the mixed solvent composed of 1,4-dioxane and acetone. In the solvent system, methanol/maleic acid solution as non-solvent and formic acid dissolved chitosan as a polymer. The higher the concentration of chitosan, the smaller the thickness of the film. With the addition of cellulose acetate, the hydrophilicity and antibacterial property of the membrane improved compared with the pure chitosan membrane. For obtaining dressing with an asymmetric porous structure, Hua et al.92 successfully combined liquid nitrogen fracturing technology with an immersion precipitation phase conversion method by glacial acetic acid/sodium hydroxide as a preparation system. The chitosan membrane dressing was composed of thick epidermis, transition zone, and the cavernous porous layer. The animal test showed that it did not cause an allergic reaction, hemolysis, cytotoxicity, and thermal effect. Poonguzhali et al.93 used an immersion precipitation phase conversion method to prepare a double-layer asymmetric chitosan dressing, which was composed of the spongy lower layer and membranous upper layer. The histological examination has evaluated its medical performance, suggesting that this asymmetric chitosan dressing could improve the proliferation of epidermal cells and make the dermis collagen tissue deposition well.
The selection of solvent and non-solvent is an essential factor that affects the liquid–liquid phase separation process and forms of dressings. High compatibility between solvent and non-solvent is the crucial point in the process of preparing chitosan dressing by immersion precipitation phase conversion method; the concentration of chitosan is also a problem to be considered in different reaction systems.
3.2 Solvent evaporation method
The solvent evaporation is a method to achieve crystal growth by increasing the concentration of solute through the evaporation of solvents to obtain a saturated solution.94 Growth can be maintained at a constant temperature by adjusting evaporation to control the solution's over-saturation. The solvent evaporation method is widely used in the preparation of chitosan-based wound dressing.To obtain the chitosan membrane with controllable microporous structures, Zeng et al.95 added silicon particles with different diameters into acetic acid dissolved chitosan solution. After solvent evaporation, the silicon particles could be inserted in the chitosan membrane. Then, the membrane was immersed in sodium hydroxide, leading the dissolution of silicon particles and the formation of the chitosan membranes with micropores. Azad et al.96 got the chitosan membrane by drying the chitosan acetic acid solution at 37 °C for 24 h and made micropores with a mesh grafting machine. Compared with the xerox gauze on the market, the chitosan dressing had a better effect on wound healing. Scholars tried to compound chitosan with other polymer materials to improve the property of chitosan-based dressing. Xu et al.97 prepared chitosan/hyaluronic acid membrane dressings by drying the solution at 50 °C for four hours with the different proportions of the mixed solution. The experimental results illustrated that with the increase of hyaluronic acid content, the water contact tentacle and the water absorption rate gradually increased while the permeability of the dressing and the adsorption of the protein decreased. Compared with vaseline, the dressing could promote wound healing more effectively. Chinese herbal medicine (CHM) has been used for treating wounds, which aims to eliminate toxins, improve circulation and dispel blood stasis, and promote wound healing.98 CHM can be used orally or topically, alone or in combination with conventional western medicine. The application of the solvent evaporation method in combining chitosan and CHM can obtain the dressing owning good medical property. Linggen et al.99 used chitosan, purple grass, angelica, pearls, and other traditional CHM to make the compound film by the solvent evaporation method. The wound healing rate of the chitosan/CHM compound film was superior to the traditional CHM – “To the raw muscle jade red paste”. Considering that the composition of compound CHM is diverse, Liu et al.100 added cork, astragalus, rhubarb, and angelica to make composite membranes. The TLC result showed that there was no interference between Chinese herbal components, which could be used as the basis for quality evaluation of chitosan film.
At present, the existing volatile solvent cannot dissolve chitosan. Thus, the solvent evaporation method in the preparation of the chitosan dressing process often needs to raise the temperature to remove the solvent for the formation of the membrane. However, heating could undoubtedly affect the properties of chitosan and composite materials. After water evaporation, the acid solvent will deposit in chitosan dressing, producing an uncertain effect on the curative effect. The above problems are the common problems in the preparation of chitosan dressing by solvent evaporation, which could affect green and efficient preparation technology of chitosan dressing. The development of a type of volatile material for dissolving chitosan to realize the solvents-volatilizing at room temperature may be a research direction.
3.3 Sedimentation method
Sedimentary is a simple and inexpensive method applied for wet chemicals. The required materials can be obtained by adding a sedimentation agent or electrodes in a chitosan solution. Electrochemical deposition and in situ deposition are two standard deposition methods in the preparation of chitosan dressing. The sedimentary method has the characteristics of uniform mixing, convenient controlling conditions, and easy industrialization.The electrochemical deposition method refers to the technology of forming a coating in a matrix under an external electric field, in which the negative ions migrate in the electrolyte solution, and the redox reaction of electrons occurs in the electrode. Based on the principle that the chitosan in a weak acidic condition presents a positive charge and not charged in alkaline states, chitosan always is deposited in the cathode by the electrochemical deposition method. The chitosan coating obtained by electrochemical deposition has the characteristics of controllable process parameters, diverse matrix shape, and uniform deposition thickness.
The process parameters of the electrochemical deposition method will affect the physical properties such as thickness and roughness of the coating. In the acidic solution, Wu et al.101 obtained a chitosan sedimentary layer on the cathode surface by electrochemical deposition, which was about four microns thick in size. Because of the different thermal expansion coefficient between the coating and the substrate, there is a certain probability that additional cracks would occur during the film deposition process, failing the film making. To solve this problem, Pang et al.102 used surface plasma spraying technology to fabricate a layer of hydroxyapatite (biomedical material) on the Ti–6Al–4V alloy evenly; the chitosan was deposited on the alloy with hyaluronic acid as electrophoresis. The results showed that the content of hydroxyapatite determined the deposition rate and sediment thickness. In the process of chitosan deposition, the tight state and shedding time of film adhesion will affect the medicinal property of the film. Liang et al.103 added the nano-gold powder to chitosan solution for enhancing the antibacterial property and studied the effect of deposition time on the adhesion performance of thin films. The shorter the deposition time, the easier the chitosan membrane could fall off the electrode. The study showed that the deposition time of five minutes could form a thin and excellent mechanical property of chitosan dressing.
The preparation of chitosan dressing by electrochemical deposition is a new technology, and the influence of process parameters on the property of the dressing is not discussed in-depth. Especially, the study of electrolyte formulation only stays in theory; the effect of bond dissociation/bonding behavior between solute and ions on the deposition action of chitosan may need to be explored. In theory, some positively charged ions will also be deposited in the cathode, creating unnecessary impurities in chitosan dressing. Process parameter regulation has become the primary consideration in the process of preparation of chitosan dressing by electrochemical deposition.
In situ precipitation is a method for preparing materials employing sedimentation agent and metal salt solution. It has the characteristics of simple operation, effortless control, multi-component precipitation, and unique positioning point deposition, which is widely used to prepare chitosan composite dressing. Qiu et al.104 used acetic acid solution to dissolve chitosan and Zn(Ac)2·2H2O. A flexible chitosan/zinc oxide nanocomposite film was prepared with sodium hydroxide solution as precipitator after sealing reaction for 1 h at 80 °C. There was a strong coordination interaction between zinc ions and chitosan. Nanometer zinc oxide is dispersed homogeneously in the chitosan membrane, which could give full play the antibacterial property of the nano-zinc oxide. In this process, the selection of sedimentation agents with high deposition efficiency and good medical effect could simplify the process steps and eliminate the damage of alkaline solvents to the human body. Ong et al.105 used phosphates as a precipitator to obtain chitosan dressing. Animal tests showed that the dressing reduced the mortality rate of Pseudomonas aeruginosa infection in mice from 90% to 14.3%. Besides, the chitosan–polyphosphate membrane had an excellent medical performance through blood coagulation test, platelet adhesion test, and thrombin generation test.
Metallic particles can improve the antibacterial property of chitosan dressing. By adding reductants to the dissolution system containing metal ions for precipitating nano-level metal particles, scholars can study the effect of metal ions on the structure of chitosan hydrogel. Nie et al.106 added different metal ions to the chitosan dissolution system and used alkaline solution as sedimentation agent to fabricate chitosan and metal ion complexes, revealing that metal ions (such as copper ions) had a strong affinity with chitosan, resulting in the volume shrinkage and multi-layer transformation of the structure. Some metal ions have a weak association with chitosan, such as calcium ions, which are natural to form precipitation in the preparation process. The addition of inorganic particles would not affect the structural characteristics of the chitosan hydrogel. Since the hydrogel dressing was generated in the liquid reaction system, the mechanical property can be enhanced by adding suitable materials before solidification. To improve the mechanical property, Shen et al.107 successfully used citric acid as a coagulant to deposit the chitosan hydrogel with apatite granules. Scanning electron microscope (SEM) images showed that the apatite particles were evenly distributed in the hydrogel. The mechanical test showed that the mechanical property was much improved than that of the pure chitosan hydrogel.
Compared to the in situ deposition method with the electrochemical deposition method, the difference between them is the catalytic mode of chitosan deposition. At present, some researchers used biomaterials as a sedimentation agent. Considering the principle of ion reduction, most sedimentation agents with an alkaline solution would cause potential harm to the human body. The electrochemical deposition has an advantage in this aspect. To obtain chitosan composite dressing with a uniform structure, multi-component, and safe green deposition is the future research direction.
3.4 Freeze-drying method
A freeze-drying method uses the sublimation principle to dry the freezing material. First, the dried stuff is frozen at low temperatures, and then the frozen water molecules directly sublimate into the vacuum environment by adjusting the temperature. The technology is mainly used in the preparation of sponge dressing, which is completed primarily in the freeze-drying machine. Ice crystals are evenly distributed in the material after frozen. The sublimation process can be evenly distributed around the content due to dehydration phenomenon, avoiding foaming, oxidation, and other side effects caused by water vapor.108 After freeze-drying, the material has a porous sponge shape, which is a suitable carrier for dressing application. Freeze-drying technology is an effective method to maintain the long-term stability of medical materials, and thus the freeze-dried method is widely used in the preparation of chitosan dressings.To obtain chitosan sponge with similar pore size, uniform texture, and good hemostatic effect, Huang et al.109 grafted 12 alkyls to the nitrogen atom of chitosan molecule and applied freeze-drying technology in dressing fabrication. The sponge chitosan obtained by freeze-dried could improve platelet aggregation in vivo experiments. The rat femoral artery hemostatic model showed that the sponge could achieve adequate hemostasis in a wound. Jaiswal et al.110 used ultrasonic technology to treat silver nitrate/chitosan solution. The freeze-dried chitosan nano-silver composite hydrogel dressing could accelerate the new angiogenesis process and granulation tissue formation rate in mice experiments. A variety of parameters in the freeze-drying process will affect the morphology and property of chitosan sponge. Berretta et al.111 proposed that the suitable freezing temperature should between −45 °C to 80 °C and the best freezing time was 2–3 days. Besides, chitosan sponge has a drug release property.
Wu et al.112 prepared chitosan sponge containing ampicillin by the freeze-drying method, which showed good antibacterial activity. Agalar et al.113 fabricated chitosan/alginate/ciprofloxacin composite sponge at −80 °C for 12 h in freeze-dryer. The death rate of Escherichia coli increased with the release of ciprofloxacin. Guan et al.114 made flavonoids/chitosan sponge. The hemostatic promotion, the growth of blood vessels, fibroblasts, and granulation showed better in the chitosan/flavonoids group than the control group of pure chitosan.
The porous chitosan sponge obtained by the freeze-drying method can maintain the stability of long-term performance and continuous drug release property. Many studies force on the drug-loading composite sponge prepared by the freeze-drying process. To enhance the curative effect of medicine, repeated tests have been used to explore the suitable compatibility, but the mechanism of action was lacking.
3.5 Freeze–thaw method
Chitosan is insoluble in water and organic solvent.41 To dissolve chitosan. The freezing–thawing method for chitosan refers to the process of adding chitosan to the alkaline solvent system for melting rapidly at room temperature after cryopreservation. The dissolution mechanism is the self-assembly of small molecules and macromolecules driven by hydrogen bonds at low temperatures, destroying the original hydrogen bond between polymers and molecules.115 For example, the NaOH/urea system can be used for dissolving chitosan by the freezing–thawing.116 Scholars have made an in-depth study on the preparation of chitosan dressing by this method.Some polymers such as poly(vinyl alcohol) (PVA) can transform into hydrogel from the solution status by freezing–thawing cyclic processing.117,118 Besides, PVA as a biomedical material can be used in wound dressings fabrication.119 Sung et al.120 added minocycline to the PVA/chitosan dissolution system and successfully made hydrogel dressing by the freeze–thaw method. Because of the weak cross-link effect between chitosan and PVA, the hydrogel had greater properties of expansibility, flexibility, and elasticity, which had a good wound healing rate. To increase the bonding strength of chitosan and other polymer materials in the freezing–thawing method, a crosslinking agent could be used before freeze–thawing.121 Electronic rays can induce polymer materials to produce the reaction of grafting, polymerization, and crosslinking, which enable chitosan dressing to improve the mechanical property. Zhao et al.122 employed electron beam irradiation in acting on PVA/carboxymethyl chitosan for fabricating blended hydrogel. The mechanical and bacteriological properties were significantly enhanced compared with the PVA hydrogel, but the biosecurity was not explored. Yang et al.123 combined the irradiation technology and freezing–thaw method to make the PVA/chitosan hydrogel dressing, which was non-toxic to L929 mouse fibroblasts, indicating that radiation–freezing–thaw technology was safe for accelerating the healing of rat trauma (11 days).
The technique of preparing chitosan dressing with the freezing–thaw method is mature, whose advantages include the gel-like formation, high water content, and the fantastic curative effect for erosion and necrotizing wound.124 The treatment effect of chitosan hydrogel dressing on the wound is superior to that of ordinary dressing. However, repeated freezing and thawing require a long operation time. The combination of ultrasonic treatment technology, electronic irradiation technology, or microfluidic technology with the freeze–thaw method can improve the morphology and property of chitosan dressing and shorten the preparation time.
3.6 Electrostatic spinning method
Electrostatic spinning technology refers to that polymer solution ejects out to form a non-woven state of nanofibers in the receiving device under a high-voltage electric field. It is a simple and effective method for the preparation of nanofibers with diameters of 5–500 nm. Compared with the traditional solution spinning method or molten spinning method for the preparation of nano-fiber, the fiber diameter made by electrostatic spinning technology can be much smaller. The non-woven fabric produced by electrospinning technology can be used as a wound dressing.To solve the problem of high viscosity and poor spinnability of chitosan, Homayoni et al.125 used NaOH to hydrolyze chitosan for reducing the molecular weight, forming the fiber more natural and stable. Adding an excellent biomedicinal material to the chitosan solution could be beneficial to the healing of the wound. Fibrin and collagen are difficult to spin, but the addition of polyethylene oxide (PEO) can improve the performance of these polymers in the spinning processing. Chen et al.126 used chitosan/collagen/polyethylene oxide as raw material and acetic acid solution as a solvent to prepare electrospinning nano-fiber dressing. Animal experiments showed that the dressing had good biocompatibility, and there was no cytotoxicity to 3T3 fibroblasts. The healing rate of the dressing was better than gauze and medical collagen sponge wound dressing. Zhou et al.127 used carboxymethyl chitosan/polyvinyl alcohol (CECS/PVA) as material and acrylic acid as a solvent to obtain electrospinning fiber dressing. They found that there was a robust molecular hydrogen bond between the two molecules; cell culture experiments showed that the dressing could promote cell adhesion and proliferation.
The technology of preparing chitosan dressing by electrostatic spinning has been developed, but there are some technical challenges for it. For example, beads will appear in the process of spraying, leading to the weak mechanical properties of fibers. It is necessary to reveal the influence of process parameters on the shape of electrospinning chitosan and solve the problem of condensation beads for improving the medicinal properties of electrospinning chitosan dressing.
The six methods above are the most used techniques in the preparation of chitosan dressing. Chitosan can be combined with different drugs or other polymer materials. Under different process preparation conditions, chitosan showed long-term stability, broadening the method of formulation optimization of chitosan dressing. However, the preparation process has pollution. The acidic solution or strong alkaline solution is often used in the preparation of chitosan solution, which may cause harm to the human body. As a kind of antibacterial material developing rapidly, nano-silver has a potential application in the formulation optimization of chitosan dressing. Combined with different treatment techniques, the advantages of different technologies can be connected to realize the green and efficient preparation process of chitosan medical dressing.
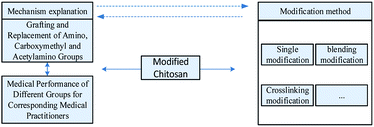
4. Research progress on modification method of chitosan dressing
Chitosan contains a large number of groups including amino, hydroxyl, and acetylamino, which can be combined or replaced in the process of modification. The modification methods of chitosan include physical modification, chemical modification, blending modification, and biological modification, among which chemical modification and blending modification are widely used in the field of medical dressing. Fig. 5 is a research framework for the modification of chitosan.The modification method for single chitosan would enhance the original medical property or obtain new therapeutic property. For example, some severe bleeding wounds often require the dressing to have a strong hemostatic property. Researchers grafted guanidine functional groups on chitosan molecules to enhance the hemostatic property of chitosan. Chitosan had a strong hemostatic effect when the deacetylation degree was 40%, and hemostatic performance increased with the decrease of deacetylation degree.128 After surface modification of chitosan by plasma, Luna et al.129 found that plasma treatment could significantly improve the biocompatibility of chitosan membrane. Eldin et al.130 modified the chitosan membrane for enhancing antibacterial property, which was prepared by grafting the polyamine to the chitosan molecular chain. Besides, the water absorption rate, steam transmittance, and mechanical property of the chitosan membrane were improved.
Modified chitosan membrane has been evaluated as a biological material for trauma repair, but the morphology of pure chitosan prepared by modified dressing is relatively single, leading to the difficult application in different types of wounds. To increase the mechanical strength or healing effect of chitosan-based dressing, scholars conducted the blending modification of chitosan using different other polymer materials. The addition of other polymer materials will enhance the mechanical strength of the dressing, thus the dressing is not easy to be destroyed in human activity. Bhattarai et al.131 blended the polycaprolactone and chitosan for receiving chitosan/polycaprolactone nanofibers, which owned the medical property of chitosan and the mechanical property of polycaprolactone. Nano-level silver ions chelating with chitosan can improve the antibacterial capacity of the dressing, but a high concentration of it will produce cytotoxicity. Zeng et al.132 used acetal reaction in alkaline solution to produce silver chitosan/polyvinyl alcohol sponge, which owned porous structure, ideal water absorption, air breathability, and moisturizing properties as a dressing. The material had no obvious toxicity to fibroblasts of mice and had an inhibitory effect on Staphylococcus aureus, Escherichia coli, Candida albicans, copper-green Pseudomonas, and Salmonella typhi.
The covalent crosslinking and the ion crosslinking play an important role as the modification methods of chitosan, which can link the molecules of different polymers into a stable reticulated structure. Zhang133 used butyric acid grafting polyethylene glycol (PVA) to prepare a type of carboxyl modified and polyvinyl alcohol crosslinked chitosan hydrogel. The mechanical property and the expansion ratio of the cross-linked hydrogel were improved obviously. The evaluation of water vapor permeability showed that crosslinked hydrogel film could keep the wound moist. The biocompatibility test showed that crosslinking hydrogel was non-cytotoxic. Wu et al.134 utilized the ion crosslinking method to prepare a quaternary ammonium chitosan material with thermal effect, which could be changed from colloidal sol state to gel state. Crosslinking can reduce the adsorption capacity of chitosan, but the loss of this ability is a prerequisite for ensuring the stability of polymers.
To sum up, a single modification method has a limited effect on enhancing the property of chitosan medicine, and blending modification is the development trend of the chitosan dressing modification method. Based on the diverse modification methods, the process of modification of chitosan dressing should be improved by selecting suitable modification methods according to the characteristics of various other polymer materials. Then, the mechanism of the replacement law of functional groups should be analyzed.
5. Data-driven mathematical models for drug release from chitosan dressing
Through the cross-linking treatment or preparation technology such as solvent evaporation and freeze-drying, chitosan-based wound dressings will present different types including hydrogel, film, sponge, etc. There are many micro/nano-pores inside these chitosan-based wound dressing, which are suitable for drug delivery.135–137 When the drug-loaded chitosan dressing is applied in the water-rich or biological liquid-rich environment, the water molecules would penetrate between molecules of chitosan, leading the volume expansion of chitosan-based dressing.138,139 Then, the drug can release from chitosan dressing. To realize a specific drug release profile and predict the drug release kinetics quantitatively, a reasonable data-driven mathematical modeling can simulate the effect of preparation parameters of chitosan dressing on drug release. Mathematical modeling of drug release can help us understand the physical and chemical changes during the drug release process. Thus, mathematical modeling can decrease experiment numbers and promote production design.The drug release profile of drug-loaded chitosan dressing can be obtained from Fick's second law, in which the diffusion coefficient of water and the shape of dressing both have an essential influence on the drug release property.140 For example, during the one-dimensional drug release of chitosan microspheres dressing, the second Fick's law of diffusion is given by:
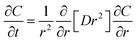 | (1) |
The original data of mathematical models for drug release in chitosan hydrogel dressing was obtained by an ultraviolet spectrophotometer or gel chromatography.142,143 For accuracy, three different mathematical models, which include zero order, first order, and some theoretical models such as Higuchi and Korsmeyer–Peppas, were commonly used to analyze the drug release rules in vitro. Liu et al.144 used three mathematical models (zero-order eqn (2)), first-order (eqn (3)), and Higuchi (eqn (4)) to fit the bFGF (recombinant basic fibroblast growth factor) release profile from the chitosan-based dressing.
| Qt = k0t | (2) |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qt = ln Qt = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Q0 − k1t Q0 − k1t | (3) |
| Qt = kht1/2 | (4) |
| Formulation code | Zero order model | First order model | Higuchi model | Korsmeyer–Peppas model | Ref. | |
|---|---|---|---|---|---|---|
| R 2 | R 2 | R 2 | R 2 | n | ||
| CC | 0.81 | 0.98 | 0.9 | 0.73 | 0.63 | 144 |
| CC-G-E | 0.83 | 0.95 | 0.92 | 0.93 | 1.00 | |
| 2.5-GSNO | 0.70 | 0.51 | 0.85 | 0.95 | 0.53 | 145 |
| 10-GSNO | 0.78 | 0.53 | 0.92 | 0.94 | 0.53 | |
| 20-GSNO | 0.71 | 0.43 | 0.84 | 0.89 | 0.70 | |
| CCS-OA | 0.89 | 0.55 | 0.90 | — | 146 | |
The experiments of drug release from chitosan-based dressing are complex and challenging to achieve. Thus, mathematical models are necessary to simulate the release rules. Parameters such as drug concentration and release time would lead to different drug release models. Some new formulation and preparative techniques of chitosan dressing can be explored from the data-driven mathematical modeling.
6. Conclusions
The preparation of chitosan medical dressing is a topic that aims to optimize the formulation to improve the curative effect under the guidance of the mechanism of medicinal property. The topic is still in the development stage, and some directions need to be paid attention to in the following situations:(1) Current results showed that the shapes of the chitosan-based dressings are of great significance for wound healing. At present, the morphology of chitosan dressing is roughly divided into the membrane, hydrogel, sponge, and asymmetric dressing. Table 2 shows the advantages and disadvantages of several typical chitosan dressing in recent years. In comparison, chitosan composite asymmetric dressing is close to the structure and function of skin epidermis and dermis and has significant benefits in wound treatment effect. The research and development of asymmetric chitosan dressing is an important direction in the future.
| Types | Main preparation process | Advantages | Disadvantages |
|---|---|---|---|
| Membrane | Solvent evaporation method | Good air permeability; cheap price; easy to fixed | Poor mechanical properties; unsuitable for large exudative wounds |
| In situ deposition method | |||
| Hydrogel | Freeze–thaw method | Provide a wet healing environment; dissolved necrotic tissue; a small amount exudate absorption | Easy to leave the residue in the wound; unsuitable for large exudative wounds; unsuitable for the infected wound |
| Ion crosslinking method | |||
| Electrochemical deposition method | |||
| Sponge | Freeze-drying method | Absorb large amount of exudate quickly; promote the growth of granulation tissue; prevent granulation tissue from edema hyperplasia | Need the secondary fixation due to the lacking viscosity to the skin; difficult to observe the wound due to opacity |
| Asymmetric dressing | Electrospinning method | Simulate the structure and function of skin for receiving excellent medical characteristics | Multi-layer deposition or coating will result uneven layers |
| Immersion precipitation phase conversion method |
(2) The modification of chitosan (blending or carboxymethyl modification), drug-loading (ciprofloxacin, silver ions), and bleeding with other polymer materials (sodium alginate, gelatin, polyvinyl alcohol) can effectively enhance the medicinal property of chitosan. However, the major studies of chitosan dressing only focused on the wound healing effect, ignoring the mechanism of the effect of composite dressing on the wound from the aspect of macromolecular or supramolecules, which could help us understand the mechanism of properties of chitosan-based dressings comprehensively. Chitosan composite dressing is one of the highlights of this topic. In addition to enhancing the medicinal property of dressing, the fiber contained in CHM can enhance the mechanical property of the dressing. However, the study of chitosan/CHM compound dressing only stays at the stage of the composite film.
(3) The chitosan-based dressing should minimize the discomfort of patients in clinical treatment. The research points should not only focus on promoting wound healing but propose methods for rapid analgesia, itching, and relieving the discomfort of patients during the healing process. From the aspect of compatibility of the dressing, the combination of drugs such as menthol, camphor, and chitosan, which have analgesic and anti-itching effect, can be used as a method to solve the problem. Due to the limitation of experimental conditions, researchers have mostly used animal experiments to evaluate the medicinal property of chitosan-based dressing which are lacking in clinical curative effect in the process of wound treatment.
(4) From this paper, chitosan has excellent biocompatibility and can be combined with various other polymer materials or drugs by proper preparation techniques. Drugs with antibacterial and anti-inflammatory can effectively work by the sustained release from the drug-loaded chitosan dressings. At present, drugs that have a conflict with chitosan are rarely reported. After chelating with metal ions, the antibacterial property of chitosan-based dressings can be greatly improved. Scholars have confirmed the non-toxicity of short-term application of metal ions in suitable concentration, not the long-term.
(5) The formulation of chitosan dressing is complex and various. To ensure the safety of application, the types of formulation and the concentration of components need to be determined by plenty of medical experiments, but most of the formulation optimization methods are not much innovative by just adding other polymer materials to the chitosan matrix. Using the existing data, the algorithm prediction model of the above parameters can be established on Python software platform147 by back-propagation (BP) neural network technology, which is characterized by the acid and alkaline of solvent, the concentration of chitosan, the main chain structure of other polymer materials, the process type, and the process length. It is necessary to simplify the cycle of innovative formulation optimization experiments of chitosan dressing.
Conflicts of interest
On behalf of all authors, the corresponding author declares no conflicts of interest.Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 51905312, 51775318), The Fundamental Research Funds of Shandong University (Grant No. 2018JC043), China Postdoctoral Science Foundation (Grant No. 2020M672051), Key Research and Development Program of Shandong Province (Grant No. 2019GGX104015), and Key Laboratory of High-efficiency and Clean Mechanical Manufacture at Shandong University, Ministry of Education.References
- X. Zhao, H. Wu, B. Guo, R. Dong, Y. Qiu and P. X. Ma, Biomaterials, 2017, 122, 34–47 CrossRef CAS.
- G. K. Zhang, X. A. Shen, S. R. Chen, L. P. Liang, Y. Luo, J. Yu and J. W. Lu, IEEE Access, 2019, 7, 140936–140945 Search PubMed.
- J. Yoon, B. Jeong, W. H. Lee and J. Kim, IEEE Access, 2018, 6, 26530–26542 Search PubMed.
- V. K. Singh, M. Abdel-Nasser, H. A. Rashwan, F. Akram, N. Pandey, A. Lalande, B. Presles, S. Romani and D. Puig, IEEE Access, 2019, 7, 130552–130565 Search PubMed.
- A. J. Singer and R. A. Clark, N. Engl. J. Med., 1999, 341, 738–746 CrossRef CAS.
- J. P. E. Junker, R. A. Kamel, E. J. Caterson and E. Eriksson, Adv. Wound Care, 2013, 2, 348–356 CrossRef PubMed.
- S.-P. Lin, H.-N. Kung, Y.-S. Tsai, T.-N. Tseng, K.-D. Hsu and K.-C. Cheng, Cellulose, 2017, 24, 4927–4937 CrossRef CAS.
- L. Fan, H. Yang, J. Yang, M. Peng and J. Hu, Carbohydr. Polym., 2016, 146, 427–434 CrossRef CAS PubMed.
- S. Boonlertnirun, C. Boonraung and R. Suvanasara, Int. J. Miner., Metall. Mater., 2017, 18, 47–52 Search PubMed.
- L. Zhang, Y. Zeng and Z. Cheng, J. Mol. Liq., 2016, 214, 175–191 CrossRef CAS.
- E. Rezvani Ghomi, S. Khalili, S. Nouri Khorasani, R. Esmaeely Neisiany and S. Ramakrishna, J. Appl. Polym. Sci., 2019, 136, 47738 CrossRef.
- S. Dhivya, V. V. Padma and E. Santhini, Biomedicine, 2015, 5, 22 CrossRef.
- M. A. Matica, F. L. Aachmann, A. Tondervik, H. Sletta and V. Ostafe, Int. J. Mol. Sci., 2019, 20, 5889 CrossRef CAS PubMed.
- H. Liu, C. Wang, C. Li, Y. Qin, Z. Wang, F. Yang, Z. Li and J. Wang, RSC Adv., 2018, 8, 7533–7549 RSC.
- M. H. Periayah, A. S. Halim and A. Z. M. Saad, Int. J. Hematol. Oncol. Stem Cell Res., 2017, 11, 319–327 Search PubMed.
- M. V. Pogorielov and V. Z. Sikora, Eur. J. Med., 2015, 2, 24–33 Search PubMed.
- H. S. Whang, W. Kirsch, Y. H. Zhu, C. Z. Yang and S. M. Hudson, J. Macromol. Sci., Polym. Rev., 2005, 45, 309–323 CrossRef.
- L. Chen, H. Deng, H. Cui, J. Fang, Z. Zuo, J. Deng, Y. Li, X. Wang and L. Zhao, Oncotarget, 2018, 9, 7204–7218 CrossRef PubMed.
- T. Takei, H. Nakahara, H. Ijima and K. Kawakami, Acta Biomater., 2012, 8, 686–693 CrossRef CAS.
- D. Fong and C. D. Hoemann, Future Sci. OA, 2018, FSO225 CrossRef CAS PubMed.
- H. J. Yoon, M. E. Moon, H. S. Park, S. Y. Im and Y. H. Kim, Biochem. Biophys. Res. Commun., 2007, 358, 954–959 CrossRef CAS.
- J. M. Reinke and H. Sorg, Eur. Surg. Res., 2012, 49, 35–43 CrossRef CAS.
- L. E. Dickinson and S. Gerecht, Front. Physiol., 2016, 7, 341 CrossRef.
- C. C. Yates, P. Hebda and A. Wells, Birth Defects Res., Part C, 2013, 96, 325–333 CrossRef.
- D. Archana, B. K. Singh, J. Dutta and P. K. Dutta, Carbohydr. Polym., 2013, 95, 530–539 CrossRef CAS.
- F. Ahmadi, Z. Oveisi, S. Mohammadi Samani and Z. Amoozgar, Results Pharma Sci., 2015, 10, 1–16 CAS.
- B. Li, C.-L. Shan, Q. Zhou, Y. Fang, Y.-L. Wang, F. Xu, L.-R. Han, M. Ibrahim, L.-B. Guo and G.-L. Xie, Mar. Drugs, 2013, 11, 1534–1552 CrossRef CAS PubMed.
- M. Beppu, R. Vieira, C. Aimoli and C. Santana, J. Membr. Sci., 2007, 301, 126–130 CrossRef CAS.
- B. Zhang, J. He, M. Shi, Y. Liang and B. Guo, Chem. Eng. J., 2020, 400, 125994 CrossRef CAS.
- F. Wahid, H.-S. Wang, C. Zhong and L.-Q. Chu, Carbohydr. Polym., 2017, 165, 455–461 CrossRef CAS PubMed.
- M. Hayes, in Marine Bioactive Compounds: Sources, Characterization and Applications, ed. M. Hayes, Springer US, Boston, MA, 2012, pp. 115–128 Search PubMed.
- S. Liu, J. Sun, L. Yu, C. Zhang, J. Bi, F. Zhu, M. Qu, C. Jiang and Q. Yang, Molecules, 2012, 17, 4604–4611 CrossRef CAS.
- B. S. Henriques, E. S. Garcia, P. Azambuja and F. A. Genta, Front. Physiol., 2020, 11, 117 CrossRef.
- H. Schmalfuss, M. N. Horst and J. A. Freeman, J. Crustac Biol., 1993, 14, 406 CrossRef.
- I. Younes and M. Rinaudo, Mar. Drugs, 2015, 13, 1133–1174 CrossRef CAS.
- H. K. No and E. Y. Hur, J. Agric. Food Chem., 1999, 46, 3844–3846 CrossRef.
- H. K. No and S. P. Meyers, J. Aquat. Food Prod. Technol., 1995, 4, 27–52 CrossRef CAS.
- M. Rolandi and R. Rolandi, Adv. Colloid Interface Sci., 2014, 207, 216–222 CrossRef CAS PubMed.
- T. Yilmaz, L. Maldonado, H. Turasan and J. Kokini, J. Food Eng., 2019, 254, 42–50 CrossRef CAS.
- C. K. S. Pillai, W. Paul and C. P. Sharma, Prog. Polym. Sci., 2009, 34, 641–678 CrossRef CAS.
- C. Qin, H. Li, Q. Xiao, Y. Liu, J. Zhu and Y. Du, Carbohydr. Polym., 2006, 63, 367–374 CrossRef CAS.
- P. Bowler, B. I. Duerden and D. Armstrong, Clin. Microbiol. Rev., 2001, 14, 244–269 CrossRef CAS PubMed.
- A. Patanaik and R. D. Anandjiwala, in Soft Computing in Textile Engineering, ed. A. Majumdar, Woodhead Publishing, 2011, pp. 246–267 Search PubMed.
- B. Ma, A. Qin, X. Li, X. Zhao and C. He, Int. J. Biol. Macromol., 2014, 64, 341–346 CrossRef CAS PubMed.
- C. Clasen, T. Wilhelms and W. M. Kulicke, Biomacromolecules, 2006, 7, 3210–3222 CrossRef CAS.
- Z. A. Yao, B. Q. Han, W. S. Liu, W. Z. Wang and L. D. Xuan, Chin. J. Biomed. Eng., 2002, 21, 256–262 CAS.
- Y. H. Ding, A. F. Huang and W. X. Li, Chin. J. Mar. Drugs, 2001, 6, 32–34 Search PubMed.
- S. Patricia Miranda, O. Garnica, V. Lara-Sagahon and G. Cárdenas, J. Chil. Chem. Soc., 2004, 49, 173–178 Search PubMed.
- P. Lu, H. Xiao, W. Zhang and G. Gong, Carbohydr. Polym., 2014, 111, 524–529 CrossRef CAS PubMed.
- T. Takahashi, M. Imai and I. Suzuki, Biochem. Eng. J., 2007, 36, 43–48 CrossRef CAS.
- K. Tomihata and Y. Ikada, Biomaterials, 1997, 18, 567 CrossRef CAS.
- A. B. Dos Reis, C. M. Pedroso Yoshida, S. O. F. Vera and W. P. Silva, Defect Diffus. Forum, 2012, 170–175 CAS.
- X. Yang and T. Xi, J. Biomed. Eng., 2001, 18, 123–128 CAS.
- E. J. Gao, B. F. Liu and L. U. Qiang, Chin. J. Rehabil. Theory Pract., 2004, 1, 34–36 Search PubMed.
- Z. Yao, H. Wu, B. Han and W. Liu, J. Biomed. Eng., 2006, 23, 800–804 CAS.
- T. Freier, H. S. Koh, K. Kazazian and M. S. Shoichet, Biomaterials, 2005, 26, 5872–5878 CrossRef CAS.
- C. Mingyu, G. Kai, L. Jiamou, G. Yandao, Z. Nanming and Z. Xiufang, J. Biomater. Appl., 2004, 19, 59–75 CrossRef PubMed.
- H. Qing, A. Qiang, D. Han, Z. Wang, W. Liu, Y. Gong and X. Zhang, Int. J. Biomed. Eng., 2012, 35, 65–69 Search PubMed.
- W. Chen, Q. Wu, J. Zhang and H. Wu, Acta Microbiol. Sin., 2008, 48, 164–168 CAS.
- M. A. Peng peng, H. E. Li qian and T. Z. Gao, J. Beijing Union Univ., 2003, 3, 27–30 Search PubMed.
- Y. C. Chung, H. L. Wang, Y. M. Chen and S. L. Li, Bioresour. Technol., 2003, 88, 179–184 CrossRef CAS PubMed.
- Z. J.-f. Heng Liying and K. Sun, J. Mater. Sci. Eng., 2000, 18, 22–24 Search PubMed.
- Y. J. Jing, Y. Hao, H. Qu, Y. Shan, D. S. Li and R. Q. Du, Chin. J. Antibiot., 2006, 31, 361–365 CAS.
- H. Wang and Y. Shen, J. `Shanghai Fish. Univ., 2001, 4, 380–382 Search PubMed.
- W. Hong-li, Cont. Med. Educ., 2006, 26, 13–19 Search PubMed.
- J. Yang, F. Tian and S. Chen, Int. J. Biomed. Eng., 2001, 24, 77–80 Search PubMed.
- D. Li, P. Li, J. Zang and J. Liu, J. Biomed. Biotechnol., 2012, 981321 Search PubMed.
- S. Sagnella and K. Mai-Ngam, Colloids Surf., B, 2005, 42, 147–155 CrossRef CAS PubMed.
- J. Yang, F. Tian, Z. Wang, Q. Wang, Y.-J. Zeng and S.-Q. Chen, J. Biomed. Mater. Res. B Appl. Biomater., 2008, 84, 131–137 CrossRef PubMed.
- L. U. Bin, L. Q. Qian and Z. L. Zhang, J. Health Res., 2010, 30, 55–58 Search PubMed.
- K. Liu, Y. Kang, Z. Wang and X. Zhang, Adv. Mater., 2013, 25, 5530–5548 CrossRef CAS PubMed.
- M. J. Webber, E. A. Appel, E. W. Meijer and R. Langer, Nat. Mater., 2016, 15, 13–26 CrossRef CAS PubMed.
- H. S. Choi and N. Yui, Prog. Polym. Sci., 2006, 31, 121–144 CrossRef CAS.
- W. Xu, Q. Song, J.-F. Xu, M. J. Serpe and X. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 11368–11372 CrossRef CAS.
- A. Dutta, S. Maity and R. K. Das, 2018, 303, 1800322.
- A. Dutta, S. Maity and R. K. Das, Macromol. Mater. Eng., 2018, 303, 1800322 CrossRef.
- M. Montiel-Herrera, A. Gandini, F. M. Goycoolea, N. E. Jacobsen, J. Lizardi-Mendoza, M. Recillas-Mota and W. M. Argüelles-Monal, Carbohydr. Polym., 2015, 128, 220–227 CrossRef CAS.
- O. Guaresti, C. García–Astrain, T. Palomares, A. Alonso–Varona, A. Eceiza and N. Gabilondo, Int. J. Biol. Macromol., 2017, 102, 1–9 CrossRef CAS PubMed.
- M. Zhang, J. Wang and Z. Jin, Int. J. Biol. Macromol., 2018, 114, 381–391 CrossRef CAS PubMed.
- Z. Zhang, T. Li, B. Chen, S. Wang and Z. Guo, J. Mater. Sci., 2017, 52, 10614–10623 CrossRef CAS.
- B. Yue, J. Glaucoma, 2014, 23, S20–S23 CrossRef PubMed.
- V. Jayawarna, M. Ali, T. A. Jowitt, A. F. Miller, A. Saiani, J. E. Gough and R. V. Ulijn, Adv. Mater., 2006, 18, 611–614 CrossRef CAS.
- M. Zhou, A. M. Smith, A. K. Das, N. W. Hodson, R. F. Collins, R. V. Ulijn and J. E. Gough, Biomaterials, 2009, 30, 2523–2530 CrossRef CAS PubMed.
- P. Chakraborty, M. Ghosh, L. Schnaider, N. Adadi, W. Ji, D. Bychenko, T. Dvir, L. Adler-Abramovich and E. Gazit, Macromol. Rapid Commun., 2019, 40, 1900175 CrossRef PubMed.
- P. Chakraborty, B. Roy, P. Bairi and A. K. Nandi, J. Mater. Chem., 2012, 22, 20291–20298 RSC.
- W. Klinkajon and P. Supaphol, Biomed. Mater., 2014, 9, 045008 CrossRef CAS.
- Z. Sun, F. Lv, L. Cao, L. Liu, Y. Zhang and Z. J. A. C. Lu, 2015, 127, 8055–8059.
- S. R. U. Rehman, R. Augustine, A. A. Zahid, R. Ahmed, M. Tariq and A. Hasan, Int. J. Nanomed., 2019, 14, 9603–9617 CrossRef PubMed.
- S. Yu and C. Gao, Membr. Sci. Technol., 2000, 5, 36–41 Search PubMed.
- F. L. Mi, S.-S. Shyu, Y.-B. Wu, S.-T. Lee, J.-Y. Shyong and R.-N. Huang, Biomaterials, 2001, 22, 165–173 CrossRef CAS.
- L. Liang, P. F. Fei, B. W. Cheng, J. Q. Meng, X. Y. Hu and J. Song, Acta Polym. Sin., 2018, 5, 607–616 Search PubMed.
- H. Hua, J. Wei and C. Liu, Compos. B Eng., 2007, 38, 311–316 CrossRef.
- R. Poonguzhali, S. K. Basha and V. S. Kumari, Int. J. Biol. Macromol., 2018, 112, 1300–1309 CrossRef CAS PubMed.
- Z. Wang, Z. Liu, L. Chen, Y. Zhao and X. Feng, Polym.-Plast. Technol. Eng., 2018, 57, 1352–1359 CrossRef CAS.
- X. Zeng and E. Ruckenstein, Ind. Eng. Chem. Res., 1996, 35, 4169–4175 CrossRef CAS.
- A. K. Azad, N. Sermsintham, S. Chandrkrachang and W. Stevens, J. Biomed. Mater. Res. B Appl. Biomater., 2004, 69, 216–222 CrossRef.
- H. Xu, L. Ma, H. Shi, C. Gao and C. Han, Polym. Adv. Technol., 2007, 18, 869–875 CrossRef CAS.
- Y. Wang, H.-J. Cao, L.-Q. Wang, C.-L. Lu, Y.-Q. Yan, H. Lu, K. Zhang, H.-M. Zhang and J.-P. Liu, Compl. Ther. Med., 2019, 44, 32–43 CrossRef PubMed.
- L. Linggen, Z. Gang and W. Mingyuan, Chin. J. Surg. Integr. Tradit. West. Med., 2002, 8, 80–83 Search PubMed.
- A. N. Yanchen, F. U. Zhengying and Z. Liu, J. Liaoning Univ. Tradit. Chin. Med., 2015, 17, 59–61 Search PubMed.
- L. Wu, A. Gadre, H. Yi, M. Kastantin, G. Rubloff, W. Bentley, G. Payne and R. Ghodssi, Langmuir, 2002, 18, 8620–8625 CrossRef CAS.
- X. Pang and I. Zhitomirsky, Mater. Chem. Phys., 2005, 94, 245–251 CrossRef CAS.
- X.-L. Luo, J.-J. Xu, Q. Zhang, G.-J. Yang and H.-Y. Chen, Biosens. Bioelectron., 2005, 21, 190–196 CrossRef CAS.
- B. Qiu, X.-F. Xu, R.-H. Deng, G.-Q. Xia, X.-F. Shang and P.-H. Zhou, Int. J. Biol. Macromol., 2019, 122, 82–87 CrossRef CAS.
- S.-Y. Ong, J. Wu, S. Moochhala, M. Tan and J. Lu, Biomaterials, 2008, 29, 4323–4332 CrossRef CAS.
- J. Nie, Z. Wang and Q. Hu, Sci. Rep., 2016, 6, 36005 CrossRef CAS.
- X. Shen, H. H. Tong, T. Jiang, Z. Zhu, P. Wan and J. Hu, Compos. Sci. Technol., 2007, 67, 2238–2245 CrossRef CAS.
- A. Reyes, A. Mahn and P. Huenulaf, Drying Technol., 2011, 29, 1076–1089 CrossRef CAS.
- Y. Huang, L. Feng, Y. Zhang, H. Liumin, C. Wang, J. Xu, J. Wu, T. Kirk, R. Guo and W. Xue, Polym. Adv. Technol., 2017, 28, 1107–1114 CrossRef CAS.
- M. Jaiswal, V. Koul and A. Dinda, J. Appl. Polym. Sci., 2016, 133, 43472 CrossRef.
- J. Berretta, J. D. Bumgardner and J. A. Jennings, in Chitosan Based Biomaterials Volume 1, ed. J. A. Jennings and J. D. Bumgardner, Woodhead Publishing, 2017, pp. 239–253 Search PubMed.
- J. Wu, C. Su, L. Jiang, S. Ye, X. Liu and W. Shao, ACS Sustainable Chem. Eng., 2018, 6, 9145–9152 CrossRef CAS.
- E. Öztürk, C. Agalar, K. Kececi and E. Denkbas, J. Appl. Polym. Sci., 2006, 101, 1602–1609 CrossRef.
- Y. Guan, Y. Tian, Z. F. Yang, M. C. Chen and A. D. Wen, China Pharm., 2011, 11, 961–963 Search PubMed.
- Y. Fang, B. Duan, A. Lu, M. Liu, H. Liu, X. Xu and L. Zhang, Biomacromolecules, 2015, 16, 1410–1417 CrossRef CAS.
- X. Hu, Y. Du, Y. Tang, Q. Wang, T. Feng, J. Yang and J. Kennedy, Carbohydr. Polym., 2007, 70, 451–458 CrossRef CAS.
- R. Ricciardi, F. Auriemma and C. De Rosa, Macromol. Symp., 2005, 222, 49–64 CrossRef CAS.
- S. R. Stauffer and N. A. Peppast, Polymer, 1992, 33, 3932–3936 CrossRef CAS.
- Y. Zhao, Z. Li, S. Song, K. Yang, H. Liu, Z. Yang, J. Wang, B. Yang and Q. Lin, Adv. Funct. Mater., 2019, 29, 1901474 CrossRef.
- J. Sung, M.-R. Hwang, J. Kim, j. h. Lee, Y. Kim, J. H. Kim, S. Chang, S. Jin, J. Kim, W. Lyoo, S. S. Han, S. Ku, C. Yong and H.-G. Choi, Int. J. Pharm., 2010, 392, 232–240 CrossRef CAS.
- L. Z. He, D. Yang and Y. Liu, Pharm. Biotechnol., 2006, 13, 45 CAS.
- L. Zhao, H. Mitomo, Z. Maolin, F. Yoshii, N. Nagasawa and T. Kume, Carbohydr. Polym., 2003, 53, 439–446 CrossRef CAS.
- X. Yang, K. Yang, S. Wu, X. Chen, F. Yu, J. Li, M. Ma and Z. Zhu, Radiat. Phys. Chem., 2010, 79, 606–611 CrossRef CAS.
- T. Wang, X.-K. Zhu, X.-T. Xue and D.-Y. Wu, Carbohydr. Polym., 2012, 88, 75–83 CrossRef CAS.
- H. Homayoni, S. A. Hosseini Ravandi and M. Valizadeh, Carbohydr. Polym., 2009, 77, 656–661 CrossRef CAS.
- J.-P. Chen, G.-Y. Chang and J.-K. Chen, Colloid. Surface. Physicochem. Eng. Aspect., 2008, 313–314, 183–188 CrossRef.
- Y. Zhou, D. Yang, X. Chen, Q. Xu, F. Lu and J. Nie, Biomacromolecules, 2008, 9, 349–354 CrossRef CAS PubMed.
- X. Huang, Y. Sun, J. Nie, W. Lu, L. Yang, Z. Zhang, H. Yin, Z. Wang and Q. Hu, Int. J. Biol. Macromol., 2015, 75, 322–329 CrossRef CAS.
- S. M. Luna, S. S. Silva, M. E. Gomes, J. F. Mano and R. L. Reis, J. Biomater. Appl., 2011, 26, 101–116 CrossRef CAS PubMed.
- M. Mohy Eldin, E. Soliman, A. Hashem and T. Tamer, Trends Biomater. Artif. Organs, 2008, 22, 158–168 Search PubMed.
- N. Bhattarai, Z. Li, J. Gunn, M. Leung, A. Cooper, D. Edmondson, O. Veiseh, M. H. Chen, Y. Zhang and R. G. Ellenbogen, Adv. Mater., 2009, 21, 2792–2797 CrossRef CAS.
- X.-f. Zeng, K. Wei, X. Han, Y. Luo, L.-j. Shu, Y. Wu and Z.-y. Shen, J. Clin. Rehabilitative Tissue Eng. Res., 2011, 15, 1413–1416 CAS.
- D. Zhang, W. Zhou, B. Wei, X. Wang, R. Tang, J. Nie and J. Wang, Carbohydr. Polym., 2015, 125, 189–199 CrossRef CAS PubMed.
- J. Wu, Y.-Q. Wang, Q. Fan, Y.-B. Wu and G.-H. Ma, J. Contr. Release, 2015, 213, e51–e52 CrossRef PubMed.
- S. M. Ahsan, M. Thomas, K. K. Reddy, S. G. Sooraparaju, A. Asthana and I. Bhatnagar, Int. J. Biol. Macromol., 2018, 110, 97–109 CrossRef CAS PubMed.
- Z. Cao, Z. Shen, X. Luo, H. Zhang, Y. Liu, N. Cai, Y. Xue and F. Yu, Carbohydr. Polym., 2017, 166, 320–328 CrossRef CAS.
- D. Ye, Z. Zhong, H. Xu, C. Chang, Z. Yang, Y. Wang, Q. Ye and L. Zhang, Cellulose, 2016, 23, 749–763 CrossRef CAS.
- L. Brannon-Peppas, in Studies in polymer science, Elsevier, 1990, vol. 8, pp. 45–66 Search PubMed.
- L. Brannon-Peppas and N. Peppas, in Studies in Polymer Science, Elsevier, 1990, vol. 8, pp. 67–102 Search PubMed.
- J.-M. Vergnaud, Controlled drug release of oral dosage forms, CRC Press, 1993 Search PubMed.
- D. Y. Arifin, L. Y. Lee and C.-H. Wang, Adv. Drug Deliv. Rev., 2006, 58, 1274–1325 CrossRef CAS.
- N. Bhattarai, J. Gunn and M. Zhang, Adv. Drug Deliv. Rev., 2010, 62, 83–99 CrossRef CAS.
- A. Bernkop-Schnürch and S. Dünnhaupt, Eur. J. Pharm. Biopharm., 2012, 81, 463–469 CrossRef.
- T. Liu, W. Dan, N. Dan, X. Liu, X. Liu and X. Peng, Mater. Sci. Eng., C, 2017, 77, 202–211 CrossRef CAS.
- J. O. Kim, J.-K. Noh, R. K. Thapa, N. Hasan, M. Choi, J. H. Kim, J.-H. Lee, S. K. Ku and J.-W. Yoo, Int. J. Biol. Macromol., 2015, 79, 217–225 CrossRef CAS PubMed.
- X. Li, S. Chen, B. Zhang, M. Li, K. Diao, Z. Zhang, J. Li, Y. Xu, X. Wang and H. Chen, Int. J. Pharm., 2012, 437, 110–119 CrossRef CAS.
- S. Raschka, Python machine learning, Packt Publishing Ltd, 2015 Search PubMed.
Footnote |
| † Shanguo Zhang and Jianyong Li have made equal contributions to the paper. |
| This journal is © The Royal Society of Chemistry 2020 |