Open Access Article
Open Access ArticleImpact of heat treatment on anti-oxidative and anti-colon cancer activities of the soluble extracts from black mulberry (Morus nigra L.) using water and ethanol–water solvents
Wen-Si Cuiab,
Qiang Zhang*a and
Xin-Huai Zhao *ab
*ab
aSchool of Biology and Food Engineering, Guangdong University of Petrochemical Technology, 525000 Maoming, PR China. E-mail: zhaoxh@gdupt.edu.cn; zhangqiang@gdupt.edu.cn
bKey Laboratory of Dairy Science, Ministry of Education, Northeast Agricultural University, Harbin 150030, PR China
First published on 17th August 2020
Abstract
Black mulberry (Morus nigra L.) is an edible fruit with various health functions in the body. In this study, the lyophilized black mulberry was extracted using water and 75% (v/v) ethanol–water, respectively; afterwards, the soluble extracts were subjected to these treatments like ethanol removal, heat treatment at 100 °C for various times, or activated carbon-mediated dephenolization. The assaying results indicated that the used heat treatment led to decreased anthocyanin but increased total phenol and flavonoid contents for the water- and ethanol-extracts, while the dephenolized extracts after the heat treatment also had increased total phenol and flavonoid contents. The performed heat treatment decreased anti-oxidative activities of the water- and ethanol-extracts, resulting in reduced scavenging activities to the DPPH and hydroxyl radicals and lower reducing power for Fe(III) ions. However, the results from cell experiments also demonstrated that the heat treatment at 100 °C for 45 min caused the water- and ethanol-extracts or dephenolized extracts with higher anti-cancer activity against human colon cancer HCT-116 cells. Overall, the heated extracts were more effective than the unheated counterparts to inhibit cell growth, alter cell morphology, generate more intracellular reactive oxygen species, enhance intracellular Ca2+ level, and reduce mitochondrial membrane potential of the cells. It is thereby concluded that the heat treatment of black mulberry might reduce its anti-oxidation but increase its anti-colon cancer effect due to the occurrence of the Maillard reaction and other unidentified reactions, which will deepen our present knowledge and provide a scientific basis to optimize storage or processing conditions of plant-based foods.
Introduction
Black mulberry (Morus nigra L.) is traditionally one of the edible fruits, and also is regarded as one of the medicinal plants in China.1 Black mulberry has various health functions such as lowering blood pressure, liver protection, anti-fatigue effect, and neuroprotection.2 Mulberry fruits are consumed as both fresh and processed products (e.g. juices, fruit salads, and dried fruits).3 Recently, the production and consumption of mulberry fruits are rapidly increasing because of their aromatic flavors, nutritional values, and various bioactive compounds.4 The main components of black mulberry are water, sugars, and organic acids.5 Black mulberry also contains various minor components including natural colorant substances anthocyanins and other phenolic/polyphenolic substances like hydroxyl-benzoic acid, chlorogenic acid, resveratrol, quercetin, and kaempferol.6 Phenols and polyphenols are natural anti-oxidants.7 Increasing evidence indicates that phenols especially polyphenols also have beneficial bioactivities in the body such as anti-cardiovascular, anti-diabetic, anti-inflammatory, and immuno-modulatory effects.2 However, both phenols and polyphenols in general are chemically instable because they can be degraded by many factors such as oxidative conditions, alkaline pH, and higher temperatures.8,9 Thereby, these compounds in plant foods after storage or during heat treatment undergo both content and bioactivity changes. It had been found that heat treatment of apple juice could decrease polyphenol content by decomposing seven proanthocyanins,10 while the sweet potato skin after cooking using microwave, boiling, and baking showed decreased phenol content and anti-oxidant activity.11 Two previous studies also revealed that drying red grape skin at higher temperatures led to decreased polyphenol content and anti-oxidant activity, while increased mashing temperature caused the reduction of polyphenol content in the wort.12,13 More importantly, a previous study from our research group verified that both heat and oxidative treatments of four polyphenols galangin, kaempferol, morin, and myricetin resulted in weakened anti-colon cancer effect on the human colon cancer HCT-116 cells.8 Overall, both oxidation and heat treatments of these polyphenols were regarded to impact their residual contents and biofunctions. However, to the best of our knowledge, the possible effect of heat treatment on beneficial biofunctions such as anti-oxidative and anti-cancer potentials of black mulberry is less-studied so far.Cancer is one of the most important causes of human death. Colorectal cancer is the third most common cancer in the United States, and is also a major health problem in other regions.14 As important dietary components, natural anti-oxidants play a critical role in cancer prevention.15 In usual, phenols and polyphenols have a non-toxic effect on normal cells; for example, they are not cytotoxic to renal cells and rat hepatocytes.16,17 However, they are capable of inhibiting the growth of various cancer cells and induce cell death. It was reported that the extract from strawberry had phenol compounds like anthocyanins and flavonoids, and could inhibit the growth of liver cancer HepG2 cells;18 moreover, the phenolic substances extracted from sweet potato leaves could inhibit the growth of human colon cancer DLD-1, leukemia HL-60, and gastric cancer Kato III cells.19 It was also found that the Mango polyphenols were able to induce the death of breast cancer MDA-MB231 cells,20 while the polyphenols from black mulberry had anti-cancer activity against prostate cancer PC-3 cells.21 When black mulberry is used as a raw material to produce these foods like juices and beverages, sterilization is a necessary step applied for the final products to ensure their shelf lives. However, whether the heat treatment of black mulberry might cause increased or decreased anti-cancer effect on cancer cells is still less-investigated. Such in vitro investigation thus deserves our consideration.
In this study, a freeze-dried black mulberry was extracted using both water and 75% (v/v) ethanol–water solvents at room temperature (20 °C), respectively, followed by various treatments like ethanol removal, dephenolization with activated carbon, or heat treatment at 100 °C for various periods. The yielded extracts were measured for their changes in phenol/polyphenol contents and anti-oxidation, and especially were assessed for their anti-cancer effect on the HCT-116 cells using growth inhibition, cell morphology, intracellular reactive oxygen species (ROS), mitochondrial membrane potential (MMP) disruption, and intracellular Ca2+ concentration as evaluation indices. This study aimed to verify how heat treatment might impact the anti-oxidation and anti-colon cancer potentials of the water- and ethanol-extracts from edible black mulberry.
Materials and methods
Materials and chemicals
Both gallic acid and quercetin (>97% of purity) were obtained from Meilun Biological Research Institute (Dalian, Liaoning, China). The Folin–Ciocalteu reagent was obtained from Solaibao Biotechnology Research Institute (Beijing, China). Both McCoy's 5A medium and 1,1-diphenyl-2-picyrl-hydrazyl (DPPH) were obtained from Sigma Chemicals Co. (St. Louis, MO, USA). Fetal bovine serum (FBS) was obtained from Wisent Inc. (Montreal, QC, Canada). The cell counting kit-8 (CCK-8) was bought from Dojindo Molecular Technologies Inc. (Kyushu, Japan), while the reactive oxygen species (ROS) assay kit, Hoechst 33258, mitochondrial membrane potential assay kit with JC-1, Fura-2 pentakis (acetoxymethyl) ester (Fura-2 Am) were provided by Beyotime Biotechnology Co. (Shanghai, China). The 5-fluorouracil (5-Fu, a chemo-therapeutic agent) was purchased from Tianjin Jinyao Pharmaceutical Co. Ltd., (Tianjin, China). Other used reagents were of analytical grade. Distilled water was used in chemical analyses, while ultrapure water generated from Milli-Q Plus (Millipore Corp., New York, NY, USA) was used in cell experiments.Black mulberry was harvested from Shandong Huali Food Co. Ltd. (Weifang, Shandong, China) in 2018. After being freeze-dried by an ALPHA 1–4 LSC plus freeze dryer (Marin Christ Co. Ltd., Osterode, Germany), the lyophilized black mulberry was ground into fine powder with a size diameter less than 150 μm and stored at −20 °C before use.
Preparation of soluble extracts for chemical and spectrometric analyses
Lyophilized black mulberry of 0.12 g was extracted with water for two times. Each extraction was performed using 10 mL water at 20 °C for 20 min with gentle stirring, while the obtained extracts were merged and added with water to a fixed volume of 20 mL. Part of the resultant water-extract was held in top-sealed glass tubes, heated for 15, 30, and 45 min at a water-bath operated at 100 °C, respectively, and cooled rapidly with the ice-water. Afterward, the unheated and heated water-extracts were measured for chemical features and anti-oxidative properties as below.At the same time, lyophilized black mulberry of 0.12 g was extracted with 75% (v/v) ethanol–water solvent for two times using the mentioned conditions as above. The obtained extracts were merged and lyophilized using the freezes dryer to remove ethanol. Lyophilized ethanol-extract was reconstituted with water of 20 mL to yield the ethanol-extract. After then, part of the ethanol-extract was heated at 100 °C as above. The unheated and heated ethanol-extracts were also analyzed for these indices as above.
Both water- and ethanol-extracts of 10 mL were added with 0.1 g of activated carbon, held at 20 °C for 20 min with gentle stirring to conduct the carbon-mediated dephenolization, and centrifuged two times at 5000g for 15 min to separate the supernatants. Part of these supernatants was heated at 100 °C for 45 min and cooled in the ice-water. Afterward, all prepared samples (dephenolized water- and ethanol-extracts) were used in later analyses.
Chemical and spectrometric analyses
Moisture and protein contents were determined using the corresponding oven-drying and Kjeldahl methods recommended by AOAC.22 A conversion factor of 6.25 was used in protein calculation. Moreover, saccharide content was determined using the phenol–sulfuric acid method.23Total phenol content was measured using the Folin–Ciocalteu method.24 In brief, the samples of 0.2 mL were mixed with the Folin–Ciocalteau reagent of 0.2 mL, kept at 20 °C for 5 min, added with 4 mL 20 g L−1 Na2CO3 and 0.6 mL water, and then held for another 30 min. The absorbance values were measured at 750 nm by a UV-visible spectrophotometer (UV-2401 PC, Shimadzu Co., Kyoto, Japan), and used to calculate total phenol content expressed as gallic acid equivalent (mg L−1). A serial of standard gallic acid solutions was used to generate a standard curve for this assay.
Total flavonoid content was assayed using a colorimetric method as previously described.25 In total, the samples of 2 mL were mixed with 0.1 mL 10 g L−1 AlCl3, 0.1 mL 1 mol L−1 potassium acetate, and 2.8 mL water, and held at 20 °C for 40 min. The absorbance values were measured at 415 nm by the spectrophotometer, and used to calculate total flavonoid content expressed as quercetin equivalent (mg L−1). A serial of standard quercetin solutions was used to generate a standard curve for this assay.
Anthocyanin content was measured using the pH differential method as previously described.26 The samples of 1 mL were mixed with 4 mL KCl buffer (25 mmol L−1, pH 1.0) or sodium acetate buffer (0.4 mol L−1, pH 4.0), held at 20 °C for 15 min, and measured for absorbance values at 515 or 700 nm by the spectrophotometer, respectively. Anthocyanin content (mg L−1) was calculated on a cyanidin-3-glucoside basis.
The endogenous fluorescence intensity was measured using the F-4500 fluorescence spectrophotometer (Hitachi, Kyoto, Japan) as previously described.27 Excitation and emission wavelengths of 347 and 415 nm together with a slit width of 10 nm were used in this assay. The UV-absorption and browning extents of the samples were assayed as previously described,28 using the spectrophotometer and suggested wavelengths of 294 and 420 nm, respectively.
Assays of free radical scavenging and reducing power
Scavenging activity for DPPH radicals was measured and calculated as previously described.29 The samples (gallic acid equivalent 100 mg L−1) of 0.2 mL were mixed with 3.8 mL DPPH solution (0.1 mmol L−1) and incubated at 30 °C for 30 min. The absorbance value of the whole reaction system was measured at 517 nm using the spectrophotometer. Meanwhile, scavenging activity for hydroxyl radicals was assayed and estimated as previously reported.30 Briefly, the samples of 1 mL (gallic acid equivalent 100 mg L−1) were mixed with 1 mL 6 mmol L−1 FeSO4 and 1 mL 6 mmol L−1 H2O2, kept at 20 °C for 10 min, added with 1 mL 6 mmol L−1 salicylic acid–ethanol solution, held at 37 °C for another 30 min, and then measured for absorbance values at 510 nm using the spectrophotometer.Reducing power was measured and calculated according to a reported method.31 The samples of 2 mL (gallic acid equivalent 20 mg L−1) were mixed with 2 mL phosphate buffer saline (PBS, 0.2 mol L−1, pH 6.6) and 2 mL 1% K3Fe(CN)6, reacted at 50 °C for 30 min, cooled rapidly in the ice-water, and added with 2 mL 10% trichloroacetic acid. The resultant supernatants of 2 mL were mixed with 2 mL water and 0.4 mL 0.1% FeCl3, held at 20 °C for 10 min, and measured for absorbance values at 700 nm using the spectrophotometer.
Preparation of soluble extracts for cell experiments
Lyophilized black mulberry of 1.2 g was extracted one time with 10 mL water at 20 °C for 20 min with gentle stirring. The obtained soluble extract was added with water to a final volume of 10 mL, and filtrated with 0.22 μm micro-pore membranes. These treatments endowed the final water-extract and extract-added cell medium with higher levels of gallic acid equivalent, to ensure reasonable value changes for these assessed indices in the forthcoming cell experiments. The water-extract was diluted with the FBS-fortified medium to reach gallic acid equivalents of 10–80 mg L−1, and then used to treat the cells. At the same time, part of the water-extract was held in a top-sealed glass tube, heated at 100 °C for 45 min, and cooled rapidly in the ice-water. The heated water-extract was also diluted with the FBS-fortified medium and used in cell experiments.Lyophilized black mulberry of 1.2 g was extracted one time as above but using 75% (v/v) ethanol–water solvent (10 mL) to replace water solvent, while the obtained extract was lyophilized to remove ethanol. The lyophilized ethanol-extract was reconstituted with water of 10 mL and filtrated with 0.22 μm micro-pore membranes to obtain the ethanol-extract. Afterward, the ethanol-extract was subjected to the same heat and dilution treatments as the water-extract.
The obtained water- and ethanol-extracts of 10 mL were added with 0.1 g of activated carbon, held at 20 °C for 20 min with gentle stirring to conduct dephenolization, and then centrifuged two times at 5000g for 15 min to separate the supernatants. The supernatants were added with water to a final volume of 10 mL, filtrated with 0.22 μm micro-pore membranes, diluted with the FBS-fortified medium to reach 40 mg L−1 gallic acid equivalent, and used in later cell experiments. Part of these dephenolized extracts was also heated at 100 °C for 45 min, diluted with the FBS-fortified medium, and then used in cell experiments.
Assay of growth inhibition
HCT-116 cells were inoculated in the 96-well plates (1 × 105 cells per well) and incubated at 37 °C for 24 h to reach 70% confluence. After medium removal, the cells were treated with the cell medium (negative control), 100 μmol L−1 5-Fu (positive control), or the samples (10–80 mg L−1 gallic acid equivalent) for 12–24 h. After medium removal and washing with PBS (10 mmol L−1, pH 7.0) for three times, 100 μL CCK-8 solution (10 μL CCK-8 in 90 μL cell medium) was added to each well, while the cells were incubated at 37 °C for 2 h. The optical density of each well was measured at 450 nm using a microplate reader (Bio Rad Laboratories, Hercules, CA, USA). Growth inhibition of the samples (expressed as percentages) was thus calculated as previously described,32 while the control cells were designed without any growth inhibition (i.e. growth inhibition of zero percentage).Assay of intracellular ROS
The cells were seeded into the 6-well plates (1 × 106 cells per well) and incubated at 37 °C for 24 h. After medium removal, the cells were treated with the cell medium (negative control) or the samples (10–80 mg L−1 gallic acid equivalents) for 12–24 h. After cell harvest and PBS washing, the cells were incubated with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA, 5 μmol L−1) at 37 °C for 20 min in the dark. The cells were washed by the serum-free medium for three times, re-suspended in the PBS of 1 mL, and detected for fluorescence intensity using a fluorescence microplate reader (Infinite 200, Tecan, Männedorf, Switzerland) and respective emission and excitation wavelengths of 488 and 525 nm. ROS levels of the treated cells were expressed as percentages of the control as previously described.33Hoechst 33258 staining
A fluorescence probe Hoechst 33258 was used for nuclear staining as previously described.34 The cells in 6-well plates grown to 70% confluence were incubated with or without the samples (80 mg L−1 gallic acid equivalent) for 24 h. After discarding the media, 1 mL 4% methanol was added to fix the cells at 4 °C for 10 min. Following three washings with PBS, the Hoechst 33258 (200 mg mL−1) of 0.5 mL was added to stain the cells for 5 min, followed by observation under a fluorescence microscope (Zeiss Axio Observer A1m, Carl Zeiss, Oberkochen, Germany). Cell images were taken at 350 nm using an objective of 40-folds.Assay of Ca2+ concentration
The cells were seeded into the 6-well plates (1 × 106 cells per well) and incubated at 37 °C for 24 h. After medium removal, the cells were treated with the cell medium (negative control) and the samples (40 mg L−1 gallic acid equivalent) for 24 h. The cells were collected and washed with the Krebs–Ringer buffer (pH 7.4) containing 137 mmol L−1 NaCl, 5 mmol L−1 KCl, 1 mmol L−1 MgCl2, 1.5 mmol L−1 CaCl2, 10 mmol L−1 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 25 mmol L−1 D-glucose. The cells were collected and incubated with the Fura-2 AM (5 μmol L−1) at 37 °C for 60 min. Afterward, the cells were washed twice and re-suspended in the Krebs–Ringer buffer, and measured for fluorescence values using the fluorescence microplate reader and respective emission and excitation wavelengths of 510 and 340–380 nm. The cells treated with 0.1% of Triton X-100 (v/v) were used to determine the maximal fluorescence, followed by an addition of 10 mmol L−1 EGTA (ethylene glycol tetra-acetic acid, pH 9.0) to determine the minimal fluorescence as previously described.35Assay of mitochondrial membrane potential (MMP) loss
The cells were seeded into the 6-well plates (5 × 105 cells per well) and incubated at 37 °C for 24 h. After medium removal, the cells were treated with the cell medium (negative control) and the samples (40 mg L−1 gallic acid equivalent) for 24 h, resuspended in 0.5 mL fresh medium, added with the JC-1 of 0.5 mL, and incubated at 37 °C for 20 min. Following three-time washing with the JC-1 stain buffer, the cells were re-suspended in the JC-1 stain buffer of 1 mL, seeded into the 96-well plates, and measured for their fluorescence intensities at the fluorescence microplate reader. MMP was expressed using the ratio of red/green fluorescence intensities as previously described.36Statistical analysis
All assays and experiments were repeated three times. The data were reported as means or means ± standard deviations, and statistically analyzed using the one-way ANOVA and Duncan multiple comparison tests by the SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Statistical significance was set at a level of p < 0.05. Correlation analysis also was performed using the SPSS 16.0 software.Results
Changes of several chemical indices of the extracts in response to heat treatment
Total phenol, total flavonoid, and anthocyanin contents of these soluble extracts with or without heat treatment were detected and estimated using the corresponding gallic acid, quercetin, and cyanidin-3-glucoside equivalents (mg L−1), to show whether the performed heat treatment might cause negative or positive effects on these chemical indices. The results (Table 1) showed that the used solvent extraction of two times resulted in total phenol, total flavonoid, and anthocyanin contents of 267.8, 66.2, and 27.6 mg L−1 for the water-extract, or 214.0, 74.2, and 31.6 mg L−1 for the ethanol-extract, respectively. Moreover, the results also demonstrated that the used heat treatment reduced anthocyanin but increased total phenol and flavonoid contents in both water- and ethanol-extracts. Overall, longer heating time consistently led to significant increases (or decreases) in total phenol and flavonoid (or anthocyanin) contents. Why the two extracts after heat treatment received increased total phenol and flavonoid contents was an unusual event to us. Thus, part of the water- and ethanol-extracts was subjected to dephenolization using activated carbon and then heated at 100 °C for 45 min. The results indicated that activated carbon had a good ability to remove phenolic substances from the two extracts, because respective total phenol and flavonoid contents were decreased to 0.9 and 0.4 mg L−1 (for the dephenolized water-extract) or 6.9 and 1.2 mg L−1 (for the dephenolized ethanol-extract) (Table 2). Furthermore, heat treatment at 100 °C for 45 min also induced the generation of phenolic or flavonoid substances in the heated dephenolized extracts (Table 2). In the case of the dephenolized water-extract, heat treatment enhanced total phenol and flavonoid contents to 1.4 and 0.6 mg L−1, respectively. For the dephenolized ethanol-extract, total phenol and flavonoid contents were increased to 8.1 and 1.5 mg L−1, respectively. It is thus suggested that heat treatment of black mulberry might impact these chemical features like total phenol and polyphenol contents, thereby might cause final products with changed anti-oxidation and anti-cancer effect.| Extract sample | Heat time (min) | Total phenols (gallic acid equivalent) | Total flavonoids (quercetin equivalent) | Anthocyanins (cyanidin-3-glucoside basis) |
|---|---|---|---|---|
| a The uppercase or lowercase letters as the superscripts after the values of the same extract in same column indicate that one-way ANOVA of the mean values differs (p < 0.05). | ||||
| Water-extract | None | 267.6 ± 0.6a | 66.2 ± 0.2a | 27.6 ± 0.1d |
| 15 | 282.9 ± 1.0b | 69.6 ± 0.2b | 22.4 ± 0.2c | |
| 30 | 291.1 ± 1.2c | 70.5 ± 0.1c | 21.7 ± 0.1b | |
| 45 | 297.8 ± 1.8d | 71.3 ± 0.2d | 18.6 ± 0.1a | |
| Ethanol-extract | None | 214.0 ± 1.4A | 74.2 ± 0.2A | 31.6 ± 0.2D |
| 15 | 218.7 ± 1.4B | 79.6 ± 0.7B | 26.5 ± 0.1C | |
| 30 | 231.1 ± 1.8C | 82.1 ± 0.2C | 22.1 ± 0.1B | |
| 45 | 237.2 ± 2.2D | 89.1 ± 0.2D | 21.3 ± 0.2A | |
| Extract sample | Total phenols (gallic acid equivalent) | Total flavonoids (quercetin equivalent) | ||
|---|---|---|---|---|
| Dephenolized extract | Heated extract | Dephenolized extract | Heated extract | |
| Water-extract | 0.9 ± 0.3 | 1.4 ± 0.4 | 0.4 ± 0.1 | 0.6 ± 0.1 |
| Ethanol-extract | 6.9 ± 0.4 | 8.1 ± 0.7 | 1.2 ± 0.1 | 1.5 ± 0.1 |
Anti-oxidation changes of the extracts in response to heat treatment
Anti-oxidative activities of these black mulberry extracts were assessed using the three indices (Table 3). Without the mentioned heat treatment, the ethanol-extract displayed higher reducing power than the water-extract of the same gallic acid equivalent (20 mg L−1) (0.574 versus 0.564). However, the water-extract showed a higher ability to scavenge DPPH and hydroxyl radicals than the ethanol-extract (75.8–83.7% versus 48.5–70.6%), when the two extracts were assayed at a fixed gallic acid equivalent of 100 mg L−1. Moreover, the conducted heat treatment consistently led to decreased radical scavenging and lower reducing power, while longer heating time (e.g. 45 min) caused much decreased values for these indices. All results declared that the conducted heat treatment indeed had an adverse impact on the anti-oxidation of the extracts. In other words, the fresh black mulberry should have good anti-oxidation than the heated black mulberry products.| Extract sample | Heat time (min) | Scavenging percentages | Reducing power | |
|---|---|---|---|---|
| DPPH radicals | Hydroxyl radicals | |||
| a Both DPPH and hydroxyl radicals were determined at gallic acid equivalent (GAE) 100 mg L−1, while reducing power was determined at GAE 20 mg L−1. The uppercase or lowercase letters as the superscripts after the values of the same extract in same column indicate that one-way ANOVA of the mean values differs (p < 0.05). | ||||
| Water-extract | None | 75.8 ± 0.6c | 83.7 ± 0.2d | 0.564 ± 0.002d |
| 15 | 74.0 ± 0.2b | 82.4 ± 0.7c | 0.553 ± 0.003c | |
| 30 | 72.6 ± 0.1a | 80.9 ± 0.7b | 0.535 ± 0.003b | |
| 45 | 72.1 ± 0.2a | 72.4 ± 0.1a | 0.525 ± 0.002a | |
| Ethanol-extract | None | 70.6 ± 0.2D | 48.5 ± 0.6C | 0.574 ± 0.002D |
| 15 | 69.9 ± 0.2C | 32.2 ± 1.2B | 0.565 ± 0.003C | |
| 30 | 65.8 ± 0.2B | 30.8 ± 0.9B | 0.544 ± 0.002B | |
| 45 | 63.3 ± 0.4A | 27.9 ± 1.2A | 0.535 ± 0.003A | |
Anti-proliferation changes of the extracts in response to heat treatment
When the water- and ethanol-extracts were used at gallic acid equivalent of 10–80 mg L−1 to treat HCT-116 cells for 12 and 24 h, CCK-8 assay results indicated that these heated or unheated extracts had anti-proliferative effect on the cells (Fig. 1), resulting in the treated cells with growth inhibition of different extents. 5-Fu as a classic chemo-therapeutic agent also exerted anti-proliferation on the cells. In general, higher extract dosage and longer cell exposure time consistently endowed the two extracts with higher inhibitory percentages in the cells, suggesting the dose- and time-dependent anti-proliferation of these extracts on the cells. The unheated ethanol-extract showed higher growth inhibition on the cells than the unheated water-extract, reflected by its higher inhibitory percentages (23.4–43.8% versus 20.3–40.6%, 12 h; or 26.7–59.8% versus 22.8–52.9%, 24 h). Moreover, further data comparison also indicated that heat treatment of these extracts at 100 °C for 45 min enhanced anti-proliferation significantly (p < 0.05). For the water-extract, heat treatment increased inhibitory percentages to 24.5–44.4% (cell treatment of 12 h) or 32.4–76.7% (cell treatment of 24 h). In the case of the ethanol-extract, heat treatment increased inhibitory percentages to 25.8–52.0% (cell treatment of 12 h) or 34.6–85.2% (cell treatment of 24 h). The calculated IC50 values of these extracts (Table 4) also reflected their different abilities to inhibit cell growth. The lower IC50 values of the extracts meant higher growth inhibition on the cells. In brief, the ethanol-extract in the cells had higher inhibition than the water-extract (IC50 values 96.9 versus 105.3 mg L−1, cell treatment of 12 h; or 54.2 versus 69.1 mg L−1, cell treatment of 24 h), while the longer time of the cells exposed to the extracts also caused greater inhibition. More importantly, the heated extracts all had reduced IC50 values (p < 0.05) and thus possessed enhanced growth inhibition. These results consistently highlighted a novel finding in this study: the performed heat treatment had a positive impact on the anti-colon cancer effect of the extracts via increasing their anti-proliferation on the cells.| Treatment time of the cells | Water-extract | Ethanol-extract | ||
|---|---|---|---|---|
| Original extract | Heated extract | Original extract | Heated extract | |
| a Different uppercase (or lowercase) letters as the superscripts after the values in the same row indicate that one-way ANOVA of the mean values differs (p < 0.05). | ||||
| 12 | 105.3 ± 2.2D | 97.9 ± 5.8C | 96.9 ± 4.2C | 72.1 ± 3.7A |
| 24 | 69.1 ± 4.6d | 38.4 ± 1.2b | 54.2 ± 3.0c | 29.5 ± 2.4a |
The morphological changes of the treated cells also provided evidence to verify the anti-proliferation of these extracts. In brief, all assessed extracts showed an effect on the cells, because the treated cells were observed to have lower cell density in the observation fields and especially morphological changes (Fig. 2). The cells exposed to these extracts showed the condensation and fragmentation of nuclei shrinkage, as well as the formation of apoptotic bodies, compared with the control cells without extract treatment. The heated extracts were observed to be more effective than the unheated ones to alter morphological features of the treated cells. Thus, heat treatment was evidenced again capable of increasing the anti-colon cancer activities of the two extracts against HCT-116 cells.
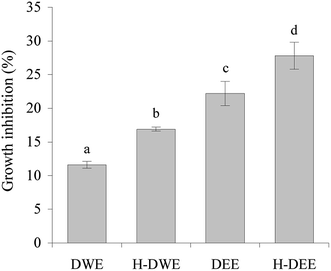
Whether a heat treatment of the dephenolized extracts at 100 °C for 45 min might also cause enhanced growth inhibition on the cells thus should be assessed, to provide extra evidence clarifying whether heat treatment of these extracts led to increased anti-proliferation. The cells were thus exposed to the dephenolized extracts or heated dephenolized extracts of 40 mg L−1 gallic acid equivalent for 24 h. The obtained data (Fig. 3) indicated that the dephenolized water- and ethanol-extracts had lower growth inhibition on the cells than the water- and ethanol-extracts (11.6–22.2% versus 41.5–47.8%), respectively. However, the heated dephenolized water- and ethanol extracts showed increased growth inhibition (16.9–27.8%). Heat treatment of the two dephenolized extracts thus brought about enhanced growth inhibition on the cells.
Why the heated extracts received increased growth inhibition on the cells might be arisen from those soluble but unidentified components in the extracts. It was speculated that these unidentified compounds underwent various reactions to produce other compounds with growth inhibition on the cells. Thus, the heated extracts obtained enhanced anti-proliferation. The fresh black mulberry was detected in this study to contain water, reducing sugars, and proteins of 896.4, 42.74, and 7.346 g kg−1, respectively. The potential non-enzymatic browning reaction between the soluble reducing sugars and proteins in these extracts was an expectable event when the extracts were heated. Afterward, the dephenolized extracts heated at 100 °C for 45 min were detected for their changes in the three indices (Table 5). The results demonstrated that the used heat treatment resulted in obvious value increases in both fluorescence and UV absorption. UV absorption at 420 nm (A420, classic brown indicator) showed value increases while that at 294 nm (A294) was also greatly enhanced, suggesting the formation of unknown aromatic substances during the heat treatment of these dephenolized extracts. This finding supported the results given in Table 1, in which both total phenol and flavonoid contents were enhanced clearly after heat treatment.
| Index | Water-extract | Ethanol-extract | ||
|---|---|---|---|---|
| Original extract | Heated extract | Original extract | Heated extract | |
| Fluorescence | 118.1 ± 1.1 | 277.0 ± 1.6 | 113.5 ± 2.3 | 274.9 ± 2.2 |
| A294 | 0.031 ± 0.002 | 0.110 ± 0.002 | 0.034 ± 0.002 | 0.125 ± 0.002 |
| A420 | 0.017 ± 0.001 | 0.024 ± 0.002 | 0.018 ± 0.001 | 0.032 ± 0.001 |
Pro-oxidation changes of the extracts in response to heat treatment
When the water- and ethanol-extracts at 10–80 mg L−1 gallic acid equivalent were used to treat HCT-116 cells for 12 and 24 h, the cells showed significant increases in intracellular ROS (Table 6), suggesting the pro-oxidation of the two extracts in the cells. In general, higher extract dosage and longer cell treatment time consistently led to higher ROS levels, revealing the dose- and time-dependent pro-oxidation of the two extracts. The ethanol-extract induced higher ROS levels than the water-extract did in each cell treatment time (105.5–157.1% versus 102.9–144.6%, 12 h; or 126.1–266.1% versus 113.1–187.9%, 24 h). The ethanol-extract thus was regarded to have higher pro-oxidation. At the same time, it was seen that heat treatment at 100 °C for 45 min enhanced pro-oxidation of the two heated extracts (p < 0.05). For the water-extract, heat treatment enhanced ROS levels to 109.2–154.4% (cell treatment of 12 h) or 123.0–228.1% (cell treatment of 24 h). In the case of the ethanol-extract, heat treatment increased ROS levels to 113.5–168.0% (cell treatment of 12 h) or 138.0–323.4% (cell treatment of 24 h). Moreover, heat treatment also increased pro-oxidation of the dephenolized extracts (Fig. 4), because the cells exposed to the heated dephenolized extracts for 24 h showed higher ROS levels than those exposed to the unheated dephenolized extracts for 24 h (105.1–122.6% versus 101.7–112.6%). It was thus proposed that the unidentified components in the extracts could undergo unidentified reactions to enhance pro-oxidation of the heated extracts.| Culture time (h) | Gallic acid equivalent (mg L−1) | Water-extract | Ethanol-extract | ||
|---|---|---|---|---|---|
| Original extract | Heated extract | Original extract | Heated extract | ||
| a Different uppercase (or lowercase) letters as the superscripts after the values in the same row (or column) indicate that one-way ANOVA of the mean values differs (p < 0.05). | |||||
| 12 | 10 | 102.9 ± 1.8Aa | 109.2 ± 1.6BCa | 105.5 ± 2.0ABa | 113.5 ± 3.4Ca |
| 20 | 116.5 ± 1.8Ab | 121.6 ± 2.5Bb | 117.6 ± 2.0Ab | 127.0 ± 1.6Cb | |
| 40 | 126.4 ± 2.0Ac | 133.6 ± 3.0Bc | 134.6 ± 2.2Bc | 143.0 ± 2.6Cc | |
| 80 | 144.6 ± 2.3Ad | 154.4 ± 1.8Bd | 157.1 ± 2.0Bd | 168.0 ± 2.4Cd | |
| 24 | 10 | 113.1 ± 2.9Aa | 123.0 ± 1.7Ba | 126.1 ± 1.6Ba | 138.0 ± 1.6Ca |
| 20 | 136.8 ± 2.2Ab | 145.7 ± 3.7Bb | 145.7 ± 3.2Bb | 155.5 ± 1.7Cb | |
| 40 | 154.9 ± 2.1Ac | 171.9 ± 2.5Bc | 191.8 ± 2.1Cc | 224.8 ± 2.5Dc | |
| 80 | 187.9 ± 2.0Ad | 228.1 ± 2.6Bd | 266.1 ± 2.2Cd | 323.4 ± 2.7Dd | |
Further analysis results showed that the measured growth inhibition and pro-oxidation (Fig. 1 and Table 6) of all extracts were significantly and positively correlated (p < 0.05). With cell treatment time of 12 h, the calculated Pearson's correlation coefficients for the water- and ethanol-extracts were 0.990 and 0.994, respectively. With cell treatment time of 24 h, the corresponding coefficients for the two extracts were 0.988 and 0.979. This correlation analysis thus highlighted an important fact: the pro-oxidation of these extracts made a direct and positive contribution to the measured growth inhibition.
Intracellular Ca2+ concentration and MMP changes induced by these extracts
The assaying results showed that when being used at 40 mg L−1 gallic acid equivalent to treat the cells for 24 h, these extracts caused increased intracellular Ca2+ concentrations (Fig. 5a). The ethanol-extract led to higher intracellular Ca2+ concentration than the water-extract did (125.6 versus 114.0 nmol L−1). Moreover, intracellular Ca2+ concentration was enhanced significantly by using the heated extracts. For example, the heated water-extract increased intracellular Ca2+ concentration to 124.0 nmol L−1, while the heated ethanol-extract promoted intracellular Ca2+ concentration up to 158.5 nmol L−1. Heat treatment thus conferred the two extracts with higher ability in the cells to increase intracellular Ca2+ concentration.At the same time, the treated cells showed significant decreases in their MMP (i.e. MMP loss) (Fig. 5b). In detail, the cells treated with the water- and ethanol-extracts had decreased MMP (cell proportion of red/green fluorescence 7.03 and 6.93), compared with the control cells without extract treatment (cell proportion of red/green fluorescence 9.14). Moreover, the heated water- and ethanol-extracts caused much MMP loss, because the resultant cell proportions of red/green fluorescence decreased to 6.61 and 6.09. That is, heat treatment of the two extracts resulted in more dramatic MMP loss. All results indicated that the two extracts were able to damage MMP while heat treatment increased the activity of the two extracts.
Discussion
Plant foods contain a large number of phenolic/polyphenolic substances that possess various health functions in the body.2 Many plant foods thus have been evaluated for their anti-oxidation and health benefits arisen from these substances. It was found that green tea contained various polyphenols that had an ability to scavenge DPPH radicals, while bayberry had anthocyanins, flavonols, and phenolic acids that were capable of scavenging ABTS radicals and reducing Fe(III) ions.24,29 Moreover, it was evident in two past studies that the anthocyanins and flavonoids from mulberry and purple maize had anti-diabetic and anti-inflammatory effects.37,38 In especial, plant extracts rich in polyphenols also had been verified to have anti-cancer effect on oral, prostate, colon, and breast cancer cells.39–42 In the most cases, plant foods are mostly consumed only after their storage or industrial/domestic processing. Reasonably, the used storage and processing conditions might have various impacts on these phenols and polyphenols in plant foods. To retain the most of these bio-active substances in the stored or processed foods, it is essential to clarify whether and how the most used food processing (i.e. heat treatment) might exert positive or negative impacts on the quantity and more importantly bio-functions of these substances. It was known from the previous studies that heat treatment of tomatoes and green vegetables such as boiling, microwave cooking, steaming, and stir-frying led to decreased polyphenol contents and reduced anti-oxidation.43,44 Sharing partly result similarity, the present study also found that heat treatment of these extracts caused reduced anti-oxidation and decreased anthocyanin content. However, opposite to these mentioned studies, this study also observed that heat treatment increased total phenol/flavonoid contents in both water- and ethanol-extracts, which resulted from those unidentified reactions.In usual, polyphenolic substances have chemical instability due to their structural characteristics (i.e. several –OH groups in their molecules) and reducing properties. These substances might undergo various chemical degradations like oxidation, hydroxylation, and ring-cleavage upon heat and oxidative treatments,45–47 and subsequently, their contents as well as bioactivities might be altered. For example, heat treatment decreased total anthocyanin contents of the grape and blueberry pomace extracts,48 or time-dependently caused reduced anthocyanin content and decreased anti-oxidation in black carrot.49 Furthermore, the fresh-cut strawberries subjected to high-oxygen atmosphere showed unavoidable air-oxidation, and thus were measured with decreased anthocyanin content and weakened anti-oxidation.50 The present results also showed that heat treatment reduced anthocyanin contents in the two extracts. From a chemical point of view, polyphenol oxidation during heat treatment will lead to the formation of these oxidized compounds like quinones and others, which do not possess good anti-oxidation than their parent compounds.51,52 It is thus reasonable to this study that the heated extracts showed decreased activities to scavenge the two radicals or to reduce Fe(III) ions. However, all heated extracts were determined with increased total phenol/flavonoid contents. A past study had found that heat processing of tomato resulted in increased lycopene content,53 which showed a conclusion consistence with the present study. Total anthocyanin contents of mulberry fruits were proposed to be closely related to their anti-oxidation.54 Furthermore, it was proposed that there was no direct correlation between total polyphenol contents of mulberry fruits and their abilities to scavenge DPPH radicals.55 Thus, it might be an accepted fact that the two extracts after heat treatment showed increased phenol/flavonoid contents (as the result of the Maillard reaction) but decreased anti-oxidation.
In the recent years, polyphenols are widely studied for their in vitro anti-cancer effects on various cancer cells, namely regulating mRNA expression of related genes, affecting cell signaling, inhibiting cell growth, inducing cell cycle arresting, and triggering apoptosis.56 The important way to control the unlimited growth of cancer cells is to inhibit cell proliferation. Thus, growth inhibition as an important index has been widely used to reflect anti-cancer effects of various substances. The anthocyanin or phenolic compounds from mulberry and blueberry were verified able to inhibit the growth of gastric cancer AGS, colon cancer HT-29, and Caco-2 cells, or able to induce cell apoptosis by various pathways.57,58 Intracellular ROS level is another index to reflect anti-cancer activities of natural compounds. ROS are highly active chemicals in cells. Under normal conditions, ROS levels are highly controlled by a variety of anti-oxidants. Once the intracellular ROS level is abnormally increased, oxidative stress occurs.59 In cancer cells, ROS levels are usually higher than those in normal cells. If chemo-therapeutic agents and natural anti-cancer compounds act on cancer cells, ROS level will be very high and consequently lead to irreversible oxidative damage and cell death.60 In a previous study, four flavonoids including apigenin, luteolin, kaempferol, and quercetin were evident capable of increasing ROS level in human hepatoma HepG2 cells.61 In general, polyphenols are suggested having pro-oxidation in cancer cells by increasing the intracellular ROS level, which then triggers cell death. The potential effect of heat treatment on anti-cancer activities of polyphenols thus was evaluated in two past studies.60,61 High-pressure cooked Thai purple rice showed unchanged growth inhibition on Caco-2 cells than the uncooked ones,62 but heat treatment of apigenin led to decreased growth inhibition and apoptosis induction in HCT-116 cells.63 However, this study obtained a conclusion contrary to that of the two mentioned studies;62,63 that is, the heated extracts had higher growth inhibition on the cells and were more able to generate intracellular ROS. Regarding the role of ROS in cancer cells, it was reasonable that the measured values of growth inhibition for the extracts (Fig. 1) were well-correlated with their pro-oxidation in the cells (Table 6).
In general, a mixture containing proteins (or their degraded products peptides and amino acids) and reducing saccharides will undergo the Maillard reaction upon heat treatment,64 which leads to the formation of many compounds (including aromatic substances) with various bioactivities. Moreover, it was found that the Maillard reaction products (MRPs) using glyceraldehydes and bovine serum albumin or casein could inhibit the growth of human promyelocytic HL-60 cells and increase intracellular ROS level,65 while the MRPs generated by sugar and tryptophan or phenylalanine also exerted growth inhibition on HCT-116 cells.66 In addition, a past study had verified that the Maillard-type products from fructose and tyrosine had anti-proliferation on human mammary cancer MCF-7, lung cancer H-460, and liver cancer HepG2 cells.67 In this study, activated carbon showed the ability to remove phenols or polyphenols from the two water- and ethanol-extracts. However, the dephenolized extracts also contained reducing sugars and proteins, and the Maillard reaction might occur during the conducted heat treatment. Thus, the heated dephenolized extracts thus contained MRPs and were more active than the unheated ones to inhibit cell growth or produce more ROS in the cells (Fig. 3 and 4). Based on this consideration, the heated water- and ethanol-extracts reasonably had higher anti-cancer effects on the cells than the unheated water- and ethanol-extracts.
Mitochondria are the center of energy metabolism, the source of most ATP in cells, and the hub of Ca2+ signaling in cells. Ca2+ overload will trigger the opening of mitochondrial permeability transition porin (MPTP), which afterward leads to mitochondrial dysfunction and cell death.68 The effect of apigenin on colon cancer HCT-116 cells was proved through increasing intracellular ROS and Ca2+ concentration, impairing MMP, causing cell morphological changes, and inducing apoptosis.69 In this study, the heated extracts were more effective than the unheated ones to decrease MMP but increase intracellular Ca2+ levels (Fig. 5), and thus possessed higher anti-cancer effect. This fact thus supported that heat treatment of the water- and ethanol-extracts led to the enhanced anti-cancer effect.
Although potential Maillard reaction during heat treatment of these extracts was briefly verified in this study using the three classic indices (Table 5), a detailed investigation is also proposed to reveal how the Maillard reaction or other unidentified reactions give their impacts on the anti-cancer effect of processed foods. Such investigation will deepen our present knowledge and provide essential scientific evidence to get optimized storage and processing conditions for plant foods. Moreover, a detailed characterization of these formed products in the heated extracts is recommended in future studies.
Conclusions
Both soluble water- and ethanol-extracts of black mulberry had in vitro anti-oxidation to scavenge DPPH and hydroxyl radicals as well as to reduce Fe(III) ions. The two extracts also had anti-colon cancer effect on the cell model (HCT-116 cells) via inhibiting cell growth, enhancing intracellular ROS level, or other ways. In brief, heat treatment of the two extracts at 100 °C would decrease their anti-oxidation and anthocyanin content but increase their total phenol and flavonoid contents. More importantly, heat treatment at 100 °C for 45 min caused increased anti-colon cancer effect including higher growth inhibition, higher intracellular ROS production, higher intracellular Ca2+ level, and higher MMP loss. The occurrence of those reactions such as the Maillard reaction and other unidentified reactions might contribute to the enhanced anti-colon cancer effect. It is thus suggested that a detailed investigation verifying whether and how heat treatment might impact anti-oxidation and anti-cancer effect of plant foods is necessary, to deepen our recent knowledge and to provide a scientific basis to optimize storage and processing conditions of these foods.Author contributions
W. S. Cui performed the experiments, analyzed the data, and wrote the original draft; X. H. Zhao obtained the funding; while X. H. Zhao and Q. Zhang conceived and designed the experiments, analyzed the data, and revised the manuscript draft.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was funded by the Key Research Project in Science and Technology of The Education Department of Heilongjiang Province (Project No. 11551z018). The authors thank the anonymous referees for their valuable advice.References
- X. M. Liu, G. S. Xiao, W. D. Chen, Y. J. Xu and J. J. Wu, J. Biomed. Biotechnol., 2004, 5, 326–331 CrossRef PubMed.
- I. Khalifa, W. Zhu, K. K. Li and C. M. Li, J. Funct. Foods, 2018, 40, 28–43 CrossRef CAS.
- A. Calin-Sanchez, J. Martinez-Nicolas, S. Munera-Picazo, A. Carbonell-Barrachina, P. Legua and F. Hernandez, Plant Foods Hum. Nutr., 2013, 68, 370–377 CrossRef CAS PubMed.
- L. H. Liang, X. Y. Wu, M. M. Zhu, W. G. Zhao, F. Li, Y. Zou and L. Q. Yong, Pharmacogn. Mag., 2012, 8, 215–224 CrossRef CAS PubMed.
- M. Imran, H. Khan, M. Shah, R. Khan and F. Khan, J. Zhejiang Univ., Sci., B, 2010, 11, 973–980 CrossRef CAS PubMed.
- P. Mena, E. M. Sanchez-Salcedo, M. Tassotti, J. J. Martinez, F. Hernandez and D. Del Rio, Food Res. Int., 2016, 89, 1116–1122 CrossRef CAS.
- Y. Shen, L. Jin, P. Xiao, Y. Lu and J. S. Bao, J. Cereal Sci., 2009, 49, 106–111 CrossRef CAS.
- B. Wang, J. Wang and X. H. Zhao, J. Food Biochem., 2017, 41, e12310 CrossRef.
- X. N. Sui, X. Dong and W. B. Zhou, Food Chem., 2014, 163, 163–170 CrossRef CAS PubMed.
- D. De Paepe, D. Valkenborg, K. Coudijzer, B. Noten, K. Servaes, M. De Loose, S. Voorspoels, L. Diels and B. Van Droogenbroeck, Food Chem., 2014, 162, 176–185 CrossRef CAS PubMed.
- M. S. Padda and D. H. Picha, Int. J. Food Sci. Technol., 2007, 43, 1404–1409 CrossRef.
- J. A. Larrauri, P. Rupérez and F. Saura-Calixto, J. Agric. Food Chem., 1997, 45, 1390–1393 CrossRef CAS.
- B. C. Nwanguma and M. O. Eze, J. Sci. Food Agric., 1996, 70, 162–166 CrossRef CAS.
- L. A. Torre, F. Bray, R. L. Siegel, J. Ferlay, J. Lortet-Tieulent and A. Jemal, Ca-Cancer J. Clin., 2015, 65, 87–108 CrossRef PubMed.
- E. A. Decker, Nutr. Rev., 1995, 53, 49–58 CrossRef CAS PubMed.
- C. Miyagawa, C. Wu, D. O. Kennedy, T. Nakatani, K. Ohtani, S. Sakanaka, M. Kim and I. Matsui-Yuasa, Biosci., Biotechnol., Biochem., 1997, 61, 1901–1905 CrossRef CAS PubMed.
- F. Hisamura, A. Kojima-Yuasa, D. O. Kennedy and I. Matsui-Yuasa, Basic Clin. Pharmacol. Toxicol., 2006, 98, 192–196 CrossRef CAS PubMed.
- Y. Shin, J. A. Ryu, R. H. Liu, J. F. Nock and C. B. Watkins, Postharvest Biol. Technol., 2008, 49, 201–209 CrossRef CAS.
- R. Kurata, M. Adachi, O. Yamakawa and M. Yoshimoto, J. Agric. Food Chem., 2007, 55, 185–190 CrossRef CAS PubMed.
- M. J. Nemec, H. Kim, A. B. Marciante, R. C. Barnes, S. T. Talcott and S. U. Mertens-Talcott, Food Funct., 2016, 7, 3825–3833 RSC.
- I. Turan, S. Demir, K. Kilinc, N. A. Burnaz, S. O. Yaman, K. Akbulut, A. Mentese, Y. Aliyazicioglu and O. Deger, Saudi Pharm. J., 2017, 25, 241–248 CrossRef PubMed.
- W. Horwitz, Official Methods of Analysis of AOAC International, 1999 Search PubMed.
- M. Dubosi, K. A. Gilles and J. K. Hamilton, Nature, 1951, 28, 167–168 CrossRef PubMed.
- C. Vasco, J. Ruales and A. Kamal-Eldin, Food Chem., 2008, 111, 816–823 CrossRef CAS.
- S. Ercisli and E. Orhan, Food Chem., 2007, 103, 1380–1384 CrossRef CAS.
- F. S. Hosseinian, W. Li and T. Beta, Food Chem., 2008, 109, 916–924 CrossRef CAS PubMed.
- J. A. Rufián-Henares, E. GuerraHernández and B. GarcíaVillanova, Food Chem., 2006, 98, 685–692 CrossRef.
- Z. M. Jiang, L. Z. Wang, W. Wu and Y. Wang, Food Chem., 2013, 141, 3837–3845 CrossRef CAS PubMed.
- N. Turkmen, F. Sari and Y. S. Velioglu, Food Chem., 2006, 99, 835–841 CrossRef CAS.
- H. Wu, J. X. Zhu, W. C. Diao and C. R. Wang, Carbohydr. Polym., 2014, 113, 314–324 CrossRef CAS PubMed.
- B. J. Chen, M. J. Shi, S. Cui, S. X. Hao, R. C. Hider and T. Zhou, Int. J. Biol. Macromol., 2016, 92, 715–722 CrossRef CAS PubMed.
- J. L. Lou, G. H. Chu, G. J. Zhou, J. Jiang, F. F. Huang, J. J. Xu, S. Zheng, W. Jiang, Y. Z. Lu, X. X. Li, Z. J. Chen and J. L. He, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2010, 697, 55–59 CrossRef CAS.
- J. J. Li, Q. Tang, Y. Li, B. R. Hu, Z. Y. Ming, Q. Fu, J. Q. Qian and J. Z. Xiang, Acta Pharmacol. Sin., 2006, 27, 1078–1084 CrossRef CAS PubMed.
- W. Chen, H. M. Su, Y. Xu, T. Bao and X. D. Zheng, Food Chem., 2016, 196, 943–952 CrossRef CAS PubMed.
- J. Tong, Y. Qi, X. M. Wang, L. Y. Yu, C. Su, W. J. Xie and J. B. Zhang, Biochim. Biophys. Acta, Mol. Cell Res., 2017, 1864, 2389–2401 CrossRef CAS PubMed.
- S. Y. Wu, L. L. Lei, Y. Song, M. T. Liu, S. B. Lu, D. Lou, Y. H. Shi, Z. B. Wang and D. F. He, Exp. Neurol., 2018, 309, 67–78 CrossRef CAS PubMed.
- K. H. Choi, H. A. Lee, M. H. Park and J. S. Han, J. Med. Food, 2016, 19, 734–745 Search PubMed.
- Q. Z. Zhang, E. G. de Mejia, D. Luna-Vital, T. Y. Tao, S. Chandrasekaran, L. Chatham, J. Juvik, V. Singh and D. Kumar, Food Chem., 2019, 289, 739–750 CrossRef CAS PubMed.
- N. P. Seeram, L. S. Adams, M. L. Hardy and D. Heber, J. Agric. Food Chem., 2004, 52, 2512–2517 CrossRef CAS PubMed.
- A. T. Holkem, C. S. Favaro-Trindade and M. Lacroix, Food Res. Int., 2020, 134, e109274 CrossRef PubMed.
- X. Huang, J. Li, M. J. Li, J. Huang, X. H. Jiang, H. F. Fu, J. M. Wu, M. Y. Bao, S. H. Wang, M. Y. Zhang and G. C. Gao, Nutr. Cancer, 2020 DOI:10.1080/01635581.2020.1792951.
- X. Wu, L. Xue, A. Tata, M. Y. Song, C. C. Neto and H. Xiao, J. Agric. Food Chem., 2020, 68, 6845–6853 CrossRef CAS.
- E. Sahlin, G. P. Savage and C. E. Lister, Food Chem., 2004, 17, 635–647 CAS.
- N. Turkmen, F. Sari and Y. S. Velioglu, Food Chem., 2005, 94, 713–718 CrossRef.
- I. Zenkevich, A. Eshchenko, S. Makarova, A. Vitenberg, Y. Dobryakov and V. Utsal, Molecules, 2007, 17, 635–647 Search PubMed.
- D. P. Makris and J. T. Rossiter, J. Agric. Food Chem., 2000, 48, 3830–3838 CrossRef CAS.
- J. S. Barnes, F. W. Foss and K. A. Schug, J. Am. Soc. Mass Spectrom., 2013, 24, 1513–1522 CrossRef CAS.
- R. C. Khanal, L. R. Howard and R. L. Prior, Food Res. Int., 2010, 43, 1464–1469 CrossRef CAS.
- E. Sadilova, R. Carle and F. C. Stintzing, Mol. Nutr. Food Res., 2007, 51, 1461–1471 CrossRef CAS.
- I. Odriozola-Serrano, R. Soliva-Fortuny and O. Martín-Belloso, J. Food Sci., 2009, 74, 184–191 CrossRef.
- J. P. Aka, F. Courtois, L. Louarme, J. Nicolas and C. Billaud, Food Chem., 2013, 138, 2–3 CrossRef PubMed.
- W. Bors, C. Michel and K. Stettmaier, Arch. Biochem. Biophys., 2000, 374, 347–355 CrossRef CAS.
- V. Dewanto, X. Z. Wu, K. K. Adom and R. H. Liu, J. Agric. Food Chem., 2002, 50, 3010–3014 CrossRef CAS.
- P. Aramwit, N. Bang and T. Srichana, Food Res. Int., 2010, 43, 1093–1097 CrossRef CAS.
- R. J. Wang and M. L. Hu, Int. J. Food Prop., 2011, 14, 1–8 CrossRef CAS.
- Q. Zhang, X. H. Zhao and Z. J. Wang, Food Chem. Toxicol., 2008, 46, 2042–2053 CrossRef CAS PubMed.
- H. P. Huang, Y. C. Chang, C. H. Wu, C. N. Hung and C. J. Wang, Food Chem., 2011, 129, 1703–1709 CrossRef CAS.
- W. G. Yi, J. Fischer, G. Krewer and C. C. Akoh, Food Chem., 2005, 53, 7320–7329 CrossRef CAS PubMed.
- S. Tan, Y. Sagara, Y. Liu, P. Maher and D. Schubert, J. Cell Biol., 1998, 141, 1423–1432 CrossRef CAS PubMed.
- T. Ozben, J. Pharm. Sci., 2007, 96, 2181–2196 CrossRef CAS.
- Q. Zhang, G. D. Cheng, H. B. Qiu, L. L. Zhu, Z. J. Ren, W. Zhao, T. Zhang and L. Liu, Food Funct., 2015, 6, 1518–1525 RSC.
- R. Chatthongpisut, S. J. Schwartz and J. Yongsawatdigul, Food Chem., 2015, 188, 99–105 CrossRef CAS PubMed.
- B. Wang and X. H. Zhao, Emirates Journal of Food and Agriculture, 2017, 29, 69–77 CrossRef.
- D. D. Muir, Int. J. Dairy Technol., 2007, 60, 59 CrossRef.
- T. Usui, S. Shizuuchi, H. Watanabe and F. Hayase, Biosci., Biotechnol., Biochem., 2004, 68, 333–340 CrossRef CAS PubMed.
- I. G. Hwang, H. Y. Kim, K. S. Woo, J. Lee and H. S. Jeong, Food Chem., 2011, 126, 221–227 CrossRef CAS.
- S. H. Lee, S. J. Jeong, G. Y. Jang, M. Y. Kim, I. G. Hwang, H. Y. Kim, K. S. Woo, B. Y. Hwang, J. Song, J. Lee and H. S. Jeong, J. Agric. Food Chem., 2016, 64, 3041–3047 CrossRef CAS PubMed.
- R. F. Feissner, J. Skalska and W. E. Gaum, Front. Biosci., Landmark Ed., 2009, 14, 1197–1218 CrossRef CAS PubMed.
- B. Wang and X. H. Zhao, Oncol. Rep., 2017, 37, 1132–1140 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |