Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hybrid structured CoNi2S4/Ni3S2 nanowires with multifunctional performance for hybrid capacitor electrodes and overall water splitting
Xiaoyun Liu *a,
Qian Lia,
Xin Zhangc and
Yueqiu Jiang*b
*a,
Qian Lia,
Xin Zhangc and
Yueqiu Jiang*b
aSchool of Science, Shenyang Ligong University, Shenyang 110159, P. R. China. E-mail: liuxy@imr.ac.cn
bDepartment of Development and Planning, Shenyang Ligong University, Shenyang 110159, P. R. China. E-mail: yueqiujiang@sylu.edu.cn
cSchool of Automobile and Transportation, Shenyang Ligong University, Shenyang 110159, P. R. China
First published on 10th September 2020
Abstract
Rational design of electrode materials plays a significant role in potential applications such as energy storage and conversion. In this work, CoNi2S4/Ni3S2 nanowires grown on Ni foam were synthesized through a facile hydrothermal approach, revealing a large capacitance of 997.2 F g−1 and cycling stability with 80.3% capacitance retention after 5000 cycles. The device was prepared using CoNi2S4/Ni3S2//AC as the positive electrode and active carbon as the negative electrode, and delivered an energy density of 0.4 mW h cm−3 at a power density of 3.99 mW cm−3 and an excellent cycle life with 79.2% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. In addition, the hybrid CoNi2S4/Ni3S2 nanowires demonstrate excellent OER performance with low overpotential of 360 mV at 30 mA cm−2 and overpotential of 173.8 mV at −10 mA cm−2 for the HER, a cell voltage of 1.43 V, and excellent cycle stability.
000 cycles. In addition, the hybrid CoNi2S4/Ni3S2 nanowires demonstrate excellent OER performance with low overpotential of 360 mV at 30 mA cm−2 and overpotential of 173.8 mV at −10 mA cm−2 for the HER, a cell voltage of 1.43 V, and excellent cycle stability.
1. Introduction
In recent years, various transition metal-based catalysts such as phosphides, oxides, nitrides, carbides, and sulfides have been widely investigated as excellent electrochemical catalysts but the improvement of bi-functional electrocatalysts with excellent HER and OER activity is still difficult and slow to implement.1–4 To date, as demonstrated by experiments, electrocatalysts with vertically well-aligned arrays on 1D nanostructures consistently lead to improved HER and OER activity and promise efficient overall water splitting.5,6 Nanostructures possess distinctive advantages, including easier electrolyte diffusion and ionic transportation, better electrical connection with substrates, and offer larger surface area to provide contact between the electrode and electrolyte.7 Special nanostructures possess interconnected networks; these hierarchical heterostructures allow for efficient electron/ion transportation and accommodate volume variation nicely. Meanwhile, heterostructures consist of highly conductive materials as the “substratum” and transition metal oxides as the “superstratum”; thus, the combination of the two types of active materials create heterostructures that inherit advantages from both the “substratum” and “superstratum”.8–10 As highlighted in recent reports, Ni(OH)2/Ni3S2 nanostructure arrays on Ni foam show excellent electrochemical activity with a low overpotential of 270 mV (OER) at 20 mA cm−2 in 1.0 M KOH but exhibited a low HER activity (211 mV at 10 mA cm−2).11 Zhao et al. prepared nitrogen-doped carbon nanomaterials as efficient oxygen evolution electrocatalysts, which exhibited an overpotential of 380 mV at a current density of 10 mA cm−2 in alkaline media.12 An effective design of catalysts integrating HER and OER activity materials is the key for overall water splitting.13 Recently, benefits from cobalt include better redox activity under mild reaction conditions, modest overpotential, and the extremely high theoretical rich polymorphism of molybdenum, Co-based electrode materials have demonstrated vastly improved electrochemical performance.14,15Among various electrode materials, spinel-structured CoNi2O4 has been investigated due to the high theoretical capacitance, high electrochemical performance and excellent electrocatalytic performance.16,17 For example, Tong et al. reported CoNi2O4 nanoneedles through a simple hydrothermal method. The as-prepared samples exhibited a specific capacitance of 1192 mF cm−2 at 2 mA cm−2 and cycle stability.18 Liu and co-workers prepared NiCo2O4 nanowires through a simple hydrothermal process; the as-prepared samples showed excellent electrocatalytic performance with an overpotential of 320 mV at 10 mA cm−2.19 However, single metal oxide electrode materials often present poor electrical conductivity, which limits the electrochemical performance. To improve the electrical conductivity, constructing a hybrid structure with metal sulfides has been considered an efficient method due to the improved electrical conductivity (about 100 times) over corresponding single metal oxides and binary materials.20 In the literature, Li et al. fabricated NiCo2S4@Co(OH)2 core–shell nanotube arrays, which showed an area capacitance of 9.6 F cm−2 at 2 mA cm−2.21 Jiang et al. prepared NiCo2S4@NiFe LDH heterostructures through a simple method, and the as-prepared electrocatalysts showed an overpotential of 201 mV and excellent cycle stability.22
Herein, we successfully synthesize CoNi2O4/Ni3S2 nanowires on Ni foam through a facile hydrothermal route. The as-fabricated samples deliver a high specific capacitance of 997.2 F g−1 at 1 A g−1 and cycle stability. In addition, the device exhibits an energy density of 0.4 mW h cm−3 at a power density of 3.99 mW cm−3 and an excellent cycle life with 79.2% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. As electrocatalysts, the hybrid CoNi2S4/Ni3S2 nanowires show excellent OER performance with a low overpotential of 360 mV and a small Tafel slope of 69.7 mV dec−1, and overpotential of 173.8 mV for the HER and Tafel slope of 98.8 mV dec−1 and excellent cycle stability.
000 cycles. As electrocatalysts, the hybrid CoNi2S4/Ni3S2 nanowires show excellent OER performance with a low overpotential of 360 mV and a small Tafel slope of 69.7 mV dec−1, and overpotential of 173.8 mV for the HER and Tafel slope of 98.8 mV dec−1 and excellent cycle stability.
2. Experimental section
2.1. Material preparation
Firstly, Ni foam was pre-treated via immersion in 0.1 M HCl, absolute ethanol and deionized water, respectively. Then, 1 mM Ni(NO3)2·6H2O, 2 mM Co(NO3)2·6H2O, 4 mM NH4F and 10 mM urea were dissolved in 60 mL deionized water. Then, the solution was transferred into an 80 mL Teflon-lined autoclave and kept at 120 °C for 8 h. Then, the samples were washed several times. Finally, the CoNi2O4 nanowires were obtained after calcination at 350 °C for 2 h (the average mass loading was 1.8 mg cm−2). Secondly, 0.35 g Na2S was dissolved into 40 mL deionized water and kept at 120 °C for 4 h. After cooling down to room temperature, the as-fabricated products were washed and dried at 60 °C. The average mass loading was 3.2 mg cm−2.2.2. Materials characterization
The crystallographic structure of the as-fabricated samples was measured through X-ray diffraction. The morphology and structure of the samples were characterized by using scanning electron microscopy (SEM, Hitachi-4800) and high resolution transmission electron microscopy (HRTEM, JEM-2100 PLUS). X-ray photoelectron spectroscopy (XPS) measurements were conducted to investigate the element composition using an ESCALAB250 with Al Kα sources.2.3. Electrochemical measurement
Electrochemical measurements of the as-prepared products including cyclic voltammetry (CV) curves, galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS) were carried out by a CHI660e electrochemical workstation in a three-electrode system in 3.0 M KOH solutions. The as-fabricated samples (d = 1 cm) were used as the working electrode, Pt foil served as the counter electrode and Hg/HgO as the reference electrode. Electrochemical impedance spectroscopy (EIS) measurements were conducted in a frequency range from 0.01 to 100 kHz at a 5 mV open circuit potential. The specific capacitance of the as-prepared samples was calculated from GCD curves via the following equations;| Cs = IΔt/mV | (1) |
2.4. Fabrication of the hybrid battery
A hybrid battery was assembled with active carbon as the negative electrode, the as-fabricated CoNi2S4/Ni3S2 nanowires as the positive electrode and PVA–KOH gel as the electrolyte. PVA–KOH gel as the electrolyte was prepared as follows: 3 g PVA was added in 25 mL deionized water at 65 °C. Then, 3 g KOH was dissolved in 5 mL deionized water and added to the above solution.2.5. Electrocatalytic performance of the hybrid battery
OER and HER performance tests of the as-prepared device were conducted in the three-electrode system. The as-fabricated samples were the working electrodes, and Ag/AgCl was the reference electrode. A graphite rod was the counter electrode. Overall water splitting was measured in a two-electrode system. All potentials were converted to reversible hydrogen electrode (RHE) though the Nernst equation ERHE = EAg/AgCl + 0.197 + 0.059 × pH, where EAg/AgCl is the measured potential.3. Results and discussion
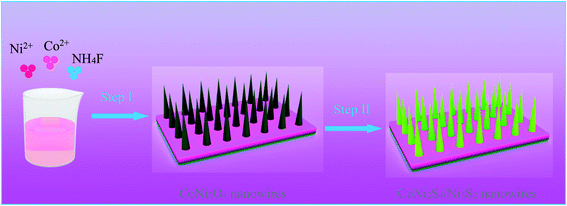
Fig. 1 shows the synthesis diagram of the hybrid CoNi2S4//Ni3S2 nanowires through a two-step hydrothermal process. Firstly, CoNi2O4 products were fabricated on the surface of Ni foam through a simple hydrothermal process. Then, the hybrid CoNi2S4/Ni3S2 products were prepared by an in situ vulcanization process.Fig. 2a shows the crystal structure of the samples; the diffraction peaks at 2θ values of 44.4, 51.6 and 76.1 degrees can be indexed to Ni foam (JCPDS no. 04-0850). The diffraction peaks at 2θ values of 31.4, 38.2, 50.3, 55.0 and 64.8 degrees could be ascribed to the (311), (400), (511), (440) and (533) crystal planes of spinel CoNi2S4 phase (JCPDS no. 24-0334). In addition, the diffraction peaks at 31.1, 37.8, 44.3, 50.1 and 55.2 degrees could be indexed to the (110), (003), (202), (211) and (300) crystal planes of Ni3S2 (JCPDS no. 44-1418), respectively, which can be attributed to the vulcanization of the Ni foam. XPS analyses were used to further investigate the chemical composition and valence. Fig. 2b shows the XPS full survey spectrum of the CoNi2S4//Ni3S2 nanowires, and the products consisted of Ni, Co and S. The Co 2p spectrum (Fig. 2c) contains two spin–orbit doublets and two shakeup satellites, the binding energy at 779.2 and 795.1 eV could be ascribed to Co3+, and the binding energies at 780.9 and 795.3 eV belong to the Co2+ phase.23 In addition, the satellite peaks reveal that Co3+ is present.24 Fig. 2d shows the Ni 2p spectrum, which can be divided into two spin–orbit doublets and two shakeup satellites. The binding energies at 854.8 and 871.6 eV can be assigned to Ni3+ and 854.3 and 871.2 eV are Ni2+.25 The sharp intense satellite peaks indicate that Ni is present as Ni2+.26 The S 2p spectrum is presented in Fig. 2e. The peak at 161.2 eV is S 2p1/2 and the peak at 162.3 eV belongs to a typical metal–sulfur bond, which improves the electrochemical activity of the as-prepared samples.27,28 Energy-dispersive X-ray spectrometry (EDX) measurements of the as-prepared CoNi2S4/Ni3S2 nanowires indicate that the as-fabricated products are composed of Ni, Co and S, as shown in Fig. 2f, and the three elements are uniformly distributed on the surface of the as-prepared samples, revealing that CoNi2S4/Ni3S2 nanowires are uniformed deposited on the surface of Ni foam.
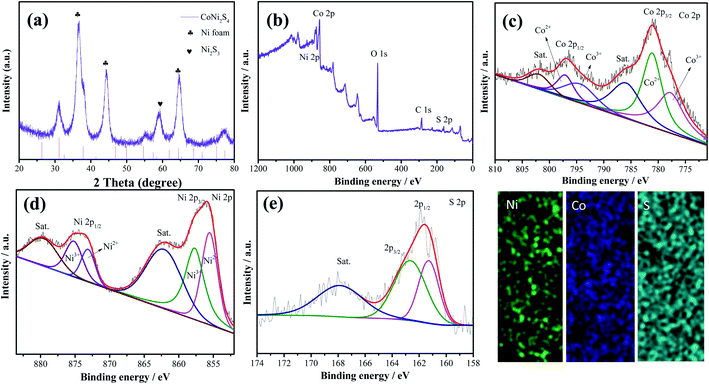 | ||
| Fig. 2 (a) XRD pattern of the as-fabricated CoNi2S4/Ni3S2 nanowires. (b) Survey spectra of the samples (c) Co 2p, (d) Ni 2p and (e) S 2p. (f) Elemental mapping. | ||
The morphology characteristics of the as-synthesized CoNi2S4/Ni3S2 nanowires were first characterized through SEM. From the low magnification SEM images in Fig. 3a, it is clearly found that CoNi2S4/Ni3S2 nanowires are uniformly located on the surface of Ni foam. From Fig. 3b, it can be found that adjacent nanowires are connected to each other to form a network structure. Fig. 3c presents high magnification SEM images, indicating that the as-prepared CoNi2S4/Ni3S2 nanowires possess an average diameter of 75 nm. TEM measurements were used to further characterize the microstructures of CoNi2S4/Ni3S2 nanowires. Fig. 3d exhibits the low-magnification TEM images of the as-fabricated samples, in which a single nanowire is made of some tiny nanoparticles and the samples show the average diameter of 70 nm. The HRTEM image shows that the lattice spacing of 0.321 nm matches well with the (220) plane of Ni3S2 phase, and the lattice spacing of 0.283 nm can be indexed to the (311) plane of CoNi2S4 spinel structure. As can be seen from Fig. 3e, it can further confirmed that the as-prepared samples contain CoNi2S4 and Ni3S2 phases, which is consistent with the XRD patterns. The SAED pattern illustrated in Fig. 3f, shows that the as-fabricated samples present polycrystalline features that possess more active sites and energy barriers for adsorbing water molecules.
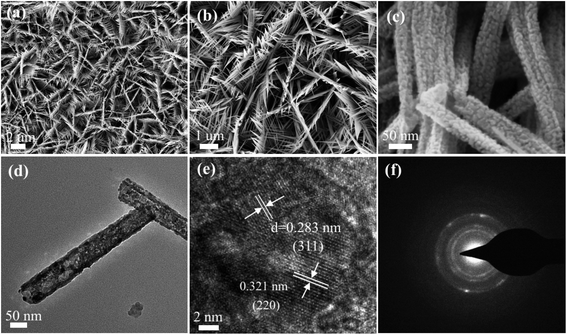 | ||
| Fig. 3 SEM images of the as-fabricated CoNi2S4/Ni3S2 nanowires. (a and b) Low magnification and (c) high magnification images. (d) TEM images. (e) HRTEM images. (f) SAED images. | ||
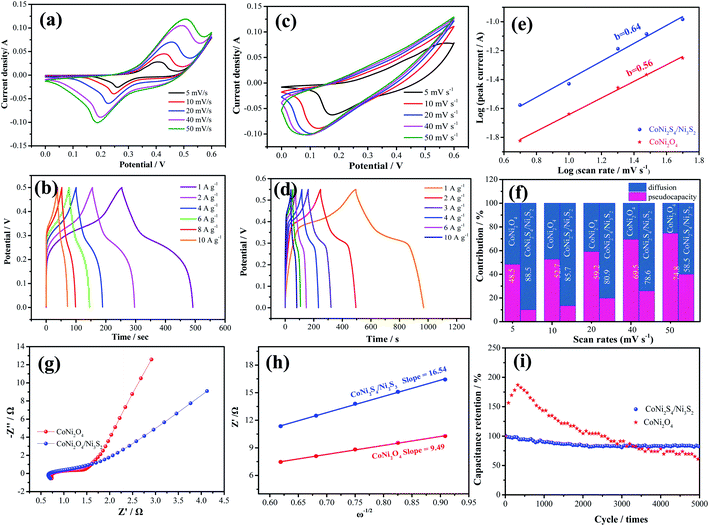
To investigate the electrochemical performances of the as-prepared samples, Fig. 4a exhibits the CV curves of CoNi2O4 samples at scan rates from 5 to 50 mV s−1. It is seen that CV curves show a pair of redox peaks, demonstrating that the products possess typical pseudocapacitive characteristics. Fig. 4b shows the GCD curves of the CoNi2S4/Ni3S2 samples, and it is seen that the samples possess a specific capacitance of 997.2 F g−1 at a current density of 1 A g−1. CV curves of the hybrid CoNi2S4/Ni3S2 samples are depicted in Fig. 4c, and it is observed that the integrated areas increase as the scan sweep increases, indicating that the as-fabricated samples possess a fast ion and electron transfer rate. Based on previous reports, Ni foam contributes little to the capacitance.29 Fig. 4d shows the GCD curves of the as-prepared electrode at different current densities from 1 A g−1 to 10 A g−1. It is found that the as-fabricated samples exhibit a high specific capacitance of 997.2 F g−1 at 1 A g−1. To further analyze the electrochemical kinetics of the as-prepared samples, the capacitance can be calculated according to following equation:
| i = avb | (2) |
| i = k1ν + k2ν1/2 | (3) |
| Z = Rs + Rct + σwω−1/2 | (4) |
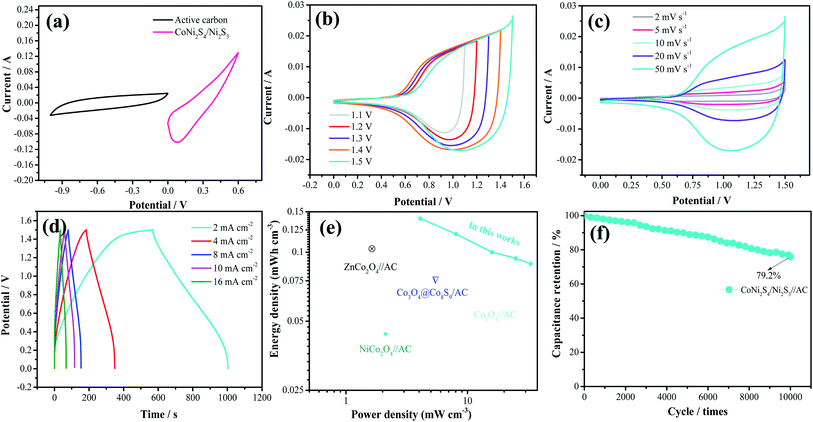
To further investigate the practical application of the as-fabricated electrode, the device is assembled with the CoNi2S4/Ni3S2 nanowires electrode as the positive electrode and active carbon as the negative electrode. The composition of CV curves of the CoNi2S4/Ni3S2 nanowires and active carbon at a current density of 50 mV s−1 are depicted in Fig. 5a. It is found that the electrode materials exhibit single potential windows of −1.0–0 V and 0–0.6 V, respectively, indicating that the device can reach a total potential of 1.6 V. Fig. 5b exhibits the CV curves of the fabricated CoNi2S4/Ni3S2 nanowires//AC device at scan rates from 2 to 50 mV s−1. It can be seen that the device can work in a voltage window of 0–1.5 V. With increasing scan rates, the CV shapes are maintained, indicating that the capacitors possess excellent rate performance. CV curves of the CoNi2S4/Ni3S2 nanowires//AC device with different potential windows at a scan rate of 50 mV s−1 are collected to investigate the extended working voltage, as illustrated in Fig. 5c, and the device shows a stable capacitive behavior with electric double-layer capacitive properties even at a voltage of up to 1.5 V. Fig. 5d shows the GCD measurement of the device, which showed that the areal capacitance of the CoNi2S4/Ni3S2 nanowires//AC device reaches 136.05 mF cm−2 at a current density of 2 mA cm−2. As the current density increased to 10 mA cm−2, the areal capacitance was maintained at 56 mF cm−2. The Ragone plots of the asymmetric supercapacitors are shown in Fig. 5e. The CoNi2S4/Ni3S2 nanowires//AC supercapacitors deliver a maximum energy density of 0.4 mW h cm−3 at a power density of 3.99 mW cm−3, which is comparable to previously reported ASC devices.31–34 The long-term cycling stability of the CoNi2S4/Ni3S2 nanowires//AC device is also evaluated by repeating charge–discharge measurements, as shown in Fig. 5f. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, there is still 90% retention of the original capacitance at a current density of 5 mA cm−2.
000 cycles, there is still 90% retention of the original capacitance at a current density of 5 mA cm−2.
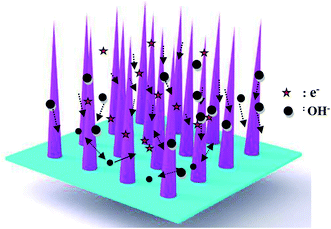
The as-fabricated samples possess excellent cycle stability, which can be ascribed to the following aspects, as shown in Fig. 6. First, the as-fabricated CoNi2S4/Ni3S2 nanowires are directly on Ni foam, which provides a lot of active sites. Secondly, the hybrid CoNi2S4/Ni3S2 structure alleviates the volume change during the electrochemical reaction process. Finally, the adjacent nanowires possess enough reaction space, which leads to a rapid electrochemical reaction.
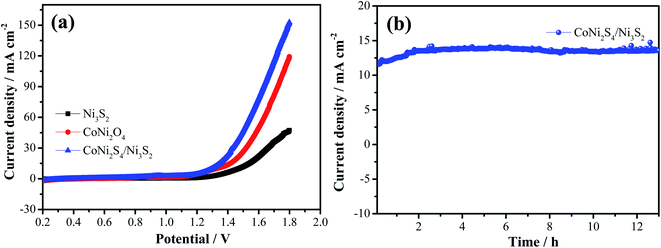
The electrocatalytic performance of the as-prepared catalysts is estimated through LSV and Tafel curves in 1.0 M KOH solution. As shown in Fig. 7a, the hybrid CoNi2S4/Ni3S2 nanowires show a lower overpotential of 360 mV compared to that of CoNi2O4 nanowires (380 mV), Ni3S2 samples (410) and Ni foam (459 mV) at a current density of 30 mA cm−2, which demonstrates that CoNi2S4/Ni3S2 nanowires possess better electrocatalytic performance. To give insight into the OER kinetic mechanism, Fig. 7b presents the Tafel slope of the catalyst. Hybrid CoNi2S4/Ni3S2 nanowires exhibit a small Tafel slope of 69.7 mV dec−1, which is lower than that of the CoNi2O4 nanowires (71.5 mV dec−1), Ni3S2 samples (83.2 mV dec−1) and Ni foam (135.3 mV dec−1). The low Tafel slope increases the charge transfer rate and provides active sites for OH adsorption.35 To further study the outstanding OER activity, the electrochemical active surface area (ECSA) was measured through the double-layer capacitance (Cdl). The value of the electrochemical double layer capacitance is shown in Fig. 7c. CoNi2S4/Ni3S2 nanowires exhibit a higher ECSA (0.039 mF cm−2) than CoNi2O4 nanowires (0.0168 mF cm−2) and Ni3S2 samples (0.0177 mF cm−2). The HER performance of the as-prepared samples is also tested at 5 mV s−1. The comparison of the LSV curves of catalysts is depicted in Fig. 7d. It is clearly found that hybrid CoNi2S4/Ni3S2 nanowires exhibit a small Tafel slope of 173.8 mV at −10 mA cm−2, which is lower than that of CoNi2O4 nanowires (192.6 mV) and Ni3S2 samples (210.8 mV). Fig. 7e shows the corresponding Tafel plots, which shows that hybrid CoNi2S4/Ni3S2 nanowires exhibit a small Tafel slope of 98.8 mV dec−1, which is lower than the CoNi2O4 nanowires (117.2 mV dec−1) and Ni3S2 samples (148.9 mV dec−1). The Tafel slope of CoNi2S4/Ni3S2 nanowires is smallest, revealing the fast reaction kinetics. Since the Tafel slope is in the range from 40 to 120 mV dec−1, this implies that the HER reaction of CoNi2S4/Ni3S2 nanowires obey the Volmer–Heyrovsky mechanism, and the Volmer step is rate-determining.36 The long-term stability measurements of the three electrode materials are conducted at a constant potential for 13 h, as shown in Fig. 7f. The results demonstrate that the as-fabricated catalysts present excellent cycle stability.
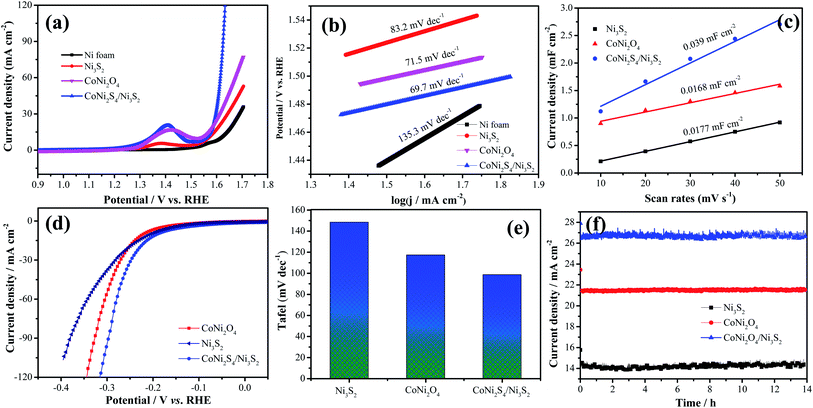 | ||
| Fig. 7 (a–c) OER of the as-prepared catalysts, (a) LSV curves, (b) Tafel, (c) electric double layer capacitor value, (d) LSV curves for HER, (e) Tafel for HER, (f) cycle stability. | ||
To further investigate the potential practical applications, overall water splitting tests are conducted through a two-electrode system with the hybrid structured catalysts as both the cathode and anode. Fig. 8a presents the LSV curve of overall water splitting with a scan rate of 5 mV s−1. It is seen that hybrid CoNi2S4/Ni3S2 samples show a small cell voltage of 1.43 V at 30 mA cm−2, which is lower than that of CoNi2S4 nanowires (1.51 V) and Ni3S2 samples (1.65 V). In order to evaluate the stability of the as-prepared catalysts, chronoamperometry measurements were conducted, as depicted in Fig. 8b. It was found that the hybrid structured CoNi2S4/Ni3S2 samples possess excellent long durability. At the same time, a large number of bubbles were formed from both the cathode and anode as the electrocatalytic test proceeded.
4. Conclusion
In summary, hybrid structured CoNi2S4/Ni3S2 nanowires were fabricated through a facile two-step hydrothermal method. The as-prepared products directly act as a supercapacitor electrode with a specific capacitance of 997.2 F g−1 at 1 A g−1 and cycle stability of 80.3% retention after 5000 cycles. The asymmetric supercapacitor shows a high energy density of 0.4 mW h cm−3 at a power density of 3.99 mW cm−3 and cycling stability of 79.2% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The as-fabricated CoNi2S4/Ni3S2 nanowires also exhibit excellent electrocatalytic performance with a low overpotential of 360 mV for the OER and a small Tafel slope of 98.8 mV dec−1 and a cell voltage of 1.43 V.
000 cycles. The as-fabricated CoNi2S4/Ni3S2 nanowires also exhibit excellent electrocatalytic performance with a low overpotential of 360 mV for the OER and a small Tafel slope of 98.8 mV dec−1 and a cell voltage of 1.43 V.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Natural Science Foundation of Liaoning Province (No. 2019-ZD-0253), Liaoning Revitalization Talents Program (No. XLYC1902095).References
- F. Shahzad, M. Alhabeb, C. B. Hatter, B. Anasori, S. Man Hong, C. M. Koo and Y. Gogotsi, Science, 2016, 353, 1137–1140 CrossRef CAS.
- D. F. Yan, Y. X. Li, J. Huo, R. Chen, L. M. Dai and S. Y. Wang, Adv. Mater., 2017, 29, 1606459 CrossRef.
- J. Y. Rao, N. S. Liu, Z. Zhang, J. Su, L. Y. Li, L. Xiong and Y. H. Gao, Nano Energy, 2018, 51, 425–433 CrossRef CAS.
- Q. Q. Qin, D. W. Ou, C. J. Ye, L. X. Chen, B. B. Lan, J. Yan and Y. C. Wu, Electrochim. Acta, 2019, 305, 403–415 CrossRef CAS.
- Y. Li, J. Xu, T. Feng, Q. F. Yao, J. P. Xie and H. Xia, Adv. Funct. Mater., 2017, 27, 1606728 CrossRef.
- M. Dakshana, S. Meyvel, M. Malarvizhi, P. Sathya, R. Ramesh, S. Prabhu and M. Silambarasan, Vacuum, 2020, 174, 109218 CrossRef.
- M. R. Lukatskaya, B. Dunn and Y. Gogotsi, Nat. Commun., 2016, 7, 12647 CrossRef.
- L. X. Xu, S. S. Xiong, S. X. Zhong, S. Bai, Y. Jiao and J. R. Chen, Vacuum, 2020, 174, 109213 CrossRef.
- J. Tian, Q. Liu, A. M. Asiri and X. Sun, J. Am. Chem. Soc., 2014, 136, 7587–7590 CrossRef.
- J. Kim, Y. Lee and S. Sun, J. Am. Chem. Soc., 2010, 132, 4996–4997 CrossRef.
- Y. L. Zhou, D. Y. Lin, X. Y. Ye and M. Y. Zhu, J. Alloys Compd., 2020, 839, 155691 CrossRef.
- Y. Zhao, R. Nakamura, K. Kamiya, S. Nakanishi and K. Hashimoto, Nat. Commun., 2013, 4, 2390 CrossRef.
- S. Bai, C. Wang, M. Deng, M. Gong, Y. Bai, J. Jiang and Y. Xiong, Angew. Chem., Int. Ed., 2014, 53, 12120–12124 CrossRef.
- K. A. Stoerzinger, L. Qiao, M. D. Biegalski and Y. Shao-Horn, J. Phys. Chem. Lett., 2014, 5, 1636–1641 CrossRef.
- Y. Pi, Q. Shao, P. Wang, J. Guo and X. Huang, Adv. Funct. Mater., 2017, 27, 1700886 CrossRef.
- S. M. Jasem and A. C. C. Tseung, J. Electrochem. Soc., 1979, 126, 1353–1360 CrossRef CAS.
- Z. Yang, H. Lv and R. Wu, Nano Res., 2016, 9, 3671–3682 CrossRef CAS.
- Y. Cheng, H. Zhao, H. Lv, T. Shi, G. Ji and Y. Hou, Adv. Electron. Mater., 2020, 6, 1900796 CrossRef CAS.
- R. Chen, H. Y. Wang, J. W. Miao, H. B. Yang and B. Liu, Nano Energy, 2015, 11, 333–340 CrossRef CAS.
- S. J. Peng, L. L. Li, C. C. Li, H. T. Tan, R. Cai, H. Yu, S. Mhaisalkar, M. Srinivasan, S. Ramakrishna and Q. Y. Yan, Chem. Commun., 2013, 49, 10178–10180 RSC.
- R. Li, S. L. Wang, Z. C. Huang, F. X. Lu and T. B. He, J. Power Sources, 2016, 312, 156–164 CrossRef CAS.
- J. Liu, J. S. Wang, B. Zhang, Y. J. Ruan, L. Lv, X. Ji, K. Xu, L. Miao and J. J. Jiang, ACS Appl. Mater. Interfaces, 2017, 9, 15336–15345 Search PubMed.
- L. J. Deng, P. H. Zhou, J. L. Xie and L. Zhang, J. Appl. Phys., 2016, 101, 103916 CrossRef.
- G. He, J. Li, W. Li, B. Li, N. Noor, K. Xu, J. Hu and I. P. Parkin, J. Mater. Chem. A, 2015, 3, 14272–14278 RSC.
- H. Lv, X. Liang, Y. Cheng, H. Zhang, D. Tang, B. Zhang, G. Ji and Y. Du, ACS Appl. Mater. Interfaces, 2015, 7, 4744–4750 CrossRef CAS.
- X. Liu, C. Hao, L. He, C. Yang, Y. Chen, C. Jiang and R. Yu, Nano Res., 2018, 11, 4169–4182 CrossRef CAS.
- X. J. Chen, D. Chen, X. Y. Guo, R. M. Wang and H. Z. Zhang, ACS Appl. Mater. Interface, 2017, 9, 18774–18781 CrossRef CAS.
- B. Y. Guan, L. Yu, X. Wang, S. Song and X. W. Lou, Adv. Mater., 2017, 29, 1605051 CrossRef.
- Q. Wu, J. Wang, H. Jin, T. Yan, G. Yi, X. Su, W. Dai and X. Wang, Mater. Lett., 2018, 244, 138–141 CrossRef.
- Y. Z. Wu, J. S. Meng, Q. Li, C. J. Niu, X. P. Wang, W. Yang, W. Li and L. Q. Mai, Nano Res., 2017, 10, 2364–2376 CrossRef CAS.
- Y. L. Tong, X. Y. Cheng, X. Y. Liu, D. L. Qi, B. Q. Chi and Y. F. Wang, J. Nanoelectron. Optoelectron., 2020, 15, 237–242 CrossRef.
- Y. L. Tong, D. L. Qi, B. Q. Chi and W. Q. Zhang, Sci. Adv. Mater., 2019, 11, 338–344 CrossRef CAS.
- S. S. Xiong, S. Y. Jiang, J. Wang, H. J. Lin, M. X. Lin, S. T. Weng, S. Liu, Y. Jiao, Y. C. Xu and J. R. Chen, Electrochim. Acta, 2020, 340, 135956 CrossRef CAS.
- Y. L. Tong, B. Q. Chi, D. L. Qi and X. Y. Liu, Sci. Adv. Mater., 2019, 11, 1087–1092 CrossRef CAS.
- Y. Sun, K. Xu, Z. Wei, H. Li, T. Zhang, X. Li, W. Cai, J. Ma, H. J. Fan and Y. Li, Adv. Mater., 2018, 30, 1802121 CrossRef.
- L. Huang, D. Chen, G. Luo, Y. Lu, C. Chen, Y. Zou, C. Dong, Y. Li and S. Wang, Adv. Mater., 2019, 31, 1901439 CrossRef.
| This journal is © The Royal Society of Chemistry 2020 |