Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fused multicyclic polyketides with a two-spiro-carbon skeleton from mangrove-derived endophytic fungus Epicoccum nigrum SCNU-F0002†
Zhangyuan Yan,
Jialin Li,
Geting Ye,
Tao Chen,
Meimei Li,
Yanmin Liang and
Yuhua Long *
*
Guangzhou Key Laboratory of Analytical Chemistry for Biomedicine, School of Chemistry, South China Normal University, Guangzhou 510006, China. E-mail: Yuhualong68@hotmail.com
First published on 3rd August 2020
Abstract
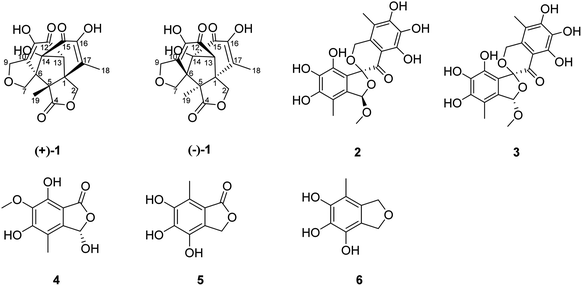
A pair of uncommon fused multicyclic polyketides with a two- spiro-carbon skeleton, (±)-isoepicolactone, (±)-1, and one new isobenzofuranone monomer (4), together with four other known biosynthetically related compounds were isolated from the fermentation of an endophytic fungus, Epicoccum nigrum SCNU-F0002, which was isolated from the fresh fruit of the mangrove plant Acanthus ilicifolius L. Comprehensive spectroscopic analysis, X-ray crystallography, together with calculated ECD, were employed to define the structures. The antibacterial and COX-2 inhibitory activities of the compounds (1–6) were evaluated. A possible biogenetic pathway of (±)-isoepicolactone was confirmed.
1. Introduction
Dimeric natural products possess fascinating molecular architectures and display remarkable bioactivities.1–4 So far, various kinds of dimeric derivatives, including polyketides,5,6 alkaloids,7 terpenoids8,9 and peptides,10 have been obtained from different species of marine mangrove endophytic fungi and terrestrial organisms. An overview of the molecular structures of dimerics reveals that the biosynthetically originate from the convergence of two monomer, which are formed mostly by intermolecular [4 + 2] cycloaddition (Diels–Alder reaction),11,12 aldol condensation reaction,13 [3 + 2] cycloaddition,14,15 radical coupling16 and [5 + 2] cycloaddition.17 Among them, dimeric isoepicolactones possessing a pentacyclic ring system based on a decalin moiety, which is bridged by two spiro-connected furan rings to form a symmetrical carbon skeleton with two spiro centers, have been isolated from the fermentation of an endophytic fungus, Epicoccum nigrum SCNU-F0002. The intricate polycyclic skeleton of 1 is unique in natural sources, and has been reported in only three literatures up to date.18–20 From the pathway analysis, we know that (±)-isoepicolactone via intermolecular [5 + 2] cycloaddition reaction complete the cascade and obtains a complex dimmer compound. Our first time characterize isoepicolactone from natural source proved the priediction of Prof. Trauner,21 who accomplished the elegant total synthesis of epicolatone using intermolecular [5 + 2] cycloaddition strategy, in that work (±)-isoepenolides are reported as by-products in racemates.During our ongoing search for bioactive polyketides derivatives from marine mangrove endophytic fungi, changes in the composition of the culture medium were employed to reinvestigate the secondary metabolites of Epicoccum nigrum SCNU-F0002. This led to the isolation of a pair of (±)-isoepicolactone, (±)-1, and one new isobenzofuranone monomer (4), together with four known biosynthetically related compounds (Fig. 1). Herein, the isolation, structural elucidation, biological evaluation, and plausible biosynthetic pathway of (±)-isoepicolactone were described.
2. Results and discussion
2.1 Structure elucidation
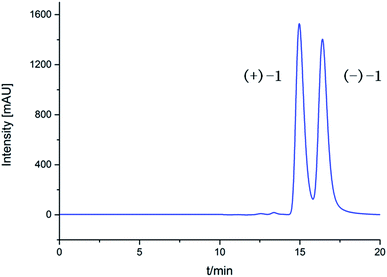
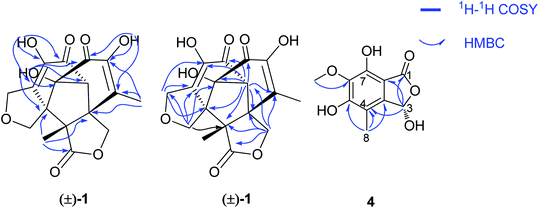
The EtOAc extract of cultures of Epicoccum nigrum SCNU-F0002 was fractionated and purified by repeated silica gel chromatography, chiral-phase HPLC, reversed-phase HPLC, and semipreparative HPLC to afford three new and four known related compounds.(±)-Iso-epicolactone, (±)-1, was obtained as white solid. The molecular formula was deduced as C17H16O18 based on the HRESIMS at m/z 347.0775 [M − H]− (calcd for C17H15O18, 347.0772), indicating 10 degrees of unsaturation. IR spectrum exhibited the absorption bands of hydroxyl group (3394 cm−1) and carbonyl groups (1757 and 1646 cm−1). The 1H NMR and HMQC spectra recorded in DMSO gave the signals of three singlets at δH 6.28 (14-OH), 8.84 (16-OH) and 8.97 (11-OH) ppm with no correlations to carbon atoms, which are thus assigned to OH, three oxymethylene protons at δH 4.21 (H-2) and 4.61 (H-2′); 3.74 (H-7) and 3.82 (H-7′); 4.28 (H-9) and 4.38 (H-9′), and two methyls at δH 1.96 (H-18), 1.03 (H-19), one methine at δH 3.08. Analysis of 13C NMR, DEPT 135°, and HMQC spectra showed 17 carbon signals that could be assigned as two α,β-unsaturated carbonyls at δC 190.1 (C-12) and 193.3 (C-15), one ester carbonyl at δC 176.5 (C-4), two methyls at δC 13.8 (C-18) and 17.5 (C-19), three methylene signals at δC 66.4 (C-2), 65.6 (C-7) and 67.8 (C-9), one methine carbon at δC 69.1 (C-13), eight quaternary carbons at δC 51.3 (C-1), 60.1 (C-5), 59.6 (C-6), 136.9 (C-10), 140.1 (C-11), 88.4 (C-14), 146.5 (C-16) and 134.7 (C-17). Their structures were identified as isoepicolactone, by analysis of spectroscopic data (1D, 2D NMR) and comparison with literature values (ESI-Table S1†).21 The lack of optical activity and cotton effects in the ECD spectrum showed that 1 was racemic. Subsequently, 1 was successfully resolved by HPLC using a chiral ND (2) column, to afford two optically pure enantiomers, (+)-1 and (−)-1, in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
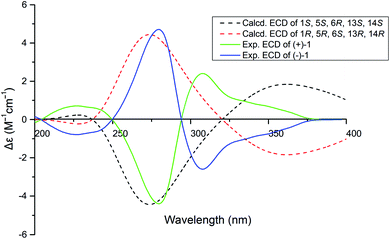
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by chiral-phase HPLC (Fig. 2). The same ECD patterns but opposite cotton effects and the opposite optical rotations of (+)-1 and (−)-1 verified their enantiomeric relationship. In the NOESY spectrum, the correlations from H-13 to H-2′/H-9/H-7′, H-19 to H-2′/H-9/H-7′, H-9 to H-2′revealed the cofacial relationship of these protons, while the cross-peaks of H-18/H-9′ and H-9′/H-2 showed these groups on the opposite side of the molecule. Thereby, the relative configuration has been assigned as 1R*,5R*,6S*,13R*,14R*. By comparing the calculated and experimental ECD spectra (Fig. 3), the absolute configurations of (+)-1 and (−)-1 were determined to be (1S,5S,6R,13S,14S) and (1R,5R,6S,13R,14R), respectively (Fig. 1).
1 by chiral-phase HPLC (Fig. 2). The same ECD patterns but opposite cotton effects and the opposite optical rotations of (+)-1 and (−)-1 verified their enantiomeric relationship. In the NOESY spectrum, the correlations from H-13 to H-2′/H-9/H-7′, H-19 to H-2′/H-9/H-7′, H-9 to H-2′revealed the cofacial relationship of these protons, while the cross-peaks of H-18/H-9′ and H-9′/H-2 showed these groups on the opposite side of the molecule. Thereby, the relative configuration has been assigned as 1R*,5R*,6S*,13R*,14R*. By comparing the calculated and experimental ECD spectra (Fig. 3), the absolute configurations of (+)-1 and (−)-1 were determined to be (1S,5S,6R,13S,14S) and (1R,5R,6S,13R,14R), respectively (Fig. 1).
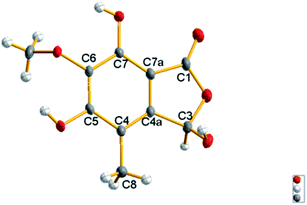
The molecular formula of epicoccone F (4) was calculated as C10H10O6 from HRESIMS. The 13C NMR data of 4 revealed the presence of 10 carbons, including one lactone carbonyl at δC 171 (C-1), one methoxy carbon at δC 61.2 (6-OMe), one methyl at δC 10.6, one oxymethine at δC 98.5 (C-3), six aromatic carbons at δC 114.3 (C-4), 142.9 (C-4a), 157 (C-5), 148.8 (C-6), 137.6 (C-7) and 104.8 (C-7a). Detailed comparison of their 1D and 2D NMR data from epicoccone D18 and F suggested evidence for the same benzofuranone subunit, but compound 4 lacked an oxymethylene proton signal at C-3. The signal was replaced by a hydroxy group (3-OH) in epicoccone F. The HMBC correlations (Fig. 5) from 6-OMe to C-6, and H-8 to C-4/C-4a/C-5 established a methyl group at C-4 and a methoxy group at C-6, which was further supported by HMBC correlations from H-3 to C-1/C-4/C-7a and X-ray single-crystal diffraction analysis (Fig. 4). Finally, all NMR data for 4 were readily assigned by HMBC analysis. Thus, compound 4 was identified as (S)-3,5,7-trihydroxy-6-methoxy-4-methylisobenzofuran-1(3H)-one (Fig. 1).
The other known compounds were identified as aspermicrone B (2),22 aspermicrone C (3),22 4,5,6-trihydroxy-7-methyl phthalide (5),23 4,5,6-trihydroxy-7- methyl-1,3-dihydroisobenzofuran (6)24 by comparison of their spectroscopic data with those reported in the literature.
2.2 Biological activity
Compounds 1–6 were tested for their antibacterial activities against some of Gram-positive and Gram-negative bacterial strains (Staphylococcus aureus ATCC 6538, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 8739, Pseudomonas Aeruginosa ATCC 9027, Salmonella ATCC 14028). Unfortunately, they showed no significant activity against the assessed organisms at a dose of 100 μg mL−1. Compounds (+)-1 and (−)-1 show weak COX-2 inhibitory activity at 5 μg mL−1, inhibition rate 28.8% and 31.2%, the positive control indomethacin inhibition rate 78.9%.2.3 Plausible biogenetic pathway of compounds (±)-1
The proposed biosynthesis pathway of compounds (±)-1 was performed in Scheme 1. Firstly, the intermediate (a) was formed by three molecules of malony-CoA and one molecular acetyl-CoA based on the HR-PKS. Then, condensation constructed a phenol (b) and the following formed intermediate (c) through the oxidation as well as methylation, which further oxidation to yield intermediate (d). Subsequently, compound 5 could be generated by esterification of intermediate (d) and the following formed compound 6 by reduction. In addition, the intermediate A was formed by the hydrolyzation, decarboxylation and oxidization of compound 5; meanwhile, the intermediate B was obtained by the hydrolyzation, reduction and oxidization of compound 5. The oxidization of epicoccine 6 gave two o-quinones intermediates (C and D). Epicolactone derivatives were formed via intermolecular [5 + 2] cycloaddition reaction of two o-quinones intermediates (A, B, C, D), followed by intramolecular lactone formation. Among them, the [5 + 2] cycloaddition of two o-quinones intermediates, A and D, obtained intermediate E, the following intramolecular nucleophilic attack of C2-hydroxyl to carbonyl C4 formed intermediate F with a lactone moiety, the following tautomerization of C12-hydroxyl and intramolecular cyclolization reaction formed C6-C14 bond, thus the (±)-iso-epicolactone, (±)-1, was formed.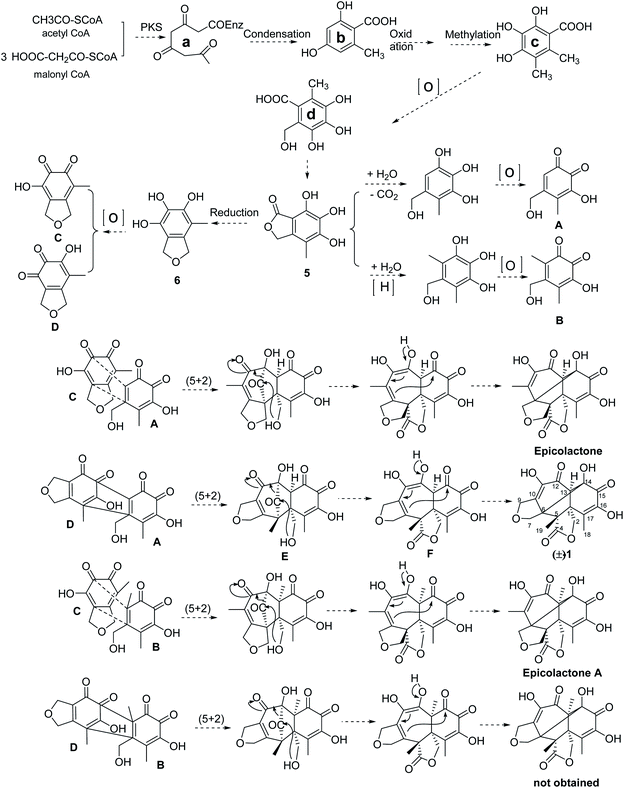 | ||
| Scheme 1 The proposed biosynthesis pathway of (±)-iso-epicolactone (a) epicolactone A18 and epicolactone19,20 were isolated as previously reported. | ||
3. Experimental section
3.1 General experimental procedures
HR-ESI-MS were acquired on a Thermofisher LTQ Orbitrp Elite LC-MS spectrometer (Thermo Fisher Scientific, Inc., Hudson, NH, USA). The 1H NMR (600 MHz), 13C NMR (150 MHz), and 2D NMR spectra were obtained on a Bruker AVANCE-600 (Bruker BioSpin Corporation, Billerica, MA, USA) using TMS as an internal reference. IR data were measured on a Nicolet 5DX-FTIR (Thermo Fisher Scientific, Inc., Hudson, NH, USA), in KBr discs. Single-crystal data were measured on an Oxford Gemini S Ultra diffractometer (Oxford Instrument, Oxfordshire, UK). Optical rotations were recorded on a Bellingham-Stanley ADP 440 + polarimeter at 25 °C. UV data were recored on a Shimadzu UV-240 spectrophotometer (Shimadzu, Kyoto, Japan). CD data were measured on a J-810 spectropolarimeter (JASCO, Tokyo, Japan). TLC analysis was carried out on silica get plates (Marine chemical Ltd, Qingdao, China). The chiral HPLC separation was accomplished over an Chiral ND (2) column (column size: 250 × 4.6 mm 5 μm; FLM Scientific Instrument Co., Ltd). Phenomenex Luna (Phenomenex, Torrance, CA, USA) C18 column (250 × 10 mm, 5 μm, 5 mL min−1) was used for semipreparative HPLC. Silica gel (200–300 mesh, Marine Chemical Ltd, Qingdao, China), and Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Stockholm, Sweden) were used for column chromatography (CC).3.2 Fungal material
The fungal strain SCNU-F0002 was isolated from the fresh fruit of the mangrove plant Acanthus ilicifolius L collected from the Qi'ao island Mangrove Nature Reserve in Guangdong province, China. The fungus was obtained using the standard protocol for isolation.25 Fungal identification was carried out using a molecular biological protocol by DNA amplification and sequencing of the ITS region.26 The sequence data obtained from the fungal strain have been deposited at GenBank with accession no. MN096740. A BLAST result revealed 100% match with the sequence of Epicoccum nigrum (compared to MH484012.1). A voucher strain was deposited in School of Chemistry and Environment, South China Normal University, Guangzhou, China, with the access code, SCNU-F0002.3.3 Fermentation, extraction and isolation
The fungus was cultured on autoclaved potato glucose water medium (250.0 g L−1 of potato, 3.0 g L−1 of artificial sea salts, 20.0 g L−1 of glucose) at room temperature under static conditions and daylight for 35 days. Following incubation, the whole liquid medium was filtered through cheesecloth to separate the liquid and mycelia. The former was extracted with EtOAc, while the latter was extracted with MeOH. The MeOH extract was evaporated under reduced pressure to afford an aqueous solution and then extracted with EtOAc. The two EtOAc extracts were combined and concentrated under reduced pressure to give an organic extract (80.0 g). The extract was isolated by column chromatography over silica gel eluting with a gradient of petroleum ether: ethyl acetate from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 0
0 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to afford five fractions (fractions 1–5). Fraction 2 (110.0 mg) was applied to Sephadex LH-20 CC and eluted with CH2Cl2/MeOH (1
1 to afford five fractions (fractions 1–5). Fraction 2 (110.0 mg) was applied to Sephadex LH-20 CC and eluted with CH2Cl2/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain 11.2 mg of compounds (±)-1 (8.0 mg). Racemic (±)-1 was separated into a pair of enantiomers (+)-1 (1.5 mg, tR = 14.9 min) and (−)-1 (1.0 mg, tR = 16.4 min) through chiral HPLC (n-hexane/isopropanol = 95
1) to obtain 11.2 mg of compounds (±)-1 (8.0 mg). Racemic (±)-1 was separated into a pair of enantiomers (+)-1 (1.5 mg, tR = 14.9 min) and (−)-1 (1.0 mg, tR = 16.4 min) through chiral HPLC (n-hexane/isopropanol = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v, flow rate: 5.0 mL min−1). Fraction 3 (12.0 mg) was purified by semipreparative RP-HPLC (70% acetonitrile/H2O) to yield compounds 2 (5.2 mg, tR = 8.5 min) and 3 (6.3 mg, tR = 11.0 min). Fraction 4 (220.0 mg) was applied to column chromatography over silica gel, eluting with CH2Cl2/MeOH (200
5, v/v, flow rate: 5.0 mL min−1). Fraction 3 (12.0 mg) was purified by semipreparative RP-HPLC (70% acetonitrile/H2O) to yield compounds 2 (5.2 mg, tR = 8.5 min) and 3 (6.3 mg, tR = 11.0 min). Fraction 4 (220.0 mg) was applied to column chromatography over silica gel, eluting with CH2Cl2/MeOH (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then further purified by Sephadex LH-20 CC eluted with CH2Cl2/MeOH (1
1) and then further purified by Sephadex LH-20 CC eluted with CH2Cl2/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to compound 5 (15.8 mg) and compound 6 (4.0 mg). Fraction 5 (40.0 mg) was applied to Sephadex LH-20 CC and eluted with CH2Cl2/MeOH (1
1) to compound 5 (15.8 mg) and compound 6 (4.0 mg). Fraction 5 (40.0 mg) was applied to Sephadex LH-20 CC and eluted with CH2Cl2/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain compound 4 (11.2 mg).
1) to obtain compound 4 (11.2 mg).
3.4 Physio–chemical properties of compounds 1and 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 302 (3.35) nm; IR (KBr) νmax: 3105, 3058, 1750, 1580, 1450, 1132, 980, 820 cm−1; HR-ESI-MS m/z 347.0775 [M − H]−(calcd for 347.0772); 1H and 13C NMR data, see Table 1.
ε): 302 (3.35) nm; IR (KBr) νmax: 3105, 3058, 1750, 1580, 1450, 1132, 980, 820 cm−1; HR-ESI-MS m/z 347.0775 [M − H]−(calcd for 347.0772); 1H and 13C NMR data, see Table 1.
| Position | 1a | 4b | ||
|---|---|---|---|---|
| δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | |
| a Recorded in DMSO-d6.b Recorded in MeOH. J in Hz. δ in ppm. | ||||
| 1 | 51.3, C | 171.0, C | ||
| 2 | 4.21, d, (J = 10.1); | 66.4, CH2 | ||
| 4.61, d, (J = 10.1) | ||||
| 3 | 6.44, s | 98.5, CH | ||
| 4 | 176.5, C | 114.3, C | ||
| 4a | 142.9, C | |||
| 5 | 60.1, C | 157.0, C | ||
| 6 | 59.6, C | 148.8, C | ||
| 6-OCH3 | 3.79, s | 61.2, CH3 | ||
| 7 | 3.74, d, (J = 10.0); | 65.6, CH2 | 137.6, C | |
| 3.82, d, (J = 10.0) | ||||
| 7a | 104.8, C | |||
| 8 | 2.15, s | 10.6, CH3 | ||
| 9 | 4.28, d, (J = 16.1); | 67.8, CH2 | ||
| 4.38, d, (J = 16.1) | ||||
| 10 | 136.9, C | |||
| 11 | 140.1, C | |||
| 11-OH | 8.97, s | — | ||
| 12 | 190.1, C | |||
| 13 | 3.08, s | 69.1, CH | ||
| 14 | 88.4, C | |||
| 14-OH | 6.27, s | — | ||
| 15 | 193.3, C | |||
| 16 | 146.5, C | |||
| 16-OH | 8.84, s | — | ||
| 17 | 134.7, C | |||
| 18 | 1.96, s | 13.8, CH3 | ||
| 19 | 1.03, s | 17.5, CH3 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 302 (3.35) nm; IR (KBr) νmax: 3105, 3058, 1750, 1580, 1450, 1132, 980, 820 cm−1; HR-ESI-MS m/z 347.0775 [M − H]−(calcd for 347.0772); 1H and 13C NMR data, see Table 1.
ε): 302 (3.35) nm; IR (KBr) νmax: 3105, 3058, 1750, 1580, 1450, 1132, 980, 820 cm−1; HR-ESI-MS m/z 347.0775 [M − H]−(calcd for 347.0772); 1H and 13C NMR data, see Table 1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 313 (4.58) nm; IR (KBr) νmax: 3134, 3033, 2920, 1750, 1450, 1145, 1040, 1060, 960, 820, 780, 762 cm−1; HRESIMS m/z 249.0371 [M + Na]+(calcd for 249.0370); 1H and 13C NMR data, see Table 1.
ε): 313 (4.58) nm; IR (KBr) νmax: 3134, 3033, 2920, 1750, 1450, 1145, 1040, 1060, 960, 820, 780, 762 cm−1; HRESIMS m/z 249.0371 [M + Na]+(calcd for 249.0370); 1H and 13C NMR data, see Table 1.3.5 X-ray crystallographic analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Crystal X-ray diffraction data were collected on an Agilent Gemini Ultra diffractometer with Cu kα radiation (λ = 1.54184 Å). The structure was solved by direct methods (SHELXS-97) and refined using full-matrix least-squares difference Fourier techniques. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were placed in the ideal geometrical positions and refined isotropically with a riding model. Crystallographic data of 4 have been deposited with the Cambridge Crystallographic Data Centre.
1, v/v). Crystal X-ray diffraction data were collected on an Agilent Gemini Ultra diffractometer with Cu kα radiation (λ = 1.54184 Å). The structure was solved by direct methods (SHELXS-97) and refined using full-matrix least-squares difference Fourier techniques. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were placed in the ideal geometrical positions and refined isotropically with a riding model. Crystallographic data of 4 have been deposited with the Cambridge Crystallographic Data Centre.![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2, Dc = 1.589 g cm−3, μ = 1.189 mm−1, and F (000) = 256.0. Crystal dimensions: 0.2 × 0.15 × 0.12 mm3. Independent reflections: 1991 (Rint = 0.0329). The final R1 values were 0.0463, wR2 = 0.1371 (I > 2σ(I)). The goodness of fit on F2 was 1.037. CCDC number: 1919077.
, Z = 2, Dc = 1.589 g cm−3, μ = 1.189 mm−1, and F (000) = 256.0. Crystal dimensions: 0.2 × 0.15 × 0.12 mm3. Independent reflections: 1991 (Rint = 0.0329). The final R1 values were 0.0463, wR2 = 0.1371 (I > 2σ(I)). The goodness of fit on F2 was 1.037. CCDC number: 1919077.3.6 Antimicrobial activity assay
The antimicrobial activities against five bacterial (S. aureus (ATCC 6538), B. subtilis (ATCC 6633), E. coli (ATCC 8739), P. aeruginosa (ATCC 9027) were evaluated in 96-well microtiter plates using a modification of the broth microdilution method.27,28 The antibacterial activity test procedure is as previously described.293.7 COX-2 inhibitory activity
The in vitro inhibitory of test compounds were evaluated using COX-2 (human) inhibitor screening assay kit (Item no. 701080) supplied by Cayman Chemicals USA. Indomethacin was used as a positive control. The inhibitory activity of the compounds was measured at each concentrations so as to calculating the IC50. The procedure for COX-2 in inhibitory immunoassay are as previously reported.30,313.8 ECD calculations
Conformational searches were carried out by means of the Spartan'14 software using Molecular Merck force field (MMFF). All density functional theory (DFT) and time-dependent (TD)-DFT calculations were performed with Gaussian 09 program. Conformers within a 10 kcal mol−1 energy window were generated and optimized by DFT calculations at the B3LYP/6-31+G (d, p) level. Conformers with a Bolzmann distribution over 3% were chose for ECD calculations by TD-DFT method at the B3LYP/6-31+G (d, p) level.32 The polarizable continuum model for MeOH was used. The calculated ECD curves were generated using the SpecDis 3.0 (University of Würzburg) and Origin Pro 8.5 (Origin Lab, Ltd) from dipole-length rotational strengths by applying Gaussian band shapes with sigma = 0.30 eV.4. Conclusions
Chemical study of Epicoccum nigrum SCNU-F0002 collected from the Qi'ao island Mangrove Nature Reserve led to the isolation and identification of three novel compounds, (±)-isoepicolactone, (±)-1, and isobenzofuranone monomer (4). Compounds (+)-1 and (−)-1 showed weak COX-2 inhibitory activity at 5 μg mL−1, inhibition rate 28.8% and 31.2%, the positive control indomethacin inhibition rate 78.9%. All compounds revealed weak or no antibacterial activity at a concentration of 100 μg mL−1. By exploring the culture conditions, we found that the yield of secondary metabolites in potato glucose water medium was lower than that in rice solid medium. The yield of dimer type compounds was significantly higher than that of rice solid medium studied before.18Conflicts of interest
No potential conflict of interest was reported by the authors.Acknowledgements
The authors gratefully acknowledge grants from the National Natural Science Foundation of China (No. 41876153, 41376149), GDME-2018C004 and the Natural Science Foundation of Guangdong Province (2017A030313088).References
- M. Moussa, W. Ebrahim, M. El-Neketi, A. Mandi, T. Kurtan, R. Hartmann, W. Lin, Z. Liu and P. Proksch, Tetrahydroanthraquinone derivatives from the mangrove-derived endophytic fungus Stemphylium globuliferum, Tetrahedron Lett., 2016, 57(36), 4074–4078 CrossRef CAS.
- S. Liu, Y. Zhao, C. Heering, C. Janiak, W. E. G. Mueller, S. H. Akone, Z. Liu and P. Proksch, Sesquiterpenoids from the endophytic fungus Rhinocladiella similis, J. Nat. Prod., 2019, 82(5), 1055–1062 CrossRef CAS PubMed.
- S.-G. Li, X.-J. Huang, M.-M. Li, Q. Liu, H. Liu, Y. Wang and W.-C. Ye, Multiflorumisides A.-G., dimeric stilbene glucosides with rare coupling patterns from the roots of Polygonum multiflorum, J. Nat. Prod., 2018, 81(2), 254–263 CrossRef CAS PubMed.
- P. Wang, Y.-F. Luo, M. Zhang, J.-G. Dai, W.-J. Wang and J. Wu, Three xanthone dimers from the Thai mangrove endophytic fungus Phomopsis sp. xy21, J. Asian Nat. Prod. Res, 2018, 20(3), 217–226 CrossRef CAS PubMed.
- Y.-N. Shi, S. Pusch, Y.-M. Shi, C. Richter, J. G. Macia-Vicente, H. Schwalbe, M. Kaiser, T. Opatz and H. B. Bode, (±)-Alternarlactones A. and B., Two Antiparasitic Alternariol-like Dimers from the Fungus Alternaria alternata P1210 Isolated from the Halophyte Salicornia sp, J. Org. Chem., 2019, 84, 11203–11209 CrossRef CAS PubMed.
- W.-M. Zhong, J.-F. Wang, X.-Y. Wei, Q. Zeng, X.-Y. Chen, Y. Xiang, X.-P. Tian, Si. Zhang, L.-J. Long and F.-Z. Wang, (+)- and (-)-Eurotone A: A pair of enantiomeric polyketide dimers from a marine-derived fungus Eurotium sp. SCSIO F452, Tetrahedron Lett., 2019, 60, 1600–1603 CrossRef CAS.
- R. Cai, H. Jiang, Z. Xiao, W. Cao, T. Yan, Z. Liu, S. 'e. Lin, Y. Long and Z. She, (-)- And (+)- Asperginulin A, a Pair of Indole Diketopiperazine Alkaloid Dimers with a 6/5/4/5/6 Pentacyclic Skeleton from the Mangrove Endophytic Fungus Aspergillus sp. SK-28, Org. Lett., 2019, 21(23), 9633–9636 CrossRef CAS PubMed.
- X.-Y. Shen, D.-P. Qin, H. Zhou, J.-F. Luo, Y.-D. Yao, C.-K. Lio, H.-B. Li, Y. Dai, Y. Yu and X.-S. Yao, Nardochinoids A.-C., Three dimeric sesquiterpenoids with specific fused-ring skeletons from Nardostachys chinensis, Org. Lett., 2018, 20(18), 5813–5816 CrossRef CAS PubMed.
- J.-W. Dong, Le. Cai, X.-J. Li, R.-F. Mei, J.-P. Wang, P. Luo, Y. Shu and Z.-T. Ding, Fermentation of Illigera aromatica with Clonostachys rogersoniana producing novel cytotoxic menthane-type monoterpenoid dimmers, RSC Adv., 2017, 7(62), 38956–38964 RSC.
- M. Bae, H. Kim, K. Moon, S.-J. Nam, J. Shin, K.-B. Oh and D.-C. Oh, Mohangamides A and B, New Dilactone-Tethered Pseudo-Dimeric Peptides Inhibiting Candida albicans Isocitrate Lyase, Org. Lett., 2015, 17(3), 712–715 CrossRef CAS PubMed.
- S.-C. Mao, E. Manzo, Y.-W. Guo, M. Gavagnin, E. Mollo, M. L. Ciavatta, R. v. Soest and G. Cimino, New diastereomeric bis-sesquiterpenes from Hainan marine sponges Axinyssa variabilis and Lipastrotethya ana, Tetrahedron, 2007, 63(45), 11108–11113 CrossRef CAS.
- M. Zhou, M. Dong, X.-Li. Zeng and X.-Z. Huang, Three new limonene-derived bis-monoterpenoids from the aerial parts of Illigera cordata, Phytochem Lett, 2019, 30, 38–42 CrossRef CAS.
- C.-L. Xie, R. Chen, S. Yang, J.-M. Xia, G.-Y. Zhang, C.-H. Chen, Y. Zhang, X.-W. Yang and A. Nesteretal, A Novel Class of Cage-Like Polyketide from Marine-Derived Actinomycete Nesterenkonia halobia, Org. Lett., 2019, 21(20), 8174–8177 CrossRef CAS PubMed.
- G.-P. Yin, Ya-R. Wu, C. Han, X.-B. Wang, H.-L. Gao, Y. Yin, L.-Yi. Kong and M.-H. Yang, Asperones A-E, five dimeric polyketides with new carbon skeletons from the fungus Aspergillus sp. AWG 1-15, Org. Chem. Front., 2018, 5(16), 2432–2436 RSC.
- J. Wu, C. Tang, L. Chen, Y. Qiao, M. Geng and Y. Ye, Dicarabrones A and B, a Pair of New Epimers Dimerized from Sesquiterpene Lactones via a [3 + 2] Cycloaddition from Carpesium abrotanoides, Org. Lett., 2015, 17(7), 1656–1659 CrossRef CAS PubMed.
- Z. Liu, S. Chen, P. Qiu, C. Tan, Y. Long, Y. Lu and Z. She, (+)- and (-)- Ascomlactone A: a pair of novel dimeric polyketides from a mangrove endophytic fungus Ascomycota sp. SK2YWS-L, Org. Biomol. Chem., 2017, 15(48), 10276–10280 RSC.
- Y. Long, Y. Ding, H. Wu, C. Qu, H. Liang, M. Zhang, X. Zhao, X. Long, S. Wang, P.-T. Puno and J. Deng, Total Synthesis of (-)-Perezoperezone through an Intermolecular [5+2] Homodimerization of Hydroxy p-Quinone, Angew Chem Int Edit, 2019, 58(49), 17552–17557 CrossRef CAS PubMed.
- Z. Yan, C. Huang, H. Guo, S. Zheng, J. He, J. Lin and Y. Long, Isobenzofuranone monomer and dimer derivatives from the mangrove endophytic fungus Epicoccum nigrum SCNU-F0002 possess α-glucosidase inhibitory and antioxidant activity, Bioorg. chem, 2020, 94, 103407 CrossRef CAS PubMed.
- F. D. d. S. Araujo, L. C. d. L. Favaro, W. L. Araujo, F. Lazzarotto de Oliveira, R. Aparicio and A. J. Marsaioli, Epicolactone-Natural Product Isolated from the Sugarcane Endophytic Fungus Epicoccum nigrum, Eur. J. Org. Chem., 2012, 5225–5230 CrossRef CAS.
- F. M. Talontsi, B. Dittrich, A. Schueffler, H. Sun and H. Laatsch, Epicoccolides: Antimicrobial and Antifungal Polyketides from an Endophytic Fungus Epicoccum sp. Associated with Theobroma cacao, Eur. J. Org. Chem., 2013, 3174–3180 CrossRef CAS.
- P. Ellerbrock, N. Armanino, M. K. Ilg, R. Webster and D. Trauner, An eight-step synthesis of Epicolactone reveals its biosynthetic origin, Nat. Chem., 2015, 7(11), 879–882 CrossRef CAS PubMed.
- N. D. Luyen, L. M. Huong, T. H. H. Tran, L. H. Cuong, T. H. Y. Duong, N. X. Nhiem, B. H. Tai, A. Gardes, K. German and V. K. Phan, Aspermicrones A-C, novel dibenzospiroketals from the seaweed-derived endophytic fungus Aspergillus micronesiensis, J. Antibiot., 2019, 72(11), 843–847 CrossRef CAS PubMed.
- N. H. Lee, J. B. Gloer and D. T. Wicklow, Isolation of chromanone and isobenzofuran derivatives from a fungicolous isolate of Epicoccum purpurascens, Bull. Korean Chem. Soc., 2007, 28, 877–879 CrossRef CAS.
- X.-Z. Huang, Y. Zhu, X.-L. Guan, K. Tian, J.-M. Guo, H.-B. Wang and G.-M. Fu, A novel antioxidant isobenzofuranone derivative from fungus Cephalosporium sp. AL031, Molecules, 2012, 17, 4219–4224 CrossRef CAS PubMed.
- S. Chen, Y. Liu and Z. Liu, Isocoumarins and benzofurans from the mangrove endophytic fungus Talaromyces amestolkiae possess α-glucosidase inhibitory and antibacterial activities, RSC Adv., 2016, 6, 26412–26420 RSC.
- Y. Liu, Q. Yang, G. Xia, H. Huang, H. Li, L. Ma, Y. Lu, L. He, X. Xia and Z. She, Polyketides with α-glucosidase inhibitory activity from a mangrove endophytic fungus, Penicillium sp. HN29-3B1, J. Nat. Prod., 2015, 78, 1816–1822 CrossRef CAS PubMed.
- M. C. Carpinella, C. G. Ferrayoli and S. M. Palacios, Antifungal synergistic effect of scopoletin, a hydroxycoumarin isolated from Melia azedarach L. fruits, J. Agric. Food Chem., 2005, 53, 2922–2927 CrossRef CAS PubMed.
- Z. Wu, Z. Xie, M. Wu, X. Li, W. Li, W. Ding, Z. She and C. Li, New antimicrobial cyclopentenones from Nigrospora sphaerica ZMT05, a fungus derived from oxya chinensis thunber, J. Agric. Food Chem., 2018, 66, 5368–5372 CrossRef CAS PubMed.
- Z. Yan, S. Wen, M. Ding, H. Guo, C. Huang, X. Zhu, J. Huang, Z. She and Y. Long, The purification, characterization, and biological activity of new polyketides from mangrove-derived endophytic fungus Epicoccum nigrum SCNU-F0002, Mar. Drugs, 2019, 17(7), 414 CrossRef CAS PubMed.
- Y.-M. Yan, H.-X. Zhang, H. Liu, Y. Wang, J.-Bo. Wu, Y.-P. Li and Y.-X. Cheng, (+/-)-Lucidumone, a COX-2 Inhibitory Caged Fungal Meroterpenoid from Ganoderma lucidum, Org. Lett., 2019, 21(21), 8523–8527 CrossRef CAS PubMed.
- P. Singh, S. Kaur, J. Kaur, G. Singh and R. Bhatti, Rational Design of Small Peptides for Optimal Inhibition of Cyclooxygenase-2: Development of a Highly Effective Anti-Inflammatory Agent, J. Med. Chem., 2016, 59(8), 3920–3934 CrossRef CAS PubMed.
- Y. Chen, Z. Liu, Y. Huang, L. Liu, J. He, L. Wang, J. Yuan and Z. She, Ascomylactams A-C, cytotoxic 12- or 13-membered-ring macrocyclic alkaloids isolated from the mangrove endophytic fungus Didymella sp. CYSK-4, and structure revisions of phomapyrrolidones A and C, J. Nat. Prod., 2019, 82(7), 1752–1758 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1919077. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra05532h |
| This journal is © The Royal Society of Chemistry 2020 |