Open Access Article
Open Access ArticleEffects of high pressure processing (HPP) on microorganisms and the quality of mango smoothies during storage
Xiufang Bi†
 *a,
Zhongyu Zhou†a,
Tingting Qinb,
Xiaoqiong Wanga,
Yuan Ma
*a,
Zhongyu Zhou†a,
Tingting Qinb,
Xiaoqiong Wanga,
Yuan Ma a,
Yage Xinga and
Zhenming Chea
a,
Yage Xinga and
Zhenming Chea
aSichuan Key Laboratory of Food Biotechnology, School of Food and Bioengineering, Xihua University, 999 Jinzhou Road, Jinniu District, Chengdu 610039, People's Republic of China. E-mail: bxf1221@163.com; Tel: +86-28-87720552
bKey Laboratory of Food Non-Thermal Technology, Engineering Technology Research Center of Food Non-Thermal, Yibin Xihua University Research Institute, Yibin 644004, China
First published on 25th August 2020
Abstract
The objective of this study is to investigate the effects of high pressure processing (HPP) on the quality of mango smoothies and the inactivation of microorganisms therein, with heat treatments used as the control. Comparative analysis was conducted on the microbiological changes in the mango smoothies subjected to HPP at 400–600 MPa for 0–15 min. The total plate count (TPC) and the yeast and mold (YM) counts were found to be significantly inactivated through increases in the pressure and treatment time (p < 0.05). Conditions of 90 °C/20 min (HT), 500 MPa/8 min (HPP-500) and 600 MPa/5 min (HPP-600) were, thus, selected as the subsequent treatment for a storage study at 4 °C for 15 days, since these conditions had similar inactivation effects on TPC and YM. After 15 days of storage, the TPC was found to have increased by 3.87, 3.54 and 3.36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cycles in the mango smoothies treated by HT, HPP-500 and HPP-600, respectively, while the YM counts remained at less than 1
log10 cycles in the mango smoothies treated by HT, HPP-500 and HPP-600, respectively, while the YM counts remained at less than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cycle in all samples. During storage, compared to the HT and HPP-600 samples, both the color and viscosity at 100 s−1 of samples treated by HPP-500 were found to be better maintained. Carotene content was better retained in storage after the HPP process than after the HT process. However, the different treatments had no effect on the pH nor on the total soluble solids (TSS) in the samples. The study ascertained that HPP-500 is able to ensure both the microbial safety and the quality of mango smoothies more effectively than HT and HPP-600.
log10 cycle in all samples. During storage, compared to the HT and HPP-600 samples, both the color and viscosity at 100 s−1 of samples treated by HPP-500 were found to be better maintained. Carotene content was better retained in storage after the HPP process than after the HT process. However, the different treatments had no effect on the pH nor on the total soluble solids (TSS) in the samples. The study ascertained that HPP-500 is able to ensure both the microbial safety and the quality of mango smoothies more effectively than HT and HPP-600.
1. Introduction
The consumption of fruit juices blended with dairy beverages – so-called smoothies – has increased in response to consumer demand for highly nutritious, healthy foods.1 Traditionally, smoothies are a mixture of liquidized ingredients usually including fruit (or, less commonly, vegetables), fruit juice, ice, yoghurt and milk.2 Smoothies are ideally suited to a market in which consumers demand the highest quality products that are convenient, nutritious, minimally processed, with fresh flavors, taste and appearance.3 To extend the shelf life of these products, smoothies are often thermally processed. Heat treatment (HT) is one of the most efficient and economical processes for achieving microbial inactivation in milk and other perishable liquid foods, however this method cannot be used to treat heat-labile compounds.4 Furthermore, high temperatures may lead to undesirable effects in milk such as off-flavors, non-enzymatic browning and the denaturation of certain vitamins and proteins.5,6 Several authors2,7,8 have reported that high pressure processing (HPP) is a proven alternative to heat treatment for the effective processing of fruit and vegetable juices and is widely accepted by consumers.9HPP is also a novel non-thermal technology used for food pasteurization.10 It promotes the destruction of vegetative microorganisms11 and can potentially stabilize bioactive compounds and antioxidant activity, thereby mitigating the loss of these important food properties. HPP can be used to create beverages with significant health benefits, while preserving nutritional and sensorial characteristics and extending their shelf life.12,13 Recently, researches have examined the effect of HPP on the qualities of fruit smoothies.10,12,14,15 Andrés, Villanueva and Tenorio12 found that orange–papaya–melon–carrot smoothies treated by HPP (450 and 600 MPa/3 min/20 °C) preserved most of their ascorbic acid, polyphenols and antioxidant activity compared to heat processing (80 °C/3 min). Hurtado et al.14 reported that, in comparison to mild heating (85 °C/7 min), HPP (350 MPa/5 min/10 °C) more effectively maintained color, viscosity and turbidity. Moreover, HPP also provides a wide margin of microbial security in blended fruit products.14 For example, pressurization at 200 MPa for 5 min produced a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction of Lactobacillus plantarum in an orange juice–milk beverage.16 HPP technology is therefore known as a treatment for the refrigerated storage of these products and further reduces the side-effects introduced by thermal processing.17 However, only a few studies have focused on changes in the rheological characteristics,18 microorganisms15 and quality attributes19 of fruit smoothies after HPP during storage, and, therefore, this treatment and the relevant changes require further investigation. Consequently, this study investigated the effect of HPP and the subsequent storage period (15 days) at 4 °C on the microorganisms and quality (pH, color, total soluble solids (TSS), viscosity, turbidity and carotenoids) in a mango smoothie, compared to the thermally treated beverage. The objective of this study was to compare these treatments and ascertain the most suitable technology for mango smoothies processing.
log reduction of Lactobacillus plantarum in an orange juice–milk beverage.16 HPP technology is therefore known as a treatment for the refrigerated storage of these products and further reduces the side-effects introduced by thermal processing.17 However, only a few studies have focused on changes in the rheological characteristics,18 microorganisms15 and quality attributes19 of fruit smoothies after HPP during storage, and, therefore, this treatment and the relevant changes require further investigation. Consequently, this study investigated the effect of HPP and the subsequent storage period (15 days) at 4 °C on the microorganisms and quality (pH, color, total soluble solids (TSS), viscosity, turbidity and carotenoids) in a mango smoothie, compared to the thermally treated beverage. The objective of this study was to compare these treatments and ascertain the most suitable technology for mango smoothies processing.
2. Materials and methods
2.1. Chemicals
Citric acid, petroleum ether (boiling range 30 °C to 60 °C), and acetone were purchased from ChengDu Chron Chemicals Co., Ltd. (Chengdu, China). Nutrient agar (NA) and rose bengal agar (RBA) broth were purchased from Beijing Aoboxing Biological Technology Co., Ltd. (Beijing, China). All other chemicals were of analytical grade and were purchased from Chengdu Kelong Chemical Reagent Factory (Chengdu, China).2.2. Preparation of mango smoothie
Mango (Kate variety), originated in Panzhihua, Sichuan and ultra-high temperature (UHT) skimmed milk (0.1% fat) (Mengniu Dairy, Chengdu Branch) were purchased from a local supermarket in China. The mango pulp was first soaked in 0.1% sodium erythorbate for 5 min before being steamed at 100 °C for 2 min and, subsequently, pressed using a juice extractor without gauze (JYL-C020E, Joyoung Co., Ltd. China). The extracted mango juice was then blended together with the UHT skimmed milk (v/v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) for 60 s using a homogenizer (JTC OmniBlend, Guangdong, China). The samples were stored at 4 °C for different treatments at the earliest opportunity.
2) for 60 s using a homogenizer (JTC OmniBlend, Guangdong, China). The samples were stored at 4 °C for different treatments at the earliest opportunity.
2.3. High-pressure and thermal processing
The smoothie was packed into 50 mL screw-cap polyethylene terephthalate (PET) bottles and processed using a 5 L HPP machine (HPP 600 MPa/3–5 L, Shanghai Wodi Intelligent Equipment Co., Ltd., China) at ambient temperature (approximately 25 °C). Distilled water was used as the pressure-transmitting fluid. HPP treatments were set at 400, 500 and 600 MPa for 0–15 min. For each HPP treatment, three bottles of samples were processed. The holding time applied in this study describes the length of time for which each sample was held at a given pressure, excluding pressurization and depressurization times. The pressurization rate was approximately 120 MPa min−1, while the depressurization was immediate (<3 s). Equivalent thermal and HPP conditions in terms of microbial safety were chosen for a clearer comparison of both treatments.20 A condition of 90 °C/20 min was, thus, selected as the subsequent heat treatment for storage study, since it had a similar inactivation effect to that of the HPP treatment.50 mL of mango smoothie was poured into glass beaker and treated with heat (HT) at the desired center temperature (90 °C) for 20 min in a water bath (DK-98-II, Tianjin Taisite Instrument Co., Ltd., Tianjin, China). After heating, the samples were immediately cooled in an ice water bath. The heating time was 16 min and the cooling time was 12 min. The HT-treated sample was aseptically transferred into 50 mL PET bottles identical to those used for the HPP. For heat treatment, three bottles of samples were prepared. Following the HPP and HT processes, all samples were stored at 4 °C for further research.
2.4. Storage conditions
The HPP and HT treated samples were stored at 4 °C for 15 days, during which they were analyzed on days 0, 3, 6, 9, 12 and 15 to assess their levels of microorganisms, viscosity, pH, color, TSS, turbidity and carotenoids.2.5. Quality analysis
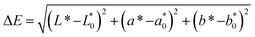 | (1) |
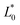 ,
, 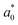 , and
, and 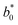 were the values for untreated samples, and L*, a*, and b* were the values of the treated sample. Each sample was measured in triplicate.
were the values for untreated samples, and L*, a*, and b* were the values of the treated sample. Each sample was measured in triplicate.| σ = k × εn | (2) |
2.6. Total carotenoid determination
Measurement of carotenoids was carried out as described by Zhou et al.23 with some modifications. 20 g sample was placed in a 100 mL stoppered conical flask with 20 mL acetone and 5 mL petroleum. The mixture was shaken for 1 min, left to stand for 5 min, then transferred to a 500 mL glass separation funnel with 100 mL of 5% sodium sulfate solution. Subsequently, 10 mL of acetone–petroleum ether (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) was mixed 2 or 3 times until the extract became colorless. The lower aqueous solution was discarded, and the mixture was washed several times with 15 mL of 5% sodium sulfate until the aqueous layer was no longer cloudy. The petroleum ether extract was filtered through anhydrous sodium sulfate (about 5 g), then placed in a 250 mL round bottom flask. The separatory funnel and anhydrous sodium sulfate were washed three times with approximately 10 mL petroleum ether, and the washing was placed in the round bottom flask. Thereafter, the petroleum ether was distilled under reduced pressure in a 40 °C water bath, until approximately 2 mL liquid remained in the bottle. The evaporation flask was subsequently removed and dried with nitrogen, immediately to which 5 mL petroleum ether was added. The liquid was concentrated to 0.1 mL, to which 4.9 mL petroleum ether was added. The absorbance was measured at 450 nm with a spectrophotometer (WFJ-7200, Unico (Shanghai) Instrument Co., Ltd., China), using petroleum ether as a blank.
7 v/v) was mixed 2 or 3 times until the extract became colorless. The lower aqueous solution was discarded, and the mixture was washed several times with 15 mL of 5% sodium sulfate until the aqueous layer was no longer cloudy. The petroleum ether extract was filtered through anhydrous sodium sulfate (about 5 g), then placed in a 250 mL round bottom flask. The separatory funnel and anhydrous sodium sulfate were washed three times with approximately 10 mL petroleum ether, and the washing was placed in the round bottom flask. Thereafter, the petroleum ether was distilled under reduced pressure in a 40 °C water bath, until approximately 2 mL liquid remained in the bottle. The evaporation flask was subsequently removed and dried with nitrogen, immediately to which 5 mL petroleum ether was added. The liquid was concentrated to 0.1 mL, to which 4.9 mL petroleum ether was added. The absorbance was measured at 450 nm with a spectrophotometer (WFJ-7200, Unico (Shanghai) Instrument Co., Ltd., China), using petroleum ether as a blank.
2.7. Microbial analysis
The microorganisms in the mango smoothie were detected using the total plate count (TPC) and yeasts and molds (YM) methods.24 Untreated and treated samples were serially diluted with sterile 0.85% NaCl, and 1.0 mL of each dilution was plated onto duplicate plates with nutrient agar medium for the TPC and rose bengal medium for the YM. Plates were incubated at 37 ± 1 °C for 48 ± 2 h for the TPC and 28 ± 1 °C for 5 days for the YM. Colonies in the smoothie samples were quantified by multiplying the reciprocals. The results were expressed as log colony-forming units (CFU mL−1) of each sample. The TPC and YM in untreated samples were 3.75 and 4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cycles CFU mL−1, respectively.
log10 cycles CFU mL−1, respectively.
2.8. Statistical analysis
All tests were conducted with three independent replicates. One-way ANOVA was applied to determine significant differences between samples by SPSS 17.0 (SPSS Inc., Chicago, IL), where the significant level was set at p < 0.05.3. Result and discussion
3.1. Effect of HPP on the inactivation of microorganisms in mango smoothie
The inactivation of microorganisms treated by HPP at 400, 500 and 600 MPa for 0–15 min is shown in Fig. 1. TPC and YM were found to be significantly inactivated as pressure increased (p < 0.05). The counts of TPC and YM decreased dramatically at 0–2 min, then decreased at a slower rate at 2–15 min. Reductions of 3.53 and 3.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cycles were achieved by treatment at 500 MPa for 8 min and 600 MPa for 5 min, respectively. The tailing-off phenomena of microorganisms during inactivation was previously reported.25 It might be due to that cells sensitive to HPP treatment might be destroyed rapidly or over a short period after exposure to a lethal treatment, while those resistant to HPP might be destroyed slowly, leading to a tailing-off on the survival curves.25 The YM counts were all below detection level after these treatment conditions (<10 CFU mL−1), thus meeting the requirements of Chinese national food safety standards for fruit and vegetable juice (GB 7101-2015, <100 CFU mL−1). These results demonstrated that the YM were more sensitive to HPP than the TPC, mainly because of their characteristic cell wall type.26 Similar results have also been previously reported.18,19 Li et al.18 found that the counts of TPC in banana smoothies were significantly reduced immediately after HPP treatment by 2.08, 2.75 and 3.06
log10 cycles were achieved by treatment at 500 MPa for 8 min and 600 MPa for 5 min, respectively. The tailing-off phenomena of microorganisms during inactivation was previously reported.25 It might be due to that cells sensitive to HPP treatment might be destroyed rapidly or over a short period after exposure to a lethal treatment, while those resistant to HPP might be destroyed slowly, leading to a tailing-off on the survival curves.25 The YM counts were all below detection level after these treatment conditions (<10 CFU mL−1), thus meeting the requirements of Chinese national food safety standards for fruit and vegetable juice (GB 7101-2015, <100 CFU mL−1). These results demonstrated that the YM were more sensitive to HPP than the TPC, mainly because of their characteristic cell wall type.26 Similar results have also been previously reported.18,19 Li et al.18 found that the counts of TPC in banana smoothies were significantly reduced immediately after HPP treatment by 2.08, 2.75 and 3.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1 at 350, 450, and 550 MPa for 10 min, respectively, while the YM counts were all below detection level after these treatments (<10 CFU mL−1). Fernandez et al.19 reported that TPC in a mixed fruit and vegetable smoothie had a reduction of 2
log10 CFU mL−1 at 350, 450, and 550 MPa for 10 min, respectively, while the YM counts were all below detection level after these treatments (<10 CFU mL−1). Fernandez et al.19 reported that TPC in a mixed fruit and vegetable smoothie had a reduction of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log cycles after HPP at 575 MPa for 9 min, while YM presented counts below the detection limit (<2.00
log cycles after HPP at 575 MPa for 9 min, while YM presented counts below the detection limit (<2.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU g−1). Conditions of 500 MPa/8 min (HPP-500) and 600 MPa/5 min (HPP-600) were, thus, selected as the subsequent HPP treatment for the storage study, since the two treatments had a similar inactivation effect.
log CFU g−1). Conditions of 500 MPa/8 min (HPP-500) and 600 MPa/5 min (HPP-600) were, thus, selected as the subsequent HPP treatment for the storage study, since the two treatments had a similar inactivation effect.
 | ||
| Fig. 1 Inactivation of microorganisms in mango smoothie by HPP for 0–15 min (A) total plate count (TPC); (B) yeast and mold (YM). | ||
3.2. Changes of microorganisms in mango smoothies by HT and HPP during storage
Differences in the growth of microorganisms in mango smoothies treated by HT, HPP-500 and HPP-600 during storage at 4 °C for 15 days are shown in Table 1. The TPC in all treated mango smoothies increased significantly as storage time progressed (p < 0.05). The counts of TPC in the HT, HPP-500 and HPP-600 treated samples were 3.87, 3.54 and 3.36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 cycles, respectively, after 15 days in storage, however, the YM counts in all treated samples were maintained at a level below 1
log10 cycles, respectively, after 15 days in storage, however, the YM counts in all treated samples were maintained at a level below 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1. These results were similar to those reported by Li et al.,18 who found that total aerobic bacteria (TAB) counts in banana smoothies after HPP at 550 MPa/10 min increased to 2.11
log10 CFU mL−1. These results were similar to those reported by Li et al.,18 who found that total aerobic bacteria (TAB) counts in banana smoothies after HPP at 550 MPa/10 min increased to 2.11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1, and that the YM counts were maintained at a level below 1
log10 CFU mL−1, and that the YM counts were maintained at a level below 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1 after 15 days at 4 °C. Hurtado et al.10 reported that the total viable counts in fruit smoothies treated by 450 MPa for 5 min, 600 MPa for 3 min, and 85 °C for 7 min increased to 2.29, 2.34, 1.25
log10 CFU mL−1 after 15 days at 4 °C. Hurtado et al.10 reported that the total viable counts in fruit smoothies treated by 450 MPa for 5 min, 600 MPa for 3 min, and 85 °C for 7 min increased to 2.29, 2.34, 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1, respectively, and that the YM counts were below the limit of detection during 30 day storage at 4 °C. In terms of pressurization effects in food matrices, HPP had been reported to induce modifications of cellular membranes and to interrupt cellular functions that were responsible for reproduction.27 In this current study, since the inactivation effects of HPP and HT on the microbes of the mango smoothies were similar, their physicochemical qualities during storage at 4 °C were compared to ascertain the most suitable processing treatment.
log10 CFU g−1, respectively, and that the YM counts were below the limit of detection during 30 day storage at 4 °C. In terms of pressurization effects in food matrices, HPP had been reported to induce modifications of cellular membranes and to interrupt cellular functions that were responsible for reproduction.27 In this current study, since the inactivation effects of HPP and HT on the microbes of the mango smoothies were similar, their physicochemical qualities during storage at 4 °C were compared to ascertain the most suitable processing treatment.
| Treatment | Time (d) | TPC log (CFU mL−1) | YM log (CFU mL−1) |
|---|---|---|---|
| a HT: 90 °C/20 min; HPP-500: high pressure processing at 500 MPa/8 min; HPP-600: high pressure processing at 600 MPa/5 min; TPC: total plate count; YM: yeast and mold, N.D.: not detected. | |||
| Untreated | 0 | 3.75 ± 0.03 | 4.60 ± 0.30 |
| HT | 0 | N.D. | N.D. |
| 3 | 0.60 ± 0.00 | N.D. | |
| 6 | 1.24 ± 0.00 | N.D. | |
| 9 | 1.51 ± 0.01 | N.D. | |
| 12 | 2.04 ± 0.00 | 0.30 ± 0.01 | |
| 15 | 3.87 ± 0.10 | N.D. | |
| HPP-500 | 0 | N.D. | N.D. |
| 3 | 0.70 ± 0.00 | N.D. | |
| 6 | 1.23 ± 0.05 | N.D. | |
| 9 | 1.47 ± 0.05 | N.D. | |
| 12 | 1.98 ± 0.03 | 0.45 ± 0.45 | |
| 15 | 3.54 ± 0.07 | 0.38 ± 0.68 | |
| HPP-600 | 0 | N.D. | N.D. |
| 3 | N.D. | N.D. | |
| 6 | 0.40 ± 0.00 | N.D. | |
| 9 | 1.24 ± 0.07 | N.D. | |
| 12 | 1.83 ± 0.05 | N.D. | |
| 15 | 3.36 ± 0.09 | N.D. | |
3.3. Changes in the viscosity of mango smoothie treated by HT and HPP during storage
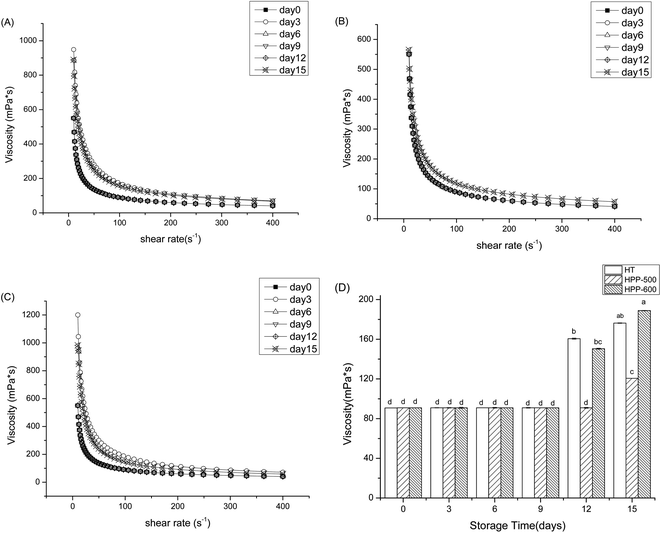
The viscosity curves of the mango smoothie after different treatments are shown in Fig. 2. The apparent viscosity in all samples was significantly decreased with increases in the shear rate (p < 0.05), exhibiting the non-Newtonian characteristics of a shear-thinning fluid. The viscosity of the mango smoothie after HT and HPP was not significantly changed compared with the control. Similarly, Anema, Lowe and Li28 found that there were no apparent differences in the viscosity curves between unheated milk samples and those treated at 90 °C for 30 min. Picouet et al.15 also reported no significant differences in the viscosity of an untreated multi-fruit smoothie and that treated at 350 MPa for 5 min. As shown in Fig. 3, the viscosity in all treated mango smoothies in this current assay increased significantly as storage time progressed (p < 0.05). The viscosity at 100 s−1 increased by 94.1%, 32.6% and 107.7% in mango smoothie treated by HT, HPP-500 and HPP-600, respectively, after 15 days (Fig. 3D). These results were similar to the findings of Zhang et al.11 and Bull et al.29 Zhang et al.11 reported that the viscosity at 100 s−1 carrot juice treated by 550 MPa/6 min and 110 °C/8.6 s was increased by 74.8% and 88.7%, respectively, after 20 days of storage at 4 °C. Bull et al.29 stated that the viscosity of orange juice treated by HPP at 600 MPa, 20 °C for 60 s and HT at 85 °C for 25 s increased 8.7% and 20.7%, respectively, after four weeks of storage at 4 °C. Bienvenue, Jiménez-Flores and Singh30 reported that the increase of viscosity in heat-treated milk could be attributed to the combined effects of increased volume fraction and increased interaction between the casein micelles. Calcium had been found in ionized or non-ionized form in milk serum, as well as associated with the casein micelles in micellar calcium phosphate form.27 Moreover, various studies have shown that HPP treatment increased the concentration of ionic calcium in milk.31 Increasing the concentration of calcium has been reported to produce casein micelles and to increase the dynamic viscosity of milk.32 Besides, the pectin in smoothies might generate low methoxyl pectin through the action of pectinmethylesterase (PME), which could react with the Ca2+ ions present to form calcium pectate and other compounds,33,34 causing the increase of viscosity in smoothies. In this study, the change in the viscosity of the HPP-500 treated sample during storage was the smallest, indicating that the taste of the samples under this condition was more like the fresh sample and would be more likely to be accepted by consumers.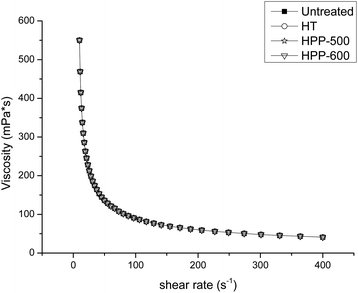 | ||
| Fig. 2 Effect of HT and HPP treatment on the viscosity in mango smoothie HT: 90 °C/20 min; HPP-500: high pressure processing at 500 MPa/8 min; HPP-600: high pressure processing at 600 MPa/5 min. | ||
3.4. Color changes in mango smoothie treated by HT and HPP during storage
The changes in the chromatic parameters of untreated, HT and HPP treated samples during the 15 day storage period are shown in Table 2. Compared with the untreated sample, the L* and b* values of the mango smoothies were increased by HT. However, these values were decreased by HPP-600, while the HT-treated mango smoothies were yellower, which could be due to an increase in browning.16 The a* value of all treated mango smoothies was reduced compared to the untreated sample. Similarly, Barba et al.16 found that orange juice mixed with milk was yellower and brighter after HT at 90 °C for 15 s than with HPP at 400 MPa for 5 min. Picouet et al.15 also reported that the L* and b* values in a multi-fruit smoothie were increased by HT at 80 °C for 7 min, however, the values were decreased by HPP at 350 MPa for 5 min. The change of L* value was not significant in HPP-500, but was noticeable (p < 0.05) in HPP-600 in this study. Kim et al.35 suggested that the decrease in L* might be due to the disintegration of casein micelles, pressurized into small fragments, that increases the translucence of milk.| Treatment conditions | Time (d) | L* | a* | b* | ΔE | pH | TSS (Brix) | Turbidity (A660) | Carotenoid (mg/100 mL) |
|---|---|---|---|---|---|---|---|---|---|
| a Different capital letters in the same column indicate significant differences (p < 0.05) among treatments at initial time (day 0). Different lowercase letters in the same column indicate significant differences (p < 0.05) between the different storage days for each treatment. HT: 90 °C/20 min; HPP-500: high pressure processing at 500 MPa/8 min; HPP-600: high pressure processing at 600 MPa/5 min; L*: lightness values; a*: redness values; b*: yellowness values; ΔE: the total color difference; TSS: total soluble solids. | |||||||||
| Untreated | — | 49.41 ± 0.28B | 0.86 ± 0.01A | 13.62 ± 0.01B | — | 4.95 ± 0.01A | 10.15 ± 0.01B | 0.45 ± 0.01C | 0.72 ± 0.01C |
| HT | 0 | 50.25 ± 0.09Aa | 0.77 ± 0.04Bf | 14.16 ± 0.06Af | 1.00 ± 0.34Be | 4.96 ± 0.01Ab | 10.27 ± 0.11A | 0.07 ± 0.01Da | 0.82 ± 0.01Ba |
| 3 | 49.26 ± 0.03b | 0.85 ± 0.01e | 14.82 ± 0.14e | 1.23 ± 0.13e | 5.02 ± 0.03a | 10.30 ± 0.07 | 0.05 ± 0.01b | 0.78 ± 0.01b | |
| 6 | 48.09 ± 0.01c | 0.95 ± 0.01d | 15.31 ± 0.13d | 2.16 ± 0.16d | 5.03 ± 0.01a | 10.27 ± 0.04 | 0.03 ± 0.01c | 0.74 ± 0.01c | |
| 9 | 47.16 ± 0.01d | 1.03 ± 0.02c | 15.71 ± 0.24c | 3.08 ± 0.19c | 5.03 ± 0.01a | 10.50 ± 0.00 | 0.03 ± 0.01c | 0.72 ± 0.01c | |
| 12 | 45.21 ± 0.05e | 1.13 ± 0.01b | 16.10 ± 0.08b | 4.89 ± 0.28b | 5.03 ± 0.01a | 10.53 ± 0.11 | 0.03 ± 0.01c | 0.68 ± 0.01d | |
| 15 | 44.34 ± 0.04f | 1.34 ± 0.02a | 16.74 ± 0.15a | 5.99 ± 0.18a | 5.00 ± 0.01a | 10.68 ± 0.04 | 0.03 ± 0.01c | 0.64 ± 0.01e | |
| HPP-500 | 0 | 49.65 ± 0.14Ba | 0.72 ± 0.01Cf | 10.54 ± 0.04Ce | 3.11 ± 0.07Ab | 4.95 ± 0.01Ab | 10.33 ± 0.04A | 0.63 ± 0.01Ba | 0.84 ± 0.01Aa |
| 3 | 47.80 ± 0.18b | 0.82 ± 0.02e | 10.93 ± 0.21d | 3.07 ± 0.16b | 5.06 ± 0.02a | 10.40 ± 0.07 | 0.60 ± 0.01b | 0.80 ± 0.01ab | |
| 6 | 46.97 ± 0.01c | 0.92 ± 0.01d | 11.41 ± 0.05c | 3.28 ± 0.22b | 5.02 ± 0.02ab | 10.55 ± 0.00 | 0.58 ± 0.01c | 0.78 ± 0.01bc | |
| 9 | 46.51 ± 0.11d | 1.00 ± 0.04c | 11.95 ± 0.08b | 3.36 ± 0.32b | 5.00 ± 0.02b | 10.60 ± 0.07 | 0.54 ± 0.01d | 0.74 ± 0.01cd | |
| 12 | 45.26 ± 0.04e | 1.10 ± 0.04b | 12.59 ± 0.28a | 4.29 ± 0.33a | 5.02 ± 0.01ab | 10.65 ± 0.00 | 0.52 ± 0.01e | 0.76 ± 0.03bc | |
| 15 | 44.81 ± 0.02f | 1.24 ± 0.04a | 12.61 ± 0.16a | 4.73 ± 0.30a | 4.99 ± 0.01bc | 10.78 ± 0.04 | 0.46 ± 0.01f | 0.70 ± 0.01d | |
| HPP-600 | 0 | 48.75 ± 0.06Ca | 0.70 ± 0.01Cd | 10.34 ± 0.06De | 3.36 ± 0.11Ac | 4.94 ± 0.01Aab | 10.40 ± 0.00A | 0.67 ± 0.01Aa | 0.86 ± 0.01Aa |
| 3 | 46.94 ± 0.02b | 0.83 ± 0.01c | 10.52 ± 0.36e | 3.97 ± 0.17b | 4.97 ± 0.01a | 10.50 ± 0.07 | 0.62 ± 0.01b | 0.84 ± 0.01ab | |
| 6 | 46.19 ± 0.10c | 0.86 ± 0.01c | 11.18 ± 0.04d | 4.06 ± 0.31b | 4.96 ± 0.01a | 10.55 ± 0.07 | 0.59 ± 0.01c | 0.82 ± 0.01b | |
| 9 | 45.48 ± 0.04d | 0.89 ± 0.10c | 11.58 ± 0.03c | 4.43 ± 0.23b | 4.95 ± 0.01ab | 10.68 ± 0.11 | 0.57 ± 0.01c | 0.76 ± 0.01c | |
| 12 | 44.40 ± 0.01e | 1.06 ± 0.04b | 12.37 ± 0.17b | 5.18 ± 0.30a | 4.98 ± 0.03a | 10.73 ± 0.04 | 0.53 ± 0.01d | 0.74 ± 0.01cd | |
| 15 | 43.95 ± 0.03f | 1.22 ± 0.02a | 14.03 ± 0.51a | 5.50 ± 0.32a | 4.95 ± 0.01ab | 10.83 ± 0.04 | 0.52 ± 0.01d | 0.72 ± 0.01d | |
During the storage period, L* values were found to decrease and a* and b* values were found to increase compared to day 0, indicating the mango smoothies becoming darker and more intensely red and yellow in color. The ΔE values in all treated mango smoothies increased significantly as storage time progressed (p < 0.05). After 12 days' storage, the ΔE values of the mango smoothies treated by HT and HPP-500 were 4.89 and 4.29, respectively, however, the ΔE values of those treated by HPP-600 were already at 4.06 after 6 days' storage, indicating that the HPP-600 treated smoothies underwent the most significant color change. These results concur with the findings of Andrés, Villanueva and Tenorio,12 who demonstrated the superior color stability of smoothies treated at 450 MPa/3 min and stored at 4 °C, compared to the samples treated by TP (80 °C/3 min) and HPP-600 (600 MPa/3 min). Color compounds of processed fruits could change during storage due to the incomplete inactivation of enzymes and microorganisms, resulting in undesirable chemical reactions in the food matrix.36,37 In conclusion, the HPP-500 treated smoothies retained their color best and underwent the least change in color during storage.
3.5. Changes in pH, TSS, turbidity and carotenoids of mango smoothie during storage
The changes in the pH, TSS, turbidity and carotenoids of the untreated, HT- and HHP-treated samples and their values during the 15 day storage period are provided in Table 2. Neither HPP nor HT caused any significant change in the pH (p > 0.05). This concurs with the findings of Hurtado et al.,10 who reported no significant change in the pH of red fruit-based smoothies treated by 350 MPa/10 °C/5 min and 85 °C/7 min. During storage, none of the samples showed significant changes in pH (p > 0.05). Similarly, Picouet et al.15 found no changes in the pH of multi-fruit smoothies treated at 80 °C/7 min and 350 MPa/5 min after storage of 21 days at 4 °C. Li et al.18 also found that there were no changes in banana smoothies treated by HPP at 550 MPa/10 min after 15 days at 4 °C. The stability of pH in the treated smoothies during storage was, thus, beneficial to the processing and subsequent sale of the products. Compared to the untreated sample, the TSS of all processing groups slightly increased (p < 0.05). Similar observations were reported by Kaushik et al.38 During storage, in the treated samples the TSS rose to between 10.15 to 10.83 °Brix, and, in this case, the observed differences were found to be statistically insignificant.19 Similarly, HPP treatment was found to have no significant effect on the TSS.10,14,18,39–41Compared to the untreated sample, turbidity in the samples treated by HT decreased by 84.4% (p < 0.05), but was increased by 40% and 48.9% by HPP-500 and HPP-600 treatments (p < 0.05), respectively. The decrease in turbidity by HT could be attributable to the denaturation of protein and the precipitate formed by protein, phenols, and polysaccharide during HT.42 The increase in turbidity by HPP suggested the gelation of pectin by pressure treatment at certain pectin contents, Brix and pH values.43 During the 15 day storage period, turbidity in the samples treated by HT decreased by 57.1% during the first 6 days and remained constant thereafter until the 15th day. However, the turbidity in the samples treated by HPP-500 and HPP-600 decreased by 27% and 22.4% respectively. Hurtado et al.14 found that the turbidity in a vegetable smoothie (comprising apple, carrot, zucchini, pumpkin and leek) treated at 85 °C/7 min and HPP (350 MPa/5 min/10 °C) decreased by 90% and 38%, respectively, after 28 days at 4 °C. A loss of turbidity in smoothies and juices had been attributed to pectin degradation through the action of pectinmethylesterase (PME).33 PME hydrolyzes methyl esters of galacturonic acid, generating low methoxyl pectin, which could react with the Ca2+ ions present in the medium to form calcium pectate and other water insoluble compounds that precipitated, involving a gradual loss of turbidity in juices.34
Compared to the untreated sample, the carotenoids in the samples treated by HT, HPP-500 and HPP-600 showed significant increases of 13.9%, 16.7% and 19.4%, respectively (p < 0.05). Similar observations have been reported in previous studies. Jacobo-Velázquez and Hernández-Brenes44 reported a significant increase of 56% in the total extractable carotenoids in avocado paste after HHP treatment at 600 MPa/3 min. Lin and Chen45 reported a pronounced increase in carotenoids in tomato juice after HT (121 °C/40 s). This increase in carotenoids may be attributed to the improvement in their extractability as a result of the compromised cell walls due to HPP or HT.46 Moreover, HPP could increase the amount of extractable carotenoids through their release after the denaturation of protein–carotenoid complexes.11
During storage, the carotenoids in all treated mango smoothies decreased significantly as storage time progressed (p < 0.05). After storage of 15 days, the retention of carotenoids was 78.04%, 83.33% and 83.72% in the mango smoothie samples treated by HT, HPP-500 and HPP-600, respectively, indicating that the carotene was better retained during storage after the HPP process than after HT. Zhang et al.11 reported that the carotenoids of carrot juice treated by HPP (550 MPa/6 min) and HT (110 °C/8.6 s) decreased by 66.7% and 73%, respectively, after 20 days of storage at 4 °C. These findings concur with several reports of a significantly higher loss in total carotenoids in HT-treated samples compared to HHP-treated samples during storage.12,20,47
These results have been widely attributed to the inactivation of the enzymes that cause a loss of carotenoids during storage, and improvement in the extraction caused by high-pressure.48 The instability and degradation of carotenoids during storage may be caused by geometric isomerization and susceptibility to oxidation.49 In fact, the phenomenon happened not only because of extrinsic factors, such as the presence or absence of light, storage temperature, or packaging, but also because of the characteristics of the food matrices, such as their chemical composition, the oxygen dissolved in the samples, the size of the particles, and the physical state of the carotenoids in the food.50,51
4. Conclusions
In this study, microbial inactivation was achieved after HPP and HT treatments, and the mango smoothies were found to be microbiologically safe (TPC < 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1 and YM < 1
log10 CFU mL−1 and YM < 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1). All samples showed a visible color change after 12 days of storage at 4 °C. Compared to the HT and HPP-600 samples, the color of the sample treated by HPP-500 was found to be better maintained. Furthermore, the study evaluated the effects of HPP on the naturally occurring microorganisms, nutritional quality and shelf life of mango smoothie, which, considering the safety standard and quality changes, could be considered to be 12 days. Overall, HPP-500 was found to effectively inhibit the growth of microorganisms in mango smoothie and to maintain its quality.
log10 CFU mL−1). All samples showed a visible color change after 12 days of storage at 4 °C. Compared to the HT and HPP-600 samples, the color of the sample treated by HPP-500 was found to be better maintained. Furthermore, the study evaluated the effects of HPP on the naturally occurring microorganisms, nutritional quality and shelf life of mango smoothie, which, considering the safety standard and quality changes, could be considered to be 12 days. Overall, HPP-500 was found to effectively inhibit the growth of microorganisms in mango smoothie and to maintain its quality.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Science and Technology Support Program of Yibin (2018ZSF002) and the Innovation Project of the Chengdu Science and Technology Bureau (2018-YF05-00095-SN, 2019-YF05-00068-SN).References
- D. E. Pszczola, Food Technol., 2005, 59, 44–61 Search PubMed.
- D. F. Keenan, N. P. Brunton, T. R. Gormley, F. Butler, B. K. Tiwari and A. Patras, Innovative Food Sci. Emerging Technol., 2010, 11, 556 CrossRef.
- I. Oey, M. Lille, A. Van Loey and M. Hendrickx, Trends Food Sci. Technol., 2008, 19, 320–328 CrossRef CAS.
- J. F. Vachon, E. E. Kheadr, J. Giasson, P. Paquin and I. Fliss, J. Food Prot., 2002, 65, 345–352 CrossRef CAS PubMed.
- A. M. J. Diels, L. Callewaert, E. Y. Wuytack, B. Masschalck and C. W. Michiels, Int. J. Food Microbiol., 2005, 101, 281–291 CrossRef CAS PubMed.
- A. M. J. Diels, E. Y. Wuytack and C. W. Michiels, Int. J. Food Microbiol., 2003, 87, 55–62 CrossRef PubMed.
- A. Landl, M. Abadias, C. Sárraga, I. Viñas and P. A. Picouet, Innovative Food Sci. Emerging Technol., 2010, 11, 564 CrossRef.
- G. Knockaert, A. D. Roeck, L. Lemmens, S. V. Buggenhout, M. Hendrickx and A. V. Loey, Food Chem., 2011, 125, 903–912 CrossRef CAS.
- P. Butz, A. F. García, R. Lindauer, S. Dieterich, A. Bognar and B. Tauscher, J. Food Eng., 2003, 56, 233–236 CrossRef.
- A. Hurtado, P. Picouet, A. Jofré, M. D. Guàrdia, J. M. Ros and S. Bañón, Food Bioprocess Technol., 2015, 8, 2470–2482 CrossRef CAS.
- Y. Zhang, X. Liu, Y. Wang, F. Zhao, Z. Sun and X. Liao, Innovative Food Sci. Emerging Technol., 2016, 33, 135–144 CrossRef CAS.
- V. Andrés, M. J. Villanueva and M. D. Tenorio, Food Chem., 2016, 192, 328–335 CrossRef PubMed.
- H. Mújica-Paz, A. Valdez-Fragoso, C. T. Samson, J. Welti-Chanes and J. A. Torres, Food Bioprocess Technol., 2011, 4, 969–985 CrossRef.
- A. Hurtado, M. D. Guàrdia, P. Picouet, A. Jofré, S. Baón and J. M. Ros, J. Food Process. Preserv., 2019, 43(10), e14139 CAS.
- P. A. Picouet, A. Hurtado, A. Jofré, S. Bañon and M. D. Guàrdia, Food Bioprocess Technol., 2016, 9, 1–14 CrossRef.
- F. J. Barba, C. Cortés, M. J. Esteve and A. Frígola, Food Bioprocess Technol., 2012, 5, 2222–2232 CrossRef CAS.
- Y. Deng, Y. Zhong, W. Yu, J. Yue, Z. Liu, Y. Zheng and Y. Zhao, J. Cereal Sci., 2013, 58, 479–487 CrossRef CAS.
- R. Li, Y. Wang, S. Wang and X. Liao, Food Bioprocess Technol., 2015, 8, 333–342 CrossRef CAS.
- M. V. Fernandez, G. I. Denoya, M. V. Agüero, R. J. Jagus and S. R. Vaudagna, Innovative Food Sci. Emerging Technol., 2018, 47, 170–179 CrossRef CAS.
- L. Vervoort, I. V. D. Plancken, T. Grauwet, R. A. H. Timmermans, H. C. Mastwijk, A. M. Matser, M. E. Hendrickx and A. V. Loey, Innovative Food Sci. Emerging Technol., 2011, 12, 477 Search PubMed.
- A. C. Sánchez-Gimeno, A. Vercet and P. López-Buesa, Innovative Food Sci. Emerging Technol., 2006, 7, 274 CrossRef.
- M. Abid, S. Jabbar, T. Wu, M. M. Hashim and X. Zeng, Ultrason. Sonochem., 2013, 20, 1182–1187 CrossRef CAS PubMed.
- L. Zhou, Y. Wang, X. Hu, J. Wu and X. Liao, Innovative Food Sci. Emerging Technol., 2009, 10, 321–327 CrossRef CAS.
- L. Chen, X. Bi, X. Cao, L. Liu and Z. Che, J. Sci. Food Agric., 2018, 98, 5378–5385 CrossRef CAS PubMed.
- X. Bi, X. Wang, Y. Chen, L. Chen, Y. Xing and Z. Che, Ultrason. Sonochem., 2020, 60, 104763 CrossRef CAS PubMed.
- F. Liu, X. Zhang, L. Zhao, Y. Wang and X. Liao, Innovative Food Sci. Emerging Technol., 2016, 34, 51–58 CrossRef CAS.
- F. J. Barba, M. J. Esteve and A. Frígola, Compr. Rev. Food Sci. Food Saf., 2012, 11, 307–322 CrossRef CAS.
- S. G. Anema, E. K. Lowe and Y. Li, Int. Dairy J., 2004, 14, 548 CrossRef.
- M. K. Bull, K. Zerdin, E. Howe, D. Goicoechea, P. Paramanandhan, R. Stockman, J. Sellahewa, E. A. Szabo, R. L. Johnson and C. M. Stewart, Innovative Food Sci. Emerging Technol., 2004, 5, 149 CrossRef.
- A. Bienvenue, R. Jiménez-Flores and H. Singh, J. Agric. Food Chem., 2003, 51, 6488–6494 CrossRef CAS PubMed.
- M. R. Zobrist, T. Huppertz, T. Uniacke, P. F. Fox and A. L. Kelly, Int. Dairy J., 2003, 15, 655–662 CrossRef.
- S. Regnault, M. Thiebaud, E. Dumay and J. Cheftel, Int. Dairy J., 2004, 14, 55–68 CrossRef CAS.
- D. N. Sila, S. V. Buggenhout, T. Duvetter, I. Fraeye, A. D. Roeck, A. V. Loey and M. Hendrickx, Compr. Rev. Food Sci. Food Saf., 2009, 8, 86–104 CrossRef CAS.
- L. Wicker, J. L. Ackerley and J. L. Hunter, Food Hydrocolloids, 2003, 17, 809–814 CrossRef CAS.
- H. Y. Kim, S. H. Kim, M. J. Choi, S. G. Min and H. S. Kwak, J. Dairy Sci., 2008, 91, 4182 CrossRef PubMed.
- A. Vega-Gálvez, J. López, M. J. Torres-Ossandón, M. J. Galotto, L. Puente-Díaz, I. Quispe-Fuentes and K. D. Scala, LWT--Food Sci. Technol., 2014, 58, 519–526 CrossRef.
- Y. Wang, F. Liu, X. Cao, F. Chen, X. Hu and X. Liao, Innovative Food Sci. Emerging Technol., 2012, 16, 326–334 CrossRef CAS.
- N. Kaushik, B. P. Kaur, P. S. Rao and H. Mishra, Innovative Food Sci. Emerging Technol., 2014, 22, 40–50 CrossRef CAS.
- D. Chen, H. Xi, X. Guo, Z. Qin, X. Pang, X. Hu, X. Liao and J. Wu, Innovative Food Sci. Emerging Technol., 2013, 19, 85–94 CrossRef CAS.
- D. Chen, X. Pang, J. Zhao, L. Gao, X. Liao, J. Wu and Q. Li, Innovative Food Sci. Emerging Technol., 2015, 32, 16–28 CrossRef.
- F. J. Barba, M. J. Esteve and A. Frigola, Food Res. Int., 2013, 50, 545–549 CrossRef CAS.
- J. Hsu, D. Heatherbell and B. Yorgey, J. Food Sci., 1989, 54, 660–662 CrossRef CAS.
- G. C. Yen and H. A. Lin, J. Food Sci., 1998, 63, 684–687 CrossRef CAS.
- D. A. Jacobo-Velázquez and C. Hernández-Brenes, Innovative Food Sci. Emerging Technol., 2012, 16, 121–128 CrossRef.
- C. H. Lin and B. H. Chen, Eur. Food Res. Technol., 2005, 221, 274–280 CrossRef CAS.
- F. Liu, Y. Wang, R. Li, X. Bi and X. Liao, Innovative Food Sci. Emerging Technol., 2014, 21, 35–43 CrossRef CAS.
- L. Plaza, C. Sánchez-Moreno, B. a. D. Ancos, P. Elez-Martínez, O. Martín-Belloso and M. P. Cano, LWT--Food Sci. Technol., 2011, 44, 839 CrossRef.
- B. A. D. Ancos, S. Sgroppo, L. Plaza and M. P. Cano, J. Sci. Food Agric., 2002, 82, 790–796 CrossRef.
- A. Zulueta, F. J. Barba, M. J. Esteve and A. Frígola, Food Bioprocess Technol., 2013, 6, 2018–2030 CrossRef CAS.
- J. G. Provesi, C. O. Dias and E. R. Amante, Food Chem., 2011, 128, 195–202 CrossRef CAS PubMed.
- A. L. Vásquez-Caicedo, S. Schilling, R. Carle and S. Neidhart, Food Chem., 2007, 102, 1172–1186 CrossRef.
Footnote |
| † Were equally the first authors. |
| This journal is © The Royal Society of Chemistry 2020 |