DOI:
10.1039/D0RA04707D
(Paper)
RSC Adv., 2020,
10, 26433-26442
Theoretical investigation of the reaction mechanisms and kinetics of CFCl2CH2O2 and ClO in the atmosphere†
Received
28th May 2020
, Accepted 19th June 2020
First published on 14th July 2020
Abstract
The reaction between CFCl2CH2O2 radicals and ClO was studied using the B3LYP and CCSD(T) methods associated with the 6-311++G(d,p) and cc-pVTZ basis sets, and subsequently RRKM-TST theory was used to predict the thermal rate constants and product distributions. On the singlet PES, the dominant reaction is the addition of the ClO oxygen atom to the terminal-O of CFCl2CH2O2 to generate adduct IM1 (CFCl2CH2OOOCl), and then dissociation to final products P1 (CFCl2CHO + HO2 + Cl) occurs. RRKM theory is employed to calculate the overall and individual rate constants over a wide range of temperatures and pressures. It is predicted that the collision-stabilized IM1 (CFCl2CH2OOOCl) dominates the reaction at 200–500 K (accounting for about 60–100%) and the dominant products are P1 (CFCl2CHO + HO2 + Cl). The yields of the other products are very low and insignificant for the title reaction. The total rate constants exhibit typical “falloff” behavior. The pathways on the triplet PES are less competitive than that on the singlet PES. The calculated overall rate constants are in good agreement with the experimental data. The atmospheric lifetime of CFCl2CH2O2 in ClO is around 2.04 h. TD-DFT calculations imply that IM1 (CFCl2CH2OOOCl), IM2 (CFCl2CH2OOClO) and IM3 (CFCl2CH2OClO2) will photolyze under sunlight.
1. Introduction
The detrimental influence of CFCs (chlorofluorocarbons) on the ozonosphere and stratosphere resulted in international agreement that the manufacturing of these chemical compounds would be discontinued by January 1996.1,2 HCFCs (hydrochlorofluorocarbons) are the suggested alternatives to CFCs since they are relatively environmentally benign. When HCFCs are released into the troposphere, they could react with OH radicals to generate haloalkyl radicals, which subsequently react with O2 to form peroxy radicals in low altitude atmosphere.1,2 With HCFC-14lb (CFCl2CH3) as a substitute for CFC, the reactions are as follows:
| CFCl2CH3 + OH → CFCl2CH2 + H2O |
| CFCl2CH2 + O2 + M → CFCl2CH2O2 + M |
To assess the atmospheric effects of CFCl2CH3, it is necessary to study the peroxy radical (CFCl2CH2O2) generated by the atmospheric reaction of CFCl2CH3. The possible degradation mechanism of the peroxy radicals includes self-reactions3–5 and reactions with free radicals, i.e. Cl, ClO, NO, HO2 and CH3O2.6–11 It is well-known that the ClO radical is the most abundant reactive halogen species in the atmosphere. It is of great atmospheric importance due to its ability to destroy ozone. Based on the catalytic cycle, ClO plays an important role in the generation of the Antarctic “ozone hole”, especially in the production of the South ozone hole.12 The generation and photolysis for the dipolymer of ClO (ClOOCl) are critical for the chemical reaction. According to the statistics, this cycle contributed to about 70% of the damage to the Antarctic ozone.13 However, previous research indicated that this gas phase chemistry alone does not result in ozone depletion due to the ozone depletion by chlorine-catalyzed reactions.14 Therefore, the reaction of ClO with CFCl2CH2O2 is a quite significant chemical reaction, which was only studied by one earlier experiment. In 1977, Wu and Carr7 studied the kinetics of the reaction between ClO and CFCl2CH2O2 by employing time-resolved mass spectrometry and a UV flash photolysis technique and measured the rate constant at 253–321 K and 4–60 torr. The obtained rate constant was (6.0 ± 0.7) × 10−12 cm3 per molecule per s at 298 K. Moreover, although studies have involved the CFCl2CH2O2 + ClO reaction,15 there have been no theoretical investigations into the ClO + CFCl2CH2O2 reaction, which may indicate the degradation of ClO with CFCl2CH2O2, affecting the stratospheric ozone consumption, which may undergo the same pathways as that of the CFCl2CH2O2 + Cl reaction. Therefore, the aim of the present theoretical investigation is to provide the mechanism and kinetics of the reaction between ClO and CFCl2CH2O2 through a description of the potential energy surfaces by means of density functional theory (DFT) theory and RRKM theory,16 which has been employed to address the complex reactions successfully,17–22 and shed light on future experimental research.
2. Computational methods
All the geometries were fully optimized using the B3LYP23,24/6-311++G(d,p) method. All stationary points were identified for local minima and transition states by vibrational analysis, and connections of the transition states between designated reactants and products were confirmed by intrinsic reaction coordinate (IRC) calculations.25,26 The energies for the singlet and triplet potential energy surfaces (PES) were refined by the single point calculations using the CCSD(T)27//cc-pVTZ method. Initially obtained PES information, involving optimum geometries, frequencies, moment of inertia and energies of the dominant reaction pathways, were ready for dynamic calculations. RRKM-TST theory was employed to gain the rate constants over a wide temperature and pressure region (200–3000 K and 10−14 to 1014 torr). The Gaussian 09 program28 was used to perform the density functional calculations, and Fortran code was used for the RRKM calculations based on the density functional data.
3. Results and discussion
The optimized geometries of all the stationary points involved on the triplet and singlet PESs in the title reaction at the B3LYP/6-311++G(d,p) level are depicted in Fig. 1 and 2, along associated with the available experimental values.29 The frequencies of HOCl, OClO, HO2, ClO, O3 and O2(3Σ) are in agreement with the experimental data (Table S1†). The reaction processes on the singlet and triplet PESs were described clearly, which are represented in Fig. 3. Table 1 summarizes the relative energies (ΔE), relative enthalpies (ΔH), Gibbs free energies (ΔG), and the ZPE for all the stationary points. Table 2 lists the excitation energy (TV), wavelength (λ) and oscillator strength (f) of the obtained intermediates. The harmonic vibrational frequencies of all the intermediates and transition states found on the PESs are listed in Table S1 as ESI.†
 |
| | Fig. 1 Optimized geometries for all the intermediates and transition states at the B3LYP/6-311++G(d,p) level for the reaction between CFCl2CH2O2 and ClO. Bond distances are given in Å. | |
 |
| | Fig. 2 Optimized geometries (length in Å and angle in degree) for all the reactants and products at the B3LYP/6-311++G(d,p) level for the reaction between CFCl2CH2O2 and ClO. Angles are given in °, and bond distances are given in Å. The values in italics are experimental data from ref. 29. | |
 |
| | Fig. 3 Potential energy surface obtained at the CCSD(T)//B3LYP level for the CFCl2CH2O2 + ClO reaction. | |
Table 1 Zero point energies (ZPE), relative energies (ΔE), relative enthalpies (ΔH) and Gibbs free energy (ΔG) for the species involved in the CFCl2CH2O2 + ClO reaction (energies in kcal mol−1)
| Species |
ZPEa |
ΔEb |
ΔHb |
ΔGb |
| At the B3LYP/6-311++G(d,p) level. The relative energies are calculated at the CCSD(T)/cc-pVTZ + ZPE level. |
| R: (CFCl2CH2O2+ClO) |
29.16 |
0.00 |
0.00 |
0.00 |
| IM1: (CFCl2CH2OOOCl) |
30.87 |
−16.28 |
−16.80 |
−5.03 |
| IM2: (CFCl2CH2OOClO) |
30.57 |
2.05 |
1.78 |
12.98 |
| IM3: (CFCl2CH2OClO2) |
31.21 |
−9.21 |
−9.86 |
2.58 |
| TS1 |
30.47 |
10.55 |
9.87 |
21.75 |
| TS2 |
29.48 |
18.92 |
18.28 |
30.16 |
| TS3 |
27.56 |
−0.86 |
−1.52 |
10.86 |
| TS4 |
28.18 |
11.83 |
11.32 |
23.16 |
| TS5 |
27.62 |
10.14 |
9.53 |
21.59 |
| TS6 |
28.32 |
57.61 |
57.44 |
68.94 |
| TS7 |
28.85 |
60.36 |
60.08 |
71.35 |
| TS8 |
26.91 |
62.12 |
62.34 |
72.17 |
| TS9 |
26.64 |
71.16 |
71.22 |
82.02 |
| TS10 |
29.08 |
47.83 |
47.45 |
59.43 |
| TS11 |
28.36 |
1.31 |
0.40 |
13.64 |
| TS12 |
26.28 |
65.73 |
66.12 |
75.21 |
| TS13 |
25.94 |
83.48 |
83.50 |
94.69 |
| TS14 |
28.57 |
65.35 |
65.18 |
76.82 |
| TS15 |
28.09 |
70.47 |
70.65 |
80.62 |
| T-h-TS1 |
25.54 |
15.73 |
15.68 |
25.48 |
| T-TS1 |
27.95 |
30.48 |
30.76 |
39.18 |
| T-TS2 |
28.36 |
43.44 |
43.33 |
53.43 |
| T-TS3 |
28.47 |
77.65 |
77.58 |
87.59 |
| P1: (CFCl2CHO + HO2 + Cl) |
27.38 |
−30.48 |
−29.60 |
−38.33 |
| P2: (or P2T): (CFCl2CHO2 + HOCl) |
28.62 |
−16.54 |
−16.42 |
−16.14 |
| P3: (CFCl2CHO + HOOCl) |
29.14 |
−57.22 |
−57.30 |
−57.48 |
| P4: (CFCl2CH2OCl + O2(1Δg)) |
29.55 |
−15.86 |
−15.76 |
−13.84 |
| P5: (CFCl2CH2Cl + O3) |
29.56 |
−12.43 |
−12.79 |
−11.94 |
| P6: (CHFClCHO + Cl2O2) |
29.64 |
−5.61 |
−5.84 |
−6.37 |
| P7: (CHCl2CHO + FClO2) |
29.31 |
2.55 |
2.26 |
1.98 |
| P8: (CFCl2CHO + HOClO) |
28.53 |
−43.06 |
−43.04 |
−43.41 |
| P9: (CHFClCHO + ClOClO) |
28.48 |
−2.86 |
−2.75 |
−4.16 |
| P10: (CHCl2CHO + FOClO) |
27.98 |
32.26 |
32.36 |
31.13 |
| P11: (CFCl2CH2ClO + O2(1Δg)) |
28.33 |
32.58 |
32.94 |
34.01 |
| P12: (CFCl2CH2OCl + O2(3Σ)) |
29.57 |
−46.01 |
−45.92 |
−44.64 |
| P13: (CFCl2CH2O + OClO) |
27.77 |
9.88 |
9.63 |
9.19 |
| P14: (CFCl2CH2OOCl + O(3P)) |
29.04 |
26.55 |
26.76 |
29.80 |
Table 2 The excitation energy TV (in eV), oscillator strength f (in atomic units) and wavelength λ (in nm) of the first five excited states of IM1 (CFCl2CH2OOOCl), IM2 (CFCl2CH2OOClO) and IM3 (CFCl2CH2OClO2) at the TD-B3LYP level of theory
| Excited states |
IM1 (CFCl2CH2OOOCl) |
IM2 (CFCl2CH2OOClO) |
IM3 (CFCl2CH2OClO2) |
| TV |
f |
λ |
TV |
f |
λ |
TV |
f |
λ |
| 1 |
3.01 |
0.0001 |
411.6 |
1.98 |
0.0000 |
625.59 |
3.48 |
0.0016 |
356.4 |
| 2 |
4.07 |
0.0001 |
304.7 |
3.47 |
0.0015 |
357.27 |
4.31 |
0.0641 |
287.3 |
| 3 |
5.02 |
0.0371 |
247.1 |
4.42 |
0.2574 |
280.47 |
4.67 |
0.0015 |
265.4 |
| 4 |
5.12 |
0.0201 |
242.1 |
4.69 |
0.0004 |
263.91 |
5.61 |
0.0663 |
221.0 |
| 5 |
5.72 |
0.1630 |
216.8 |
5.57 |
0.0013 |
222.45 |
5.71 |
0.0611 |
217.0 |
3.1. Generation of adducts on the singlet PES
The generation of the initial adduct involves the addition process of ClO to CFCl2CH2O2, which could be described as the approach of the O atom of ClO to the terminal-O atom of CFCl2CH2O2 along the O–O reaction coordinate, resulting in the IM1 (CFCl2CH2OOOCl) intermediate. Since ClO and CFCl2CH2O2 are both radicals, the first association step is expected to be a barrierless process. Moreover, the relaxed scan along the reactive O–O bond confirmed that the first step is a barrierless process. The forming O–O bond is 1.320 Å, and the O–O bond energy is calculated to be 16.28 kcal mol−1. The intermediate IM1 (CFCl2CH2OOOCl) can overcome the TS1 barrier, resulting in IM2 (CFCl2CH2OOClO), where the terminal Cl atom is shifted to the middle-O atom of the –OOO– skeleton, and the O–O bond is broken simultaneously. The breaking O–O bond in the triangle structure TS1 is elongated to 2.307 Å and the forming Cl–O bond is 2.253 Å. The energy barrier for the rearrangement IM1 (CFCl2CH2OOOCl) → TS1 → IM2 (CFCl2CH2OOClO) is 26.83 kcal mol−1. The conformer IM2 (CFCl2CH2OOClO) can easily isomerize to a minimum energy IM3 (CFCl2CH2OClO2), resulting from the –ClO group shifting to the middle-O atom of the –COO– skeleton, while the O–O bond is broken. The breaking O–O bond in the triangle structure TS2 is elongated to 2.168 Å and the forming Cl–O bond is 2.414 Å. The energy barrier for IM2 (CFCl2CH2OOClO) → TS2 → IM3 (CFCl2CH2OClO2) is 16.87 kcal mol−1. It should be noted that several conformers for IM1, IM2 and IM3 exist. We used the lowest-energy conformers in the following discussion. To summarize, three adducts IM1 (CFCl2CH2OOOCl), IM2 (CFCl2CH2OOClO) and IM3 (CFCl2CH2OClO2) are generated on the singlet PES with the energy of −16.28, 2.05 and −9.21 kcal mol−1, which can further generate many products by isomerization or dissociation before being quenched by collisions, as will be discussed below.
3.2. Decomposition pathways from IM1 (CFCl2CH2OOOCl), IM2 (CFCl2CH2OOClO) and IM3 (CFCl2CH2OClO2)
Starting from IM1 (CFCl2CH2OOOCl), five dissociation pathways were identified. Three intramolecular H-migration channels can be competitive as they possess low barriers and generate stable products. 1,4-H migration from the –CH2 group to the middle-O atom of the –OOCl skeleton, respectively associated with breaking the O–O and O–Cl bonds or O–O bond through TS3 or TS4, gives rise to P1 (CFCl2CHO + HO2 + Cl) or P2 (CFCl2CHO2 + HOCl). In TS3, the breaking C–H bond (1.306 Å), O–O bond (2.121 Å) and the O–Cl (2.086 Å) bond are elongated by 0.217, 0.633 and 0.244 Å, compared to the intermediate IM1 (CFCl2CH2OOOCl) (1.089, 1.488 and 1.842 Å, respectively), and the forming O–H bond is 1.349 Å. In TS4, the breaking C–H bond (1.177 Å) and the O–O bond (2.580 Å) are elongated by 0.088 and 1.260 Å, and the forming O–H bond is 1.520 Å. Alternatively, 1,3-H migration from the –CH2 group to the middle-O atom of the –OOO– skeleton in IM1, along with breakage of the O–O bond via TS5, results in P3 (CFCl2CHO + HOOCl). The above three decomposition channels need to overcome 15.42, 28.11 and 26.42 kcal mol−1 energy barriers, respectively. The overall exothermicities of generating the P1 (CFCl2CHO + HO2 + Cl), P2 (CFCl2CHO2 + HOCl) and P3 (CFCl2CHO + HOOCl) channels are estimated to be 29.60, 16.42 and 57.30 kcal mol−1, respectively, implying that the most significant reaction pathway is the generation of P1 (CFCl2CHO + HO2 + Cl), and the pathways to generate P2 (CFCl2CHO2 + HCl) and P3 (CFCl2CHO + HOOCl) compete with each other.
When we considered the other direct dissociation channels from IM1 (CFCl2CH2OOOCl), one four-center and five-center transition state (TS6 and TS7) were identified. TS6 connects the IM1 (CFCl2CH2OOOCl) and P4 (CFCl2CH2OCl + O2(1Δg)) end products, whereas P5 (CFCl2CH2Cl + O3) is generated from IM1 (CFCl2CH2OOOCl) passing through TS7. These two channels can be attributed to the –ClO group or terminal-Cl atom shifting to the carbon atom, and the O2(1Δg) or O3 leaving simultaneously, respectively. TS6 and TS7, with imaginary frequencies of 386i and 439i cm−1, respectively, are first-order saddle points, which was confirmed by vibrational frequency analysis. The energy barriers of IM1 (CFCl2CH2OOOCl) → TS6 → P4 (CFCl2CH2OCl + O2(1Δg)) and IM1 (CFCl2CH2OOOCl) → TS7 → P5 (CFCl2CH2Cl + O3) are 73.89 and 76.64 kcal mol−1. Thus, neither the O2(1Δg) or O3 elimination channels from IM1 are favorable judging from the high barrier height.
Two elimination mechanisms were located from IM2 (CFCl2CH2OOClO) through a dicyclo-transition state TS8 or TS9 to generate P6 (CHFClCHO + Cl2O2) or P7 (CHCl2CHO + FClO2); this occurred through one of the H atoms in the –CH2 group migrating to another carbon atom, and one of the Cl atoms or F atom in the -CFCl2 group shifting to the Cl atom in the –OOClO skeleton, accompanied by the O–O bond breaking. The breaking C–H, C–Cl and O–O bonds are elongated to 1.207, 2.925 and 2.529 Å, and the forming C–H and Cl–Cl bond are 1.672 and 2.502 Å for TS8. The breaking C–H, C–F and O–O bonds are elongated to 1.202, 2.326 and 2.554 Å, and the forming C–H and F–Cl bonds are 1.676 and 1.976 Å for TS9. Vibrational frequency analysis of TS8 and TS9 reveals one imaginary frequency of 661i and 699i cm−1, respectively. The activation barriers for IM2 (CFCl2CH2OOClO) → TS8 → P6 (CHFClCHO + Cl2O2) and IM2 (CFCl2CH2OOClO) → TS9 → P7 (CHCl2CHO + FClO2) are 60.07 and 69.11 kcal mol−1. The high barriers restrain these two dissociation pathways from proceeding.
We also considered the dissociation pathway from IM2 (CFCl2CH2OOClO) resulting in the P4 (CFCl2CH2OCl + O2(1Δg)) products, and transition state TS10 was located for this channel. TS10 is a COOClO five-center transition state, that involves the C–O and Cl–O bonds breaking and forming another C–O bond, accompanied with the dissociation of O2(1Δg). The energy barrier is 45.78 kcal mol−1, suggesting that the conversion of IM2 (CFCl2CH2OOClO) → TS10 → P4 (CFCl2CH2OCl + O2(1Δg)) was inefficient.
IM3 (CFCl2CH2OClO2) involves a 1,4-H shift from the carbon atom of the –CH2 group to the oxygen atom, along with breakage of the O–Cl bond leading to P8 (CFCl2CHO + HOClO) via TS11. TS11 presents a non-planar and loose HCOClO five-membered ring structure with long C–H, O–Cl and O–H distances, that is, r(C–H) = 1.217 Å, r(O–Cl) = 2.238 Å, and r(O–H) = 1.498 Å. The IM3 (CFCl2CH2OClO2) → TS11 → P8 (CFCl2CHO + HOClO) transformation barrier is 10.52 kcal mol−1 and TS11 is only 1.31 kcal mol−1 higher than the reactants. Therefore, this dissociation pathway may be important for the reaction.
IM3 (CFCl2CH2OClO2) could also undergo a respective 1,5-Cl shift or 1,5-F shift from the carbon atom to the oxygen atom, and a 1,2-H shift from the carbon atom of the –CH2 group to another carbon atom as well, which is associated with breaking the O–Cl bond (i.e., TS12 and TS13 together generate product P9 (CHFClCHO + (ClO)2) and P10 (CHCl2CHO + FOClO)). The loose ClCCOClO and FCCOClO six-membered rings are nonplanar and found in TS12 and TS13, respectively. In TS12, the breaking C–H, C–Cl and O–Cl bonds and the forming C–H and Cl–O bonds are 1.191, 3.005, 2.720, 1.720 and 2.064 Å, respectively. In TS13, the C–H, C–F and O–Cl bonds that will be broken and the C–H and F–O bond that will be formed are 1.451, 1.837, 2.534, 1.330 and 2.312 Å, respectively. The decomposition barriers of IM3 (CFCl2CH2OClO2) → TS12 → P9 (CHFClCHO + (ClO)2) and IM3 (CFCl2CH2OClO2) → TS13 → P10 (CHCl2CHO + FOClO) are 74.94 and 92.69 kcal mol−1, which are quite high, making the generation of P9 (CHFClCHO + (ClO)2) and P10 (CHCl2CHO + FOClO) highly impossible.
3.3. SN2 displacement pathways on the singlet PES
Another type of mechanism (SN2 displacement reaction) could take place for the CFCl2CH2O2 + ClO reaction. Two different transition states were identified. The two SN2 displacement channels can be attributed to the O atom or the Cl atom in ClO respectively attacking the C atom of the –CH2 group in CH3CFClO2, along with the O2(1Δg) group leaving via TS14 or TS15, leading to P4 (CFCl2CH2OCl + O2(1Δg)) or P11 (CFCl2CH2ClO + O2(1Δg)). The forming and breaking C–O bonds in TS14 and the forming C–Cl bond and breaking C–O bond in TS15 are respectively 2.046, 1.794 Å, and 2.414, 2.004 Å. The processes of R → TS14 → P4 (CFCl2CH2OCl + O2(1Δg)) and R → TS15 → P11 (CFCl2CH2ClO + O2(1Δg)) require quite high energy barriers of 65.35 and 70.47 kcal mol−1. Thus, these two SN2 displacement channels are prohibited kinetically.
3.4. The pathways on the triplet PES
On the triplet surface, both H-abstraction and SN2 displacement reaction mechanisms were identified. Surmounting T-h-TS1, T-TS1, T-TS2 and T-TS3, P2T (CFCl2CHO2 + HCl), P12 (CFCl2CH2OCl + O2(3Σ)), P13 (CFCl2CH2O + OClO) and P14 (CFCl2CH2OOCl + O(3P)) are produced, and the relative energy is −16.54, −46.01, 9.88 and 26.55 kcal mol−1, respectively. The barriers of these four channels are 15.73, 30.48, 43.44 and 77.65 kcal mol−1, respectively. Compared with the addition/elimination pathways on the singlet PES, the pathways on the triplet PES are energetically less convenient because of the higher barrier heights.
3.5. RRKM-TST calculations of the rate constants
Because the energy barriers are much higher than the obtained dominant pathways (Scheme 1), we ignore the pathways to produce P4 (CFCl2CH2OCl + O2(1Δ(g))), P5 (CFCl2CH2Cl + O3), P6 (CHFClCHO + Cl2O2), P7 (CHCl2CHO + FClO2), P9 (CHFClCHO + (ClO)2), P10 (CHCl2CHO + FOClO), P11 (CFCl2CH2ClO + O2 (1Δg)), P12 (CFCl2CH2OCl + O2(3Σ)), P13 (CFCl2CH2O + OClO) and P14 (CFCl2CH2OOCl + O(3P)) in the RRKM calculations. The predicted rate constants of IM1 (CFCl2CH2OOOCl), IM2 (CFCl2CH2OOClO), IM3 (CFCl2CH2OClO2), P1 (CFCl2CHO + HO2 + Cl), P2 (CFCl2CHO2 + HOCl), P3 (CFCl2CHO + HOOCl), and P8 (CFCl2CHO + HOClO) are denoted as kIM1, kIM2, kIM3, kP1, kP2, kP3 and kP8, and the total rate constant is denoted as ktot = kIM1 + kIM2 + kIM3 + kP1 + kP2 + kP3 + kP8 at 200–3000 K, 12 torr N2, which are presented in Fig. 4. The estimated values of ktot are consistent with the experimental data (e.g. ktot = 6.39 × 10−12 cm3 per molecule per s vs. 6.70 × 10−12 cm3 per molecule per s at 298 K). ktot appears to decrease first and then increase as the temperature increases. The branching ratios are presented Fig. S1.† The low-temperature (200–500 K) association is dominated by the production of IM1 (CFCl2CH2OOOCl), and the production of P1 (CFCl2CHO + HO2 + Cl) quickly becomes dominant with the rise of temperature (500–3000 K). The P2 (CFCl2CHO2 + HOCl) product channel contributes to the reaction at T > 1200 K. The P3 (CFCl2CHO + HOOCl) product pathway generating from IM1 (CFCl2CH2OOOCl), the P8 (CFCl2CHO + HOClO) product pathway generating from IM3 (CFCl2CH2OClO2) and the pathways of the IM2 (CFCl2CH2OOClO) and IM3 (CFCl2CH2OClO2) collisional stabilization rarely occur at any temperature.
 |
| | Scheme 1 The primary pathways of the CFCl2CH2O2 + ClO reaction. | |
 |
| | Fig. 4 Plots of the rate coefficients for total and primary reaction channels versus 1000/T (K−1) at 200–1000 K associated with the available experimental value. | |
The rate constants for the generation of branched products of the ClO + CFCl2CH2O2 reaction at different pressures are shown in Fig. 5. Due to the competition between decomposition and stabilization, kIM1, kIM2 and kIM3 are for the generation of IM1, IM2 and IM3 by collisional deactivation, and kP1, kP2, kP3 and kP8 are for the generation of P1, P2, P3 and P8, which demonstrate strong dependence on pressure. The pattern of the pressure dependence of IM1 (10−10 to 1010 atm), IM2 (10−10 to 102 atm) and IM3 (10−10 to 102 atm) is contrary to the dissociation process and IM2 (102 to 1010 atm) and IM3 (102 to 1010 atm). kIM1, kIM2 and kIM3 are very small and not competitive at lower pressure; when the pressure exceeds 10−4 atm, kIM1 is near the high pressure limit at T > 1000 K. In addition, kIM1 displays strong negative dependence on temperature at 200–3000 K, owing to the reduction of the collision inactivation rate. The rate constants for the dissociation procedure kP1 display positive dependence on temperature and negative dependence on pressure. At low temperatures and high pressures, kP1, kP2, kP3 and kP8 become negligible.
 |
| | Fig. 5 Predicted rate coefficients for the total reaction and each individual product pathway of the reaction between CFCl2CH2O2 and ClO at 200–3000 K and 10−10 to 1010 atm. | |
The branching ratios of the individual product pathways of the CFCl2CH2O2 + ClO reaction at the low pressure limit (10−10 atm), atmospheric pressure (1 atm) and high pressure limit (1010 atm) are presented in Fig. 6. Seven primary product pathways dominate noticeably – the competitive inactivation and dissociation that generate IM1, IM2, IM3, P1, P2, P3 and P8, respectively. At the low and high pressure limits, the production of P1 (CFCl2CHO + HO2 + Cl) and IM1 (CFCl2CH2OOOCl) predominate the reaction at 200–3000 K, respectively. At atmospheric pressure and high temperatures, the generation of P1 (CFCl2CHO + HO2 + Cl) dominates the reaction; conversely, at moderate and low temperatures, the collision inactivation of IM1 (CFCl2CH2OOOCl) takes over the reaction.
 |
| | Fig. 6 Predicted branching ratios for the CFCl2CH2O2 + ClO reaction at the low pressure limit, atmospheric pressure and high pressure limit. | |
The three-parameter Arrhenius equations for the rate constants of generating IM1 (CFCl2CH2OOOCl) (kIM1) and P1 (CFCl2CHO + HO2 + Cl) (kP1) at the low pressure limit, atmospheric pressure and high pressure limit of N2 can be represented by:
kIM10(CFCl2CH2OOOCl)/(cm3 per molecule per s) = 4.23 × 10−23T−4.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(7517.87/T) (200 ≤ T ≤ 3000 K) exp(7517.87/T) (200 ≤ T ≤ 3000 K) |
k0P1(CFCl2CHO + HO2 + Cl)/(cm3 per molecule per s) = 1.01 × 10−16T0.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(1001.83/T) (200 ≤ T ≤ 500 K) = 9.45 × 10−15T0.69 exp(1001.83/T) (200 ≤ T ≤ 500 K) = 9.45 × 10−15T0.69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−1327.18/T) (500 < T ≤ 3000 K) exp(−1327.18/T) (500 < T ≤ 3000 K) |
kIM1(CFCl2CH2OOOCl)/(cm3 per molecule per s) = 1.84 × 10−7T−1.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(62.58/T) (200 ≤ T ≤ 3000 K) exp(62.58/T) (200 ≤ T ≤ 3000 K) |
kP1(CFCl2CHO + HO2 + Cl)/(cm3 per molecule per s) = 2.38 × 10−15T0.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−990.60/T) (200 ≤ T ≤ 3000 K) exp(−990.60/T) (200 ≤ T ≤ 3000 K) |
k∞IM1(CFCl2CH2OOOCl)/(cm3 per molecule per s) = 5.43 × 10−18T2.36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(47.22/T) (200 ≤ T ≤ 1800 K) = 1.08 × 10−9T−0.33 exp(47.22/T) (200 ≤ T ≤ 1800 K) = 1.08 × 10−9T−0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(2682.26/T) (1800 < T ≤ 3000 K) exp(2682.26/T) (1800 < T ≤ 3000 K) |
k∞P1(CFCl2CHO + HO2 + Cl)/(cm3 per molecule per s) = 1.14 × 10−10T−1.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(8395.50/T) (200 ≤ T ≤ 3000 K) exp(8395.50/T) (200 ≤ T ≤ 3000 K) |
We also examined the pressure effect of the total rate constants at selected low (298 K), moderate (500 K and 1000 K), and high (3000 K) temperatures (Fig. 7), and established the branching ratios of the individual product pathways of the CFCl2CH2O2 + ClO reaction (Fig. 8). Fig. 7 displays the typical “S” behavior, and the “S” region moves to the high pressure range as the temperature increases. The “S” regions are 10−4 to 104, 10−1 to 106, 102–109 and 105–1010 torr at selected temperatures, respectively. At each respective temperature, the branching ratio for P1 (CFCl2CHO + HO2 + Cl) (kP1/ktot) possesses the largest values in the pressure ranges of 10−14 to 10−2, 10−14 to 101, 10−14 to 104, and10−14 to 107 torr, respectively. But at higher pressures, the branching ratio for the energized IM1 (CFCl2CH2OOOCl) intermediate, which is stabilized by collisions, becomes dominant.
 |
| | Fig. 7 Pressure dependence of the total rate coefficients for the CFCl2CH2O2 + ClO reaction at 298, 1000 and 3000 K. | |
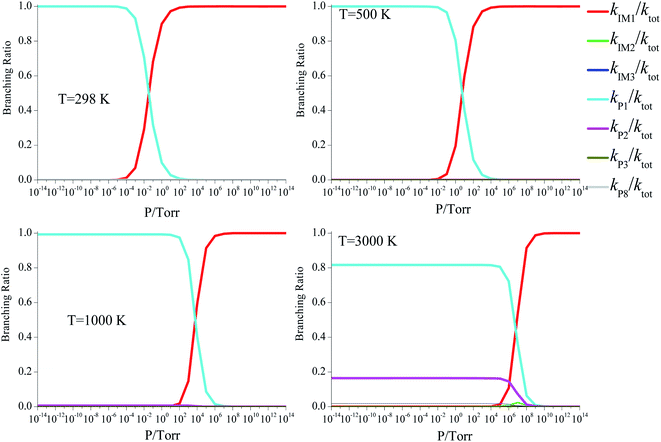 |
| | Fig. 8 Branching ratios for the CFCl2CH2O2 + ClO reaction at 10−14 to 1014 torr at 298, 1000 and 3000 K. | |
3.6. Atmospheric lifetimes of CFCl2CH2O2
The atmospheric lifetime of CFCl2CH2O2 can be deduced by the following formula: The calculated average daytime atmospheric concentration of chlorine monoxide radical (ClO) is 1 × 107 molecules per cm3 (Tang, 2004),30 and kClO = 1.36 × 10−11 cm3 per molecule per s was estimated. The atmospheric lifetime of CFCl2CH2O2 is approximately 2.04 h, which suggests that the ClO-initiated reaction with CFCl2CH2O2 plays an important role in some special areas and the marine boundary layer.
The calculated average daytime atmospheric concentration of chlorine monoxide radical (ClO) is 1 × 107 molecules per cm3 (Tang, 2004),30 and kClO = 1.36 × 10−11 cm3 per molecule per s was estimated. The atmospheric lifetime of CFCl2CH2O2 is approximately 2.04 h, which suggests that the ClO-initiated reaction with CFCl2CH2O2 plays an important role in some special areas and the marine boundary layer.
3.7. Vertical excitation energy of IM1 (CFCl2CH2OOOCl), IM2 (CFCl2CH2OOClO) and IM3 (CFCl2CH2OClO2)
The photo-oxidation of compounds containing chlorine is significant for Cl atmospheric chemistry and might influence the stratosphere and troposphere. To gain new insight into the photolytic stability of the Cl-containing compounds, the vertical excitation energy (TV) of the first five excited states for IM1 (CFCl2CH2OOOCl), IM2 (CFCl2CH2OOClO) and IM3 (CFCl2CH2OClO2) were calculated by TDDFT employing B3LYP/6-311++G(d,p), and the results, including wavelength (λ), excitation energy (TV) and oscillator strength (f), are listed in Table 2. Compounds will typically photolyze if the TV value is smaller than 4.13 eV or the wavelength is longer than 300 nm. It is seen from Table 2 that the TV value of the first two excited states of IM1 (CFCl2CH2OOOCl) are 3.01 eV (411.6 nm) and 4.07 eV (304.7 nm) and the oscillator strengths are 0.0001 and 0.0001; the second excited state of IM2 (CFCl2CH2OOClO) is 3.47 eV (357.27 nm) and the oscillator strength is 0.0015; the TV value and oscillator strength of the first excited state of IM3 (CFCl2CH2OClO2) are 3.48 eV (356.4 nm) and 0.0016, respectively, indicating that the photolysis of IM1 (CFCl2CH2OOOCl), IM2 (CFCl2CH2OOClO) and IM3 (CFCl2CH2OClO2) could occur under sunlight.
4. Conclusions
The ClO-initiated oxidation reaction of CFCl2CH2O2 was researched by means of density functional theory (DFT) and RRKM theory to understand the mechanism and product distribution. On the singlet PES, the addition of the O atom of ClO to the terminal-O of CFCl2CH2O2 to generate IM1 (CFCl2CH2OOOCl) is more favorable at 200–500 K, followed by intramolecular 1,4-H shifts along with breakage of the O–Cl bond to generate P1 (CFCl2CHO + HO2 + Cl), which dominates the reaction at high temperatures. The kinetics simulations revealed that the total rate constants exhibit typical “falloff” behavior. The pathways on the triplet PES are less preferred over the pathways on the singlet PES. The ClO-determined lifetime of CFCl2CH2O2 is 2.04 h. IM1 (CFCl2CH2OOOCl), IM2 (CFCl2CH2OOClO) and IM3 (CFCl2CH2OClO2) will photolyze under the sunlight.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by the Natural Science Foundations of China (No. 21707062).
References
- E. C. Tuazon and R. Atkinson, Potential Replacements for the Chlorofluorocarbons and Halons, American Chemical Society, Washington, DC, 1995 Search PubMed.
- J. Sehested, Int. J. Chem. Kinet., 1994, 26, 1023–1039 CrossRef CAS.
- R. Atkinson, D. L. Baulch, R. A. Cox, R. F. Hampson Jr, J. A. Kerr, M. J. Rossi and J. Troe, J. Phys. Chem. Ref. Data, 1997, 26, 521–1011 CrossRef CAS.
- P. D. Lightfoot, R. A. Cox, J. N. Crowley, M. Destriau, G. D. Hayman, M. E. Jenkin, G. K. Moortgat and F. Zabel, Atmos. Environ., Part A, 1992, 26, 1805–1961 CrossRef.
- T. J. Wallington and O. J. Nielsen, Int. J. Chem. Kinet., 1991, 23, 785–798 CrossRef CAS.
- R. W. Carr, D. G. Peterson and F. K. Smith, J. Phys. Chem., 1986, 90, 607 CrossRef CAS.
- F. X. Wu and R. W. Carr, J. Phys. Chem., 1995, 99, 3128–3136 CrossRef CAS.
- J. Sehested, O. J. Nielsen and T. J. Wallington, Chem. Phys. Lett., 1993, 213, 457–464 CrossRef CAS.
- F. X. Wu and R. W. Carr, Int. J. Chem. Kinet., 1996, 28, 9–19 CrossRef CAS.
- G. D. Hayman and F. Battin-Leclerc, J. Chem. Soc., Faraday Trans., 1995, 91, 1313–1323 RSC.
- D. E. Shallcross, M. T. Raventos-Duran, M. W. Bardwell, A. Bacak, Z. Solman and C. J. Percival, Atmos. Environ., 2005, 35, 763–771 CrossRef.
- S. Y. Du, J. S. Francisco, G. K. Schenter and B. C. Garrett, J. Am. Chem. Soc., 2009, 131, 14778–14785 CrossRef CAS.
- J. G. Anderson, D. Toohey and W. Brune, Science, 1991, 251, 39–46 CrossRef CAS PubMed.
- F. D. Pope, J. C. Hansen, K. D. Bayes, R. R. Friedl and S. P. Sander, Cheminform, 2007, 111, 4322–4332 CAS.
- Y. J. Zhang, B. He and Y. X. Sun, J. Mol. Graphics Modell., 2020, 99, 107618 CrossRef CAS PubMed.
- K. A. Holbrook, M. J. Pilling, and S. H. Robertson, Unimolecular Reactions, J. Wiley, Chichester, UK, 1996 Search PubMed.
- H. Hou, B. S. Wang and Y. S. Gu, J. Phys. Chem. A, 2000, 104, 320 CrossRef CAS.
- H. Hou and B. S. Wang, J. Chem. Phys., 1999, 103, 8021–8029 CrossRef.
- Y. J. Zhang, J. Y. Sun, K. Chao, H. Sun, F. Wang, S. W. Tang, X. M. Pan, J. P. Zhang and R. S. Wang, J. Phys. Chem. A, 2012, 116, 3172 CrossRef CAS PubMed.
- J. Y. Sun, R. S. Wang and B. S. Wang, Phys. Chem. Chem. Phys., 2011, 13, 16585 RSC.
- S. H. Mousavipour, S. Ramazani and Z. Shahkolahi, J. Phys. Chem. A, 2009, 113, 2838 CrossRef CAS PubMed.
- Y. J. Zhang, K. Chao, J. Y. Sun, Z. M. Su, X. M. Pan, J. P. Zhang and R. S. Wang, J. Phys. Chem. A, 2013, 117, 6629–6640 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- C. Lee, W. Yang and R. G. Par, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS PubMed.
- C. Gonzalez and H. B. Schlegel, J. Chem.
Phys., 1989, 90, 2154–2161 CrossRef CAS.
- C. Gonzalez and H. B. Schlegel, J. Phys. Chem., 1990, 94, 5523–5527 CrossRef CAS.
- K. Raghavachari, G. W. Trucks, J. A. Pople and M. Head-Gordon, Chem. Phys. Lett., 1989, 157, 479–483 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian, Inc, Wallingford CT, 2009.
- NIST Computational Chemistry Comparison and Benchmark Database, http://srdata.nist.gov/cccbdb/ Search PubMed.
- C. T. Chang, T. H. Liu and F. T. Jeng, Environ. Res., 2004, 94, 67–74 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra04707d |
|
| This journal is © The Royal Society of Chemistry 2020 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a and
Bing Heb
*a and
Bing Heb






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(7517.87/T) (200 ≤ T ≤ 3000 K)
exp(7517.87/T) (200 ≤ T ≤ 3000 K)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(1001.83/T) (200 ≤ T ≤ 500 K) = 9.45 × 10−15T0.69
exp(1001.83/T) (200 ≤ T ≤ 500 K) = 9.45 × 10−15T0.69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−1327.18/T) (500 < T ≤ 3000 K)
exp(−1327.18/T) (500 < T ≤ 3000 K)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(62.58/T) (200 ≤ T ≤ 3000 K)
exp(62.58/T) (200 ≤ T ≤ 3000 K)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−990.60/T) (200 ≤ T ≤ 3000 K)
exp(−990.60/T) (200 ≤ T ≤ 3000 K)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(47.22/T) (200 ≤ T ≤ 1800 K) = 1.08 × 10−9T−0.33
exp(47.22/T) (200 ≤ T ≤ 1800 K) = 1.08 × 10−9T−0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(2682.26/T) (1800 < T ≤ 3000 K)
exp(2682.26/T) (1800 < T ≤ 3000 K)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(8395.50/T) (200 ≤ T ≤ 3000 K)
exp(8395.50/T) (200 ≤ T ≤ 3000 K)

 The calculated average daytime atmospheric concentration of chlorine monoxide radical (ClO) is 1 × 107 molecules per cm3 (Tang, 2004),30 and kClO = 1.36 × 10−11 cm3 per molecule per s was estimated. The atmospheric lifetime of CFCl2CH2O2 is approximately 2.04 h, which suggests that the ClO-initiated reaction with CFCl2CH2O2 plays an important role in some special areas and the marine boundary layer.
The calculated average daytime atmospheric concentration of chlorine monoxide radical (ClO) is 1 × 107 molecules per cm3 (Tang, 2004),30 and kClO = 1.36 × 10−11 cm3 per molecule per s was estimated. The atmospheric lifetime of CFCl2CH2O2 is approximately 2.04 h, which suggests that the ClO-initiated reaction with CFCl2CH2O2 plays an important role in some special areas and the marine boundary layer.

