 Open Access Article
Open Access ArticleEffects of high mechanical treatment and long-term annealing on crystal structure and thermal stability of Ti2O3 nanocrystals
Albina A. Valeeva *ab,
Svetlana Z. Nazarovaa,
Hartmuth Schröttnerc,
Evgeny Yu. Gerasimovd and
Andrey A. Rempelabe
*ab,
Svetlana Z. Nazarovaa,
Hartmuth Schröttnerc,
Evgeny Yu. Gerasimovd and
Andrey A. Rempelabe
aInstitute of Solid State Chemistry of the Ural Branch of the Russian Academy of Sciences, 620990 Ekaterinburg, Russia. E-mail: anibla_v@mail.ru
bUral Federal University, 620002 Ekaterinburg, Russia
cInstitute for Electron Microscopy and Nanoanalysis, Graz University of Technology, A-8010 Graz, Austria
dBoreskov Institute of Catalysis SB RAS, 630090 Novosibirsk, Russia
eInstitute of Metallurgy of the Ural Branch of the Russian Academy of Sciences, 620016 Ekaterinburg, Russia
First published on 7th July 2020
Abstract
The effect of high-energy milling and long-term annealing on the stability of Ti2O3 nanocrystals was studied using a magnetic susceptibility method. In situ temperature dependences revealed that the crystal size greatly affects the magnetic susceptibility value. According to XRD, SEM and TEM data, Magnéli phases Ti9O10, Ti4O7, Ti7O19 and Ti3O5 are formed.
The titanium–oxygen (Ti–O) system is currently important from both scientific and applied viewpoints.1 Titanium compounds depending on nonstoichiometry2–5 are a promising functional material for a wide range of applications, such as a capacitors, photocatalysts for the degradation of organic pollutants in air and water, promising functional materials for renewable energy sources (e.g., solar batteries, photochemical water decomposition and hydrogen generation devices), efficient photoelectric converters, memristor memory elements, etc.6–12 Titanium oxide Ti2O3 with unusual properties has a narrow homogeneity region from TiO1.49 to TiO1.51.13 There is a d-metal–semiconductor electronic phase transition at 420–550 K without any change in the symmetry and crystal structure. The strong deformation of the crystal lattice with increasing temperature caused the band gap closure.14–16 Also, Ti2O3 exhibits thermoelectrical properties,17 and doped Ti2O3 films have high negative magnetoresistance.18
The aim of this work is to study the effect of mechanical treatment (high-energy milling) and subsequent high-temperature annealing in a vacuum on the crystal structure and thermal stability of Ti2O3 nanocrystals in the temperature range from 300 to 1200 K with the use of a magnetic susceptibility method.
The initial titanium(III) oxide Ti2O3 microcrystals with corundum structure (sp. gr. R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) with an average size of about 25 μm were synthesized by solid phase sintering from a mixture of Ti and TiO2 powders in a vacuum of 10−3 Pa at 1770 K. Titanium(III) oxide Ti2O3 nanocrystals have been obtained by high-energy milling of Ti2O3 microcrystals in a Retsch PM 200 planetary ball mill. The mass ratio of grinding balls made of zirconium dioxide (ZrO2) stabilized with yttrium oxide (Y2O3) to Ti2O3 powder in the experiment was 10
c) with an average size of about 25 μm were synthesized by solid phase sintering from a mixture of Ti and TiO2 powders in a vacuum of 10−3 Pa at 1770 K. Titanium(III) oxide Ti2O3 nanocrystals have been obtained by high-energy milling of Ti2O3 microcrystals in a Retsch PM 200 planetary ball mill. The mass ratio of grinding balls made of zirconium dioxide (ZrO2) stabilized with yttrium oxide (Y2O3) to Ti2O3 powder in the experiment was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Isopropyl alcohol was used as a grinding liquid. The following milling regime was employed to produce nanopowders: the duration of milling was 15, 30, 60, 120, 240 and 480 min, reversal of rotation direction was each 15 min, the interval between rotation direction reversal was 5 s, the rotation velocity of milling pot backing plate was 500 rpm.
1. Isopropyl alcohol was used as a grinding liquid. The following milling regime was employed to produce nanopowders: the duration of milling was 15, 30, 60, 120, 240 and 480 min, reversal of rotation direction was each 15 min, the interval between rotation direction reversal was 5 s, the rotation velocity of milling pot backing plate was 500 rpm.
X-ray phase analysis of all powders was performed in CuKα1,2-radiation on a Shimadzu XRD-7000 diffractometer in Bragg–Brentano geometry in stepwise scanning mode with Δ(2θ) = 0.02° in 2θ angle interval from 10 to 120°. To identify phases, the powder diffraction database ICDD, USA, Release 2016 was used. The phases were analyzed with the use of the Powder Cell 2.4 program. For a full-profile description of X-ray diffraction reflections, the pseudo-Voigt function was used. The diameter of nanocrystals D was determined from diffraction reflection broadening with the Williamson–Hall method.19,20 The CSR size was obtained by extrapolating the dependence β*(s) to the value s = 0, and the magnitude of microstrain was determined from the slope of this dependence.21,22
The microstructure of nanocrystals was studied with the high-resolution scanning electron microscopy (SEM) on a ZEISS Ultra 55 microscope. The working distance (WD) was 3.9–4.3 mm, the electron high tension (EHT) was 3–5 keV, and the beam width ranged from 2 to 6 μm depending on magnification. In order to avoid excessive electrization of powder during electron microscope imaging, the examined powder was deposited on a conducting adhesive tape and then was covered with a chromium layer of about 2 to 4 nm thick; the chromium coating did not affect the quality of visualization of powder morphology.
The structure of titanium oxide nanocrystals was determined by using the high-resolution transmission electron microscopy (HRTEM) on a JEM 2010 electron microscope (JEOL, Japan) with accelerating voltage of 200 kV and ultimate lattice resolution of 140 pm. Imaging was performed by means of CCD matrix of Soft Imaging System (Germany). The device was equipped with a Phoenix (EDAX, USA) energy-dispersive characteristic X-ray radiation (EDX) spectrometer with a semiconducting Si(Li)-detector with energy resolution of 130 eV. Ti2O3 particles were placed into alcohol and were further deposited on perforated carbon substrates (diameter of holes of about 1 μm) fixed on copper grids. Particles were deposited with the use of a UZD-1UCh2 ultrasonic disperser, which allowed uniform particle distribution on the substrate surface. After the grids were extracted from alcohol, the alcohol evaporated.
Thermal stability and phase transformations with long-term exposure for complete running of processes in the system and structure stabilization were studied with the analysis of magnetic susceptibility variation by using the Faraday method on a pendulum magnetic Domenikalli-type balance in vacuum of about 10−3 Pa.23 Magnetic susceptibility χ of Ti2O3 was measured in the temperature interval from 300 to 1200 K in magnetic fields with intensity from 7.2 to 8.8 kOe. The heating and cooling rate of the samples during susceptibility measurements was about 1 K min−1. The powder mass and the crystal structure of Ti2O3 were controlled before and after χ measurements. The accuracy of χ measurements was about ±0.05 × 10−6 emu g−1. The absence of ferromagnetic impurities in the powders was confirmed by measurements at different magnetic field values.
Detailed analysis of X-ray diffraction patterns of ball milled Ti2O3 (Fig. 1a) showed that the crystal structure of nanopowder coincides with the crystal structure of microcrystal (Fig. 1a), i.e. the structure of Ti2O3 is highly stable with respect to high-energy milling, and fragmentation does not lead to changing of crystal symmetry. After high-energy milling, broadening of reflections is observed on the X-ray diffraction patterns, which is related to a small grain size and the presence of microstrains in the system due to high-energy milling. The full-profile analysis of X-ray diffraction reflections showed a decrease in coherent scattering region (CSR) from 25 μm to 10 nm with an increase of milling time from 15 to 480 minutes, while the microstrain increase up to 0.26%.
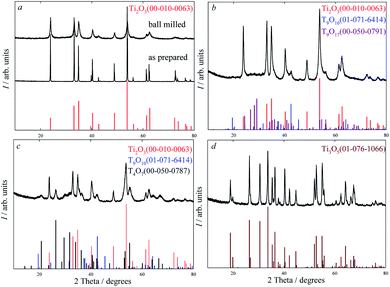 | ||
| Fig. 1 XRD pattern of powders at room temperature: (a) as prepared microcrystals and ball milled nanocrystals; after magnetic susceptibility measurements: (b) at 673 K; (c) at 873 K; (d) at 1200 K. | ||
Analysis of SEM micrographs of ball milled Ti2O3 nanocrystals revealed that the particles are uniformly distributed in the bulk and have a granular or rounded shape (Fig. 2). It was found that small granules of about 10–30 nm in size adhere to each other and form agglomerates. Fig. 3 displays a HRTEM image of the ball milled Ti2O3 nanocrystal. According to HRTEM data, the sample consists mainly of crystallites of sizes from 50 to 500 nm (Fig. 3a).
 | ||
| Fig. 2 SEM image of ball milled Ti2O3. Small granules of about 10 to 30 nm in size adhere to each other and form agglomerates. | ||
According to the observed dhkl (Fig. 3b), this phase corresponds to Ti2O3 (PDF 00-010-0063). Thus, the size of particles found with SEM and HRTEM methods coincide, which indicates that the size of CSR of particles determined from XRD data is accurate enough.
The experimental data obtained earlier show that the magnetic susceptibility value of nanocrystals is twice as small in absolute magnitude as that of microcrystals.24 The crystal size affects greatly not only the value of magnetic susceptibility, but also the temperature behaviour of magnetic susceptibility. The temperature dependence of magnetic susceptibility for Ti2O3 microcrystals has a classical form of s-shaped curve typical of first-order phase transitions without considerable hysteresis. Fig. 4 shows in situ temperature dependences of magnetic susceptibility for Ti2O3 nanocrystals. In the temperature region from 300 to 400 K, the structure of initial Ti2O3 nanocrystal remains trigonal (sp. gr. R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c); the temperature dependence exhibits a reverse behavior of magnetic susceptibility, which shows that the state of nanocrystals in this temperature region is metastable. Annealing of Ti2O3 nanocrystals at temperature above 400 K leads to phase transformations and, as a result, to magnetic susceptibility enhancement. X-ray diffraction analysis shows that after annealing to 673 K the powder contains additional phases of Ti9O10 (sp. gr. Immm) – 3 mass% and Ti9O17 (sp. gr. I
c); the temperature dependence exhibits a reverse behavior of magnetic susceptibility, which shows that the state of nanocrystals in this temperature region is metastable. Annealing of Ti2O3 nanocrystals at temperature above 400 K leads to phase transformations and, as a result, to magnetic susceptibility enhancement. X-ray diffraction analysis shows that after annealing to 673 K the powder contains additional phases of Ti9O10 (sp. gr. Immm) – 3 mass% and Ti9O17 (sp. gr. I![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) – 11 mass% alongside with Ti2O3 phase (sp. gr. R
) – 11 mass% alongside with Ti2O3 phase (sp. gr. R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) in the amount of 86 mass% (Fig. 1b). Further annealing and increasing annealing temperature to 873 K result in variation of phases and phase proportions. X-ray diffraction analysis (Fig. 1c) also shows that the powder contains Ti2O3 (sp. gr. R
c) in the amount of 86 mass% (Fig. 1b). Further annealing and increasing annealing temperature to 873 K result in variation of phases and phase proportions. X-ray diffraction analysis (Fig. 1c) also shows that the powder contains Ti2O3 (sp. gr. R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) – 38 mass%, Ti9O10 (sp. gr. Immm) – 2 mass%, Ti4O7 (sp. gr. A
c) – 38 mass%, Ti9O10 (sp. gr. Immm) – 2 mass%, Ti4O7 (sp. gr. A![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) – 60 mass%.
) – 60 mass%.
It is noteworthy that the Ti9O10 phase (sp. gr. Immm), which was first obtained during annealing of nonstoichiometric TiOy nanocrystal and annealing of TiOy/HAP nanocomposite,25–27 is formed during annealing of only titanium oxide nanocrystals with superstoichiometric composition. According to quantum chemical calculations, the Ti9O10 phase (sp. gr. Immm) in a microcrystalline state is unfavorable in comparison with the disordered cubic phase of the same TiO9/10 composition, therefore its formation is due to size effects.28,29
When the annealing temperature rises, the stable Ti3O5 with monoclinic structure (sp. gr. I2/c) is formed (Fig. 1d). Judging by the temperature dependence of magnetic susceptibility (Fig. 4), the formation of the Ti3O5 phase occurs at about 1073 K. At further heating from 300 to 1200 K and cooling from 1200 to 300 K, a reverse behavior of magnetic susceptibility is observed, which indicates that the system reaches the equilibrium state. Thus, the experimental results show that the phase stability and phase transitions in Ti2O3 are greatly affected above all by the crystal sizes.
Fig. 5 demonstrates the HRTEM micrographs of nanocrystalline powder after annealing experiments. The sample consists of plate-like particles 10 nm to 1 μm in size (Fig. 5a). Regions with well crystallized structure are observed where the dimensions of blocks are 100 nm. According to the observed interplanar spacings, the phase formed corresponds to the Ti3O5 phase (Fig. 5b) with monoclinic structure (sp. gr. I2/c) (PDF 01-076-1066).
Thus, Ti2O3, subjected to mechanical treatment (high-energy milling) and heat treatment in the temperature range from 300 to 1200 K in vacuum, is an unstable phase since it changes during thermal treatment. According to the X-ray diffraction analysis, SEM and HRTEM data, the Ti9O10 phase and Magnelli phases Ti4O7, Ti7O19 and Ti3O5 are formed depending on the annealing temperature. In addition, the in situ temperature dependences of magnetic susceptibility showed that the crystal size greatly affects the value of magnetic susceptibility; the magnetic susceptibility value of nanocrystals is twice as small in absolute magnitude as that of microcrystals.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The reported study was funded by RFBR according to the research project no. 19-03-00051a.References
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891 CrossRef CAS PubMed.
- A. A. Valeeva, S. Z. Nazarova and A. A. Rempel, Phys. Solid State, 2016, 58(4), 771 CrossRef CAS.
- A. A. Valeeva and A. A. Rempel, Mendeleev Commun., 2010, 20, 101 CrossRef.
- A. A. Valeeva, A. A. Rempel and A. I. Gusev, Dokl. Phys., 2002, 47, 39 CrossRef CAS.
- M. G. Kostenko, A. A. Valeeva and A. A. Rempel, Mendeleev Commun., 2012, 22, 245 CrossRef CAS.
- V. Schöllmann, J. Johansson, K. Andersen and D. V. Haviland, J. Appl. Phys., 2000, 88, 6549 CrossRef.
- A. A. Rempel, E. A. Kozlova, T. I. Gorbunova, S. V. Cherepanova, E. Y. Gerasimov, N. S. Kozhevnikova, A. A. Valeeva, E. Y. Korovin, V. V. Kaichev and Y. A. Shchipunov, Catal. Commun., 2015, 68, 61 CrossRef CAS.
- A. A. Valeeva, E. A. Kozlova, A. S. Vokhmintsev, R. V. Kamalov, I. B. Dorosheva, A. A. Saraev, I. A. Weinstein and A. A. Rempel, Sci. Rep., 2018, 8, 9607 CrossRef CAS PubMed.
- A. A. Valeeva, I. B. Dorosheva, E. A. Kozlova, R. V. Kamalov, A. S. Vokhmintsev, D. S. Selishchev, A. A. Saraev, E. Y. Gerasimov, I. A. Weinstein and A. A. Rempel, J. Alloys Compd., 2019, 796, 293 CrossRef CAS.
- D. Recatalá, R. Llusar, A. L. Gushchin, E. A. Kozlova, Y. A. Laricheva, P. A. Abramov, M. N. Sokolov, R. Gómez and T. Lana-Villarreal, ChemSusChem, 2015, 8, 148 CrossRef PubMed.
- N. S. Kolobov, D. A. Svintsitskiy, E. A. Kozlova, D. S. Selishchev and D. V. Kozlov, Eng. J., 2017, 314, 600 CAS.
- A. S. Vokhmintsev, I. A. Weinstein, R. V. Kamalov and I. B. Dorosheva, Bull. Russ. Acad. Sci.: Phys., 2014, 78, 932 CAS.
- S. Andersson, B. Collén, U. Kuylenstierna and A. Magnéli, Acta Chem. Scand., 1957, 11, 1641 CrossRef CAS.
- H. Fan, M. Wang, Z. Yang, X. Ren, M. Yin and S. Liu, Appl. Phys. A: Mater. Sci. Process., 2016, 122, 964 CrossRef.
- J. Wang, Y. Li, L. Deng, N. Wei, Y. Weng, S. Dong, D. Qi, J. Qiu, X. Chen and T. Wu, Adv. Mater., 2016, 29, 1603730 CrossRef PubMed.
- A. I. Poteryaev, A. I. Lichtenstein and G. Kotliar, Phys. Rev. Lett., 2004, 93, 086401 CrossRef CAS PubMed.
- I. Veremchuk, I. Antonyshyn, C. Candolfi, X. Feng, U. Burkhardt, M. Baitinger, J. T. Zhao and Y. Grin, Inorg. Chem., 2013, 52, 4458 CrossRef CAS PubMed.
- Z. Wang, Y. Hong, J. Tang, C. Radu, Y. Chen, L. Spinu, W. Zhou and L. D. Tung, Appl. Phys. Lett., 2004, 86, 7384 Search PubMed.
- W. H. Hall, Proc. Phys. Soc., London, Sect. A, 1949, 62, 741 CrossRef.
- G. K. Williamson and W. H. Hall, Acta Metall., 1953, 1, 22 CrossRef CAS.
- A. A. Valeeva, H. Schroettner and A. A. Rempel, Russ. Chem. Bull., 2014, 63, 2729 CrossRef CAS.
- A. A. Valeeva, S. Z. Nazarova and A. A. Rempel, Phys. Status Solidi B, 2016, 253, 392 CrossRef CAS.
- A. A. Valeeva, S. Z. Nazarova and A. A. Rempel, JETP Lett., 2015, 101, 258 CrossRef CAS.
- A. A. Valeeva, S. Z. Nazarova and A. A. Rempel, J. Alloys Compd., 2020, 817, 153215 CrossRef CAS.
- S. V. Rempel, A. A. Valeeva, E. A. Bogdanova, H. Schroettner, N. A. Sabirzyanov and A. A. Rempel, Mendeleev Commun., 2016, 26, 543 CrossRef CAS.
- S. V. Rempel, D. A. Eselevich, E. Y. Gerasimov and A. A. Valeeva, J. Alloys Compd., 2019, 800, 412 CrossRef CAS.
- S. V. Rempel, E. A. Bogdanova, A. A. Valeeva, H. Schroettner, N. A. Sabirzyanov and A. A. Rempel, Inorg. Mater., 2016, 52, 476 CrossRef CAS.
- A. A. Valeeva, M. G. Kostenko, S. Z. Nazarova, E. Y. Gerasimov and A. A. Rempel, Inorg. Mater., 2018, 54, 568 CrossRef CAS.
- M. G. Kostenko and A. A. Valeeva, Mendeleev Commun., 2019, 29, 405 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |



