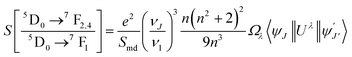Open Access Article
Open Access ArticleSpectroscopic analysis of Eu3+ doped silica–titania–polydimethylsiloxane hybrid ORMOSILs†
Manju Gopinath R. J.,
Subash Gopi,
Sanu Mathew Simon,
A. C. Saritha,
P. R. Biju,
Cyriac Joseph and
N. V. Unnikrishnan
and
N. V. Unnikrishnan *
*
School of Pure and Applied Physics, Mahatma Gandhi University, Kottayam, Kerala 686560, India. E-mail: nvu100@yahoo.com
First published on 27th May 2020
Abstract
Eu3+ doped silica–titania–polydimethylsiloxane hybrid ORMOSILs were synthesized via a non-hydrolytic sol–gel route. The structural and thermal analyses of the samples confirmed that the matrix structure remains unaffected by doping with different concentrations of Eu3+ ions. Photoluminescence (PL) studies performed at 394 nm on Eu3+ doped ORMOSILs imply that they emit broad blue host emission and the characteristic Eu3+ red emissions simultaneously. Also, the samples were excited at the charge transfer (CT) band and this confirmed the existence of an energy transfer path from the host to the Eu3+ ions via Ti4+–O2−–Eu3+ bonds. The phonon energy of the host matrix was estimated by phonon sideband (PSB) analysis and the results were substantiated by Raman analysis. Judd–Ofelt (JO) parameters were also evaluated which give details about the local surroundings of the Eu3+ ions in the system and these parameters were further used for predicting the radiative properties of 5D0 → 7F1,2,4 transitions of Eu3+ ions. Furthermore, the quantum efficiency and CIE co-ordinates were evaluated and it was found that Eu3+ doped silica–titania–polydimethylsiloxane ORMOSIL has an intense pinkish red emission with a quantum efficiency of 30.7%.
1. Introduction
Research has been progressing for decades on the development of luminescent materials and their applications in fabricating optical devices. Materials doped with rare-earths (REs) have drawn considerable attention due to their excellent fluorescent properties. Cerium, samarium, europium, gadolinium, terbium, dysprosium, erbium, and ytterbium are some of the rare-earths commonly used for luminescence applications. But the intensity of emission by these rare-earths is greatly influenced by the site and its neighbourhood occupied by the RE ions in the host matrix.1 Europium (Eu3+) ions emit radiation in the red region resulting from the 5D0 → 7F2 hypersensitive transition and its intensity is highly influenced by the symmetry of the positions occupied by Eu3+ ions in the host matrix. Consequently, the Eu3+ ions are effectively used to explore the symmetry and bonding nature around these dopant ions in the system.2 Hence new host matrices are developed to incorporate these RE ions and the study of the performance of the RE ions in these new systems is a fascinating field of research in material science. Thus a new family of organically modified silicate is introduced as host matrix in the present work.The electrons that are excited to the 5D1,2,3 levels of the Eu3+ ions de-excites non-radiatively to the 5D0 level by multiphonon relaxation. Studies show that the multiphonon decay rate increases with increase in phonon energy and electron–phonon coupling strength.3,4 Hence it is also important to estimate the phonon energy associated with the present glass system.
In many recent works, silica is effectively used as the host matrix to incorporate the rare-earth ions so that these ions can be protected from the environmental effects and can enhance emission intensity.5 Also, the binary matrices such as TiO2–SiO2 and TiO2–ZrO2 doped with RE ions were widely examined during the last decades as they find applications in lasers, lighting and displays, up-conversion and white light emission.6–9 However, the surface defects and lattice distortions in these host matrices adversely influence the luminescence properties of the RE ions which leads to quenching of luminescence.4 Hence the decay life time and luminescence intensity of these host matrices have to be improved. To achieve this goal, organics are incorporated into the inorganic matrix which leads to the formation of new Organically Modified Silicates (ORMOSILs) and Organically Modified Ceramers (ORMOCERs) with improved mechanical, thermal and optical properties.10 In the light of the above investigations, the present work aims at the preparation and characterisation of polydimethylsiloxane modified silica–titania (SiO2–TiO2–PDMS) ORMOSIL matrix doped with Eu3+ ions. Here, PDMS is selected as the organic polymer for modifying the silica–titania inorganic matrix because both PDMS and SiO2 have similar –Si–O–Si– chemical bond and hence forms covalently bonded network.11,12 Here non-hydrolytic route of sol–gel method is adopted for the sample preparation since the organic polymer PDMS is insoluble in water and TiO2 precipitates in the hydrolytic process.13
Interestingly, the silica–titania–polydimethylsiloxane (SiO2–TiO2–PDMS) host matrix has a broad blue emission peaking at 470 nm when excited with UV light.14 Thus the photoluminescence emission of the Eu3+ doped SiO2–TiO2–PDMS hybrid matrix is the resultant of the blue emission of the host matrix and the red emission of the Eu3+ ions which finds application in WLEDs, novel luminescent phosphors, displays etc.
2. Experimental details
The inorganic precursors for synthesising the samples were tetraethyl orthosilicate/TEOS (Merck) and titanium(IV) isopropoxide/TIP (Aldrich) which contains SiO2 and TiO2 respectively. The organic polymer for modifying the inorganic was OH-terminated polydimethylsiloxane/PDMS (MW = 4200) (Alfa Aesar). Here, ethanol (Merck) was the solvent and hydrochloric acid (HCl) was the catalyst. The source of Eu3+ ions for doping was europium(III) nitrate hexahydrate (Alfa Aesar). The molar ratio of (SiO2 + PDMS)/TiO2 is taken as 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The SiO2 and PDMS were taken together since the organic polymer PDMS has the same –Si–O–Si– bonding as that of SiO2.
1. The SiO2 and PDMS were taken together since the organic polymer PDMS has the same –Si–O–Si– bonding as that of SiO2.
Mixtures 1 and 2 were prepared during synthesis. Mixture 1 contains 5.2 mL of TEOS, 0.1 mL of PDMS and 5.8 mL of ethanol and the molar ratio of EtOH/TEOS is kept as 4. Mixture 2 consists of 1.9 mL of TIP and 1.8 mL of ethanol where the molar ratio of EtOH/TIP is kept as 5. After stirring these mixtures separately for 30 minutes, they were added together and the stirring was further continued for another one hour. Europium(III) nitrate hexahydrate dissolved in ethanol was then added to the resultant SiO2–TiO2–PDMS mixture in various concentrations ranging from 1 to 4 wt% and hydrochloric acid was also added in very small quantity. This mixture is vigorously stirred to obtain a clear and homogenous sol which is transferred to polypropylene petridish and covered with para film. These dishes were kept without any disturbance until transparent glass samples were formed. All the synthesis procedures were carried out at room temperature and normal atmospheric conditions. In this discussion undoped ORMOSIL host matrix SiO2–TiO2–PDMS is denoted as Eu0 and the Eu3+ doped samples were denoted as Eu1, Eu2, Eu3 and Eu4 for 1, 2, 3 and 4 wt% doping respectively.
X-Ray powder diffraction analysis and Fourier transform infra-red characterization were carried out for the analysis of the sample structure using PANalytical X'Pert PRO and Perkin Elmer Spectrum 400 respectively. The thermal stability of the samples was studied using PerkinElmer STA 8000 thermo gravimetric analyser. Also, Flurolog-3 Spectrofluorometer (Horiba Scientific) was used for photoluminescence characterisations and Fluoromax-4, TCSPC Fluorescence Lifetime Measurement System (Horiba Scientific) for lifetime measurements. The Raman spectra of the samples were also recorded for analysing the phonon side band (PSB) using WITec Alpha 300 RA Confocal Raman Spectrometer.
3. Results and discussions
3.1 XRD analysis
X-Ray diffraction analyses were carried out on the pure host matrix Eu0 and samples doped with varying Eu3+ concentrations. All the samples show identical diffraction pattern and hence the patterns of representative samples Eu0, Eu2 and Eu4 are depicted in Fig. S1.† The X-ray diffraction pattern has a broad hump between 15° and 30° which is a characteristic of the SiO2 component of the host matrix.15,16 Here, the peaks of TiO2 are not distinctly visible as the amorphous nature of SiO2 is prominent. The diffraction patterns of all the samples also show similar behaviour which confirms that the structure of the host matrix remains unaltered even after Eu3+ doping.3.2 FTIR analysis
The FTIR spectroscopy can be adopted as an effective tool for analysing the chemical and structural changes of the host matrix with Eu3+ doping. The FTIR spectra of the pure (Eu0) and Eu3+ doped samples (Eu2 and Eu4) were recorded from 400 cm−1 to 4000 cm−1 and are shown in Fig. 1.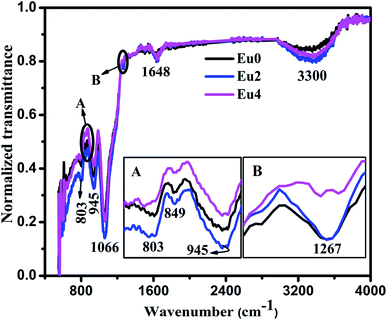 | ||
| Fig. 1 FTIR spectra of pure and Eu3+ doped SiO2–TiO2–PDMS ORMOSIL samples (inset figures (A and B) shows the peaks in the range 750 cm−1 to 975 cm−1 and 1240 cm−1 to 1275 cm−1 respectively). | ||
The FTIR spectra of pure and Eu3+ doped samples show identical nature in respect of peak positions and intensity. The small peak at 849 cm−1 in the spectra establishes that copolymerisation reaction has occurred between Si–OH groups of hydrolysed TEOS and PDMS molecules. Also, the peak located at 945 cm−1 is ascribed to the Si–O–Ti bond formed during condensation reaction. The asymmetric vibration of Si–O–Si bonds results in the absorption band at 1066 cm−1.17 Also, the band at 803 cm−1 is assigned to the net effect of Si–C stretching and CH3 rocking in Si–CH3 and that at 1267 cm−1 is due to the symmetric deformation of CH3 in Si–CH3 group.18,19 Mackenzie et al. have suggested in the report on SiO2–PDMS ORMOSIL that the –CH3 groups in Si–CH3 performs in a way similar to the non-bonding oxygen in silicate glasses.20 Thus it can be concluded that the FTIR spectroscopy confirms the presence of oxygen related defects in the host matrix which plays an important role in the luminescence properties of the SiO2–TiO2–PDMS ORMOSIL host matrix.14 Absorption bands were also present at 1648 cm−1 and 3300 cm−1 which occurs by the stretching and bending vibrations of surface adsorbed water and OH groups respectively.21 The similarity in the FTIR spectra of both pure and Eu3+ doped samples further confirms that the matrix structure remains unchanged by doping with different concentrations of Eu3+ ions.
3.3 TG analysis
Thermo gravimetric (TG) analysis was done in the nitrogen atmosphere up to 700 °C to study the thermal stability of pure and Eu3+ doped samples and the curves are illustrated in Fig. S2.†The TG curves depicts that a weight loss of only 15% occurred on heating up to 150 °C for all the samples. This is due to the evaporation of adsorbed water and trapped solvents in the matrix, whose presence is confirmed in FTIR analysis.21 The curves become almost straight in the range 150–700 °C and is indicating that the samples are thermally stable up to 700 °C. The similar nature of TG curves for the pure and doped samples confirms that the matrix structure remains unaffected by Eu3+ doping.
3.4 Photoluminescence analysis
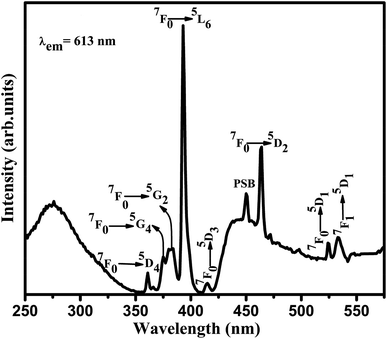
The photoluminescence (PL) analysis is used as an efficient tool for investigating the energy level transitions in Eu3+ doped SiO2–TiO2–PDMS ORMOSILs. The excitation spectra of the samples were recorded fixing λem at 613 nm which is the prominent emission wavelength of Eu3+ ions. The excitation spectra of all the samples are depicted in Fig. S3† and as a representative case, the spectrum of 2 wt% Eu3+ doped SiO2–TiO2–PDMS ORMOSIL (Eu2) is shown in Fig. 2.The excitation spectrum of Eu2 sample consists of a broad band peaking at 276 nm which is due to the overlapping of the O–Eu charge transfer (CT) band and the SiO−–Ti ligand-to-metal electron transfer [LMET] band. This indicates an energy transfer from the O–Ti charge transfer band to the Eu3+ ions in the SiO2–TiO2–PDMS system.22,23 The lines at 360, 374, 380, 394, 414, 464 and 524 nm are assigned to the electronic transitions of Eu3+ ions from the ground 7F0 level to 5D4, 5G4, 5G2, 5L6, 5D3, 5D2 and 5D1 levels respectively. Also, there is a peak at 534 nm which corresponds to the 7F1 → 5D1 energy level transition of Eu3+ ions.2 The peak at 450 nm is the phonon sideband of the host matrix which will be discussed in the forthcoming section. The prominent excitation transition at 394 nm and the charge transfer band were chosen for the investigation of the photoluminescence characteristics of Eu3+ doped SiO2–TiO2–PDMS ORMOSILs.
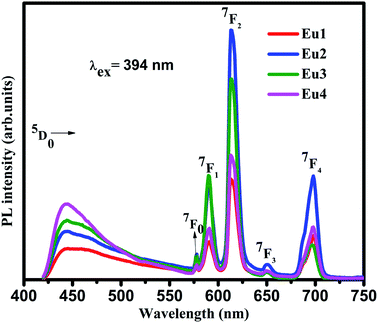
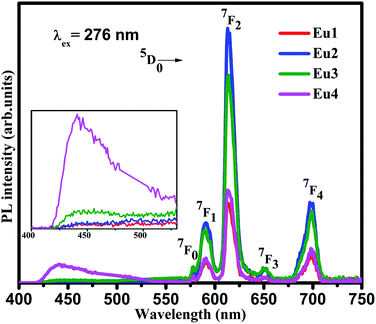
The emission spectra of all the samples were obtained upon excitation at 394 nm and are shown in Fig. 3. Here, the spectra consist of a broad emission peaking at 450 nm which is assigned to the oxygen related defects in the host matrix.21,24 The authors reported in their previous work that the pure SiO2–TiO2–PDMS host matrix has a weak excitation around 276 nm and a strong excitation around 396 nm, monitoring the emission at 470 nm.14 Hence, when the Eu3+ doped system is excited at 394 nm, both the host matrix and the Eu3+ ions were excited giving host emission along with the characteristic emissions of Eu3+ ions as shown in the spectra. It is observed that the host emission intensity increases with increase in Eu3+ doping concentration. As the Eu3+ ions were embedded in the host matrix, its lattice gets distorted due to the difference in the radius of Eu3+ ions (0.098 nm) and Ti4+ ions (0.061 nm). Also, a charge imbalance exists between Eu3+ and Ti4+ ions which leads to the formation of oxygen vacancies.6,25 Thus as the Eu3+ ion concentration increases, these defects also increases which in turn enhances the host emission.
The line emissions in the spectra correspond to 5D0 → 7F0 (577 nm), 5D0 → 7F1 (590 nm), 5D0 → 7F2 (613 nm), 5D0 → 7F3 (651 nm) and 5D0 → 7F4 (698 nm) transitions of Eu3+ ions.2,6 Though the electrons were excited to the higher energy levels of Eu3+ ions, only radiative transitions from 5D0 level to the lower f levels were observed in the emission spectra. This indicates the existence of non-radiative transition from the higher energy levels to 5D0 level. Even though 5D0 → 7F0 and 5D0 → 7F3 transitions are present in the spectra, they are forbidden according to the Judd–Ofelt (JO) theory. The presence of these transitions were due to J-mixing which is enhanced by strong crystal-field effects of the host matrix.2 Hence it can be confirmed that the Eu3+ ions have asymmetric vicinity in the matrix. Furthermore, the 5D0 → 7F0 transition is narrow which confirms that the Eu3+ ions occupy asymmetric sites in the host matrix which is a characteristic of amorphous hybrid samples.26
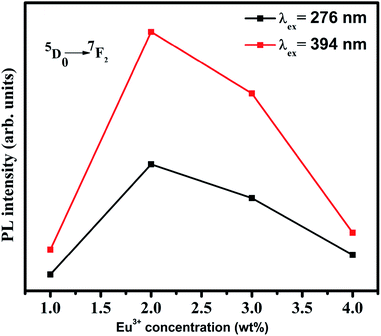
The 5D0 → 7F1 transition is a magnetic dipole transition while the 5D0 → 7F2 transition is an electric dipole transition. Hence the 5D0 → 7F1 transition does not depend on local environment of the Eu3+ ions while the 5D0 → 7F2 transition is affected by the local surroundings of the Eu3+ ions and the nature of the ligands in the host matrix. Thus the 5D0 → 7F2 transition is a hypersensitive transition which can be used as a probe to measure the asymmetry of Eu3+ occupied site. On exciting the samples at 394 nm, the peak corresponding to 5D0 → 7F2 transition at 613 nm is very strong compared to 5D0 → 7F1 transition at 590 nm. As the Eu3+ ions occupy asymmetric sites in the host matrix the electric dipole transition dominates the magnetic dipole transition.27 Hence it can be concluded from the photoluminescence studies that the Eu3+ ions occupy asymmetric sites in the SiO2–TiO2–PDMS ORMOSIL host matrix. The luminescence intensity is found to increase with an increase in the Eu3+ ion concentration up to 2 wt%. The glass exhibits luminescence quenching above this concentration and hence 2 wt% Eu3+ is the optimum doping concentration in the present system. As the number of Eu3+ activator ions increase, the interionic distance decreases which results in a fast energy transfer between the ions. Thus a part of the excitation energy is non-radiatively dissipated resulting in the quenching of luminescence.28
The emission spectra were also recorded on exciting the Eu3+ doped samples in the CT band (276 nm) and are shown in Fig. 4. The spectra shows similar nature as that of the directly excited samples which clarifies that the Eu3+ ions in the same site are excited in both cases. The Eu3+ activator ions are not directly excited in the CT band but the high emission intensity of the Eu3+ ions may be due to the efficient energy transfer from the host matrix to the Eu3+ activator ions via Ti4+–O2−–Eu3+ bond.29 When the samples are excited at 276 nm, the electrons in the O 2p states are excited to the Ti 3d states of the host matrix which is transferred to the 4f state of the Eu3+ ions.25 Thus the Eu3+ activator ions are efficiently sensitized by the host. Since the excited electrons in the host matrix are transferred to the Eu3+ ions, the host emission intensity is not prominent in the spectra. The magnified image in the inset of Fig. 4 shows that though the host emission intensity is feeble, the trend shown is same as that of the directly excited samples. This confirms that the Eu3+ insertion in to the host matrix generates defects which in turn increases the host emission. The variation in the photoluminescence intensity of 5D0 → 7F2 transition with Eu3+ concentration in both the excitation wavelengths are also depicted in Fig. 5.
 | ||
| Fig. 4 Emission spectra of Eu3+ doped SiO2–TiO2–PDMS ORMOSILs excited at 276 nm (inset shows the magnified image of the host emission in the range 400–550 nm). | ||
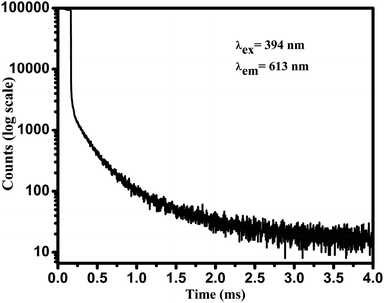
The luminescence life time of 2 wt% Eu3+ doped SiO2–TiO2–PDMS ORMOSIL (Eu2) was evaluated by monitoring the decay profile of 613 nm emission of the sample on exciting with 394 nm and the luminescence time spectrum is shown in Fig. 6.
The average fluorescence lifetime (τ) of the energy level can be obtained from the formula, 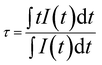 , where I(t) represents the luminescence intensity at time t.30 The average lifetime of Eu2 ORMOSIL glass was found to be 0.90 ms. A relatively longer lifetime of the sample shows that the Eu3+ ions are evenly embedded in the host matrix so that ion clustering is reduced.29 The nature of the decay curve also indicates that the Eu3+ ions are present in an asymmetric environment in the host matrix.31 A comparative study with the lifetimes of other Eu3+ doped samples are tabulated in Table 1.
, where I(t) represents the luminescence intensity at time t.30 The average lifetime of Eu2 ORMOSIL glass was found to be 0.90 ms. A relatively longer lifetime of the sample shows that the Eu3+ ions are evenly embedded in the host matrix so that ion clustering is reduced.29 The nature of the decay curve also indicates that the Eu3+ ions are present in an asymmetric environment in the host matrix.31 A comparative study with the lifetimes of other Eu3+ doped samples are tabulated in Table 1.
The asymmetric ratio (R) is another effective tool to analyse the symmetry of site occupied by Eu3+ ions in the host matrix. It is the ratio between the integrated emission intensity of 5D0 → 7F2 and 5D0 → 7F1 energy level transitions.6 As discussed earlier, 5D0 → 7F2 transition is due to the electric dipole mechanism and 5D0 → 7F1 transition due to magnetic dipole mechanism which is not affected by the local surroundings of the ions. Hence the intensity of 5D0 → 7F1 transition is taken as the reference. Thus the R values can be used to understand the asymmetry in the neighbourhood of Eu3+ ions in the host matrix and also the degree of covalency between Eu3+ and O2− ions.1,3 The R values obtained in the present study are 3.35, 3.44, 3.37 and 2.5 for Eu1, Eu2, Eu3 and Eu4 samples respectively. The larger values of R indicates that the asymmetry and covalency effect around the Eu3+ ion site is higher in the present system. Julian et al. have reported that the R value is in between 3 and 6 for Eu3+ doped silica glasses, polymers or crystals.34 Also as the Eu3+ ion concentration increases, the R value reaches a maximum for Eu2 sample and decreases above that concentration. This confirms the cluster forming tendency of Eu3+ ions at higher concentration which leads to luminescence quenching.35
3.5 Phonon sideband analysis
The Eu3+ ions doped in the SiO2–TiO2–PDMS host matrix are excited to 5D1,2,3,4, 5L6, 5G2 energy levels. But the phonon energy of the sample hinders the radiative emission from these higher energy levels to the ground state. Hence, all these excited electrons of Eu3+ ions can populate the lowest excited 5D0 energy level after non-radiative decay. From this 5D0 level, the excited electrons return to the 7FJ (J = 1, 2, 3, 4) ground state emitting its characteristic radiation. The excitation spectrum of Eu3+ doped SiO2–TiO2–PDMS ORMOSIL contains a peak in the higher energy side of the pure electronic band (PEB) transition, 7F0 → 5D2 and this peak is assigned as the phonon sideband (PSB) as shown in Fig. 7. The PSB analysis is carried out to study the multiphonon relaxation in Eu3+ doped SiO2–TiO2–PDMS ORMOSIL host matrix.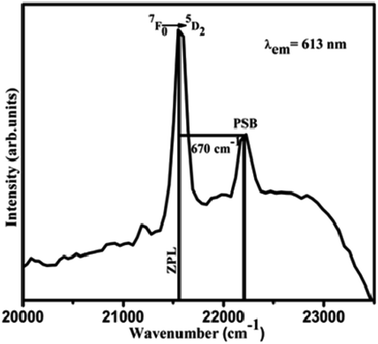 | ||
| Fig. 7 Phonon sideband spectrum of 2 wt% Eu3+ doped SiO2–TiO2–PDMS ORMOSIL with 7F0 → 5D2 excited state transition. | ||
The PSB includes simultaneous vibrational and electronic transitions and its presence in the excitation spectrum is an indication of the coupling of excited electrons with the excess energy of vibrational modes around the Eu3+ ions. The difference in energy between the PEB and PSB band corresponds to the phonon energy, ħω and here it is estimated to be 670 cm−1. The coupling of a particular vibrational mode to an electronic transition is termed as electron-phonon coupling strength, g and is estimated to be 0.2984 for Eu3+ doped SiO2–TiO2–PDMS ORMOSIL. Similar high values of g was also reported for Eu3+ doped ZrO2–PEG composites35 and for Eu3+ doped SiO2–TiO2–ZrO2 glasses.4 Also, L. Skuja has reported the significance of electron-phonon coupling constant (g) in optical transition. That is if the g value is below ∼4, the optical absorption or luminescence spectra contains zero-phonon and vibronic lines even at low temperatures.36 The relative non-radiative decay rate Wmp/W0 were also calculated for the 5D1, 5D2 and 5D3 levels of the sample and are tabulated in Table 2. The equations and methods of PSB analysis have been published previously3,37 and are included in Section S1 of the ESI.†
| ħω (cm−1) | g | 7F0 → 5D1 | 7F0 → 5D2 | 7F0 → 5D3 | |||
|---|---|---|---|---|---|---|---|
| α (×10−3 cm) | Wmp/W0 | α (×10−3 cm) | Wmp/W0 | α (×10−3 cm) | Wmp/W0 | ||
| 670 | 0.2984 | 1.667 | 0.0541 | 2.169 | 0.0044 | 2.35 | 0.0014 |
The observed PSB band for the Eu3+ ions in the SiO2–TiO2–PDMS ORMOSIL glass is correlated with its Raman spectra which is shown in Fig. 8.
The assignments of chemical bonds in the host matrix that produces the vibrational frequencies in the Raman spectrum are given in Table 3.
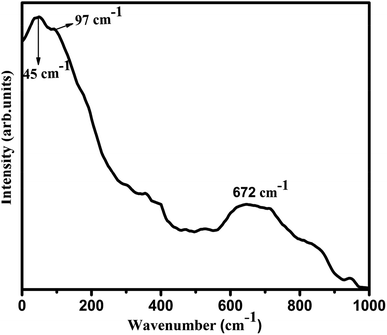
Thus it is evident from Raman spectrum that the most energetic vibrational mode which substantially contributes to the appearance of phonon sideband is at 672 cm−1 and is correlated with the stretching vibration of Ti–O bond of the host matrix. Furthermore, the presence of Ti–O bond in the Eu2 sample is confirmed in FTIR analysis and also the possibility of charge transfer from Ti–O bond to Eu3+ ions is confirmed. Marchese et al. have also reported similar photoluminescence emissions in grafted Ti-MCM41 silicas.41 Thus the features of the phonon side band of Eu2 glass is complementary with its Raman spectrum.
3.6 Judd–Ofelt (JO) analysis
The chemical surroundings of the site occupied by the Eu3+ ions in the host matrix has a strong effect on its emission intensity. Judd–Ofelt (JO) theory is a very efficient tool to study the variation in emission intensity of Eu3+ ions with its varying chemical environment in the host medium.4,27 Usually, the JO parameters are determined from the absorption spectrum using the least square fit method. However, in several cases of Eu3+ ions, there are difficulties to derive the JO parameters due to (i) thermal corrections42 (ii) zero magnitude of U(4) matrix element28 (iii) poor intensity of 7F0 → 5D2 level transition.43 Hence as an alternative method the JO intensity parameters can be calculated from the emission spectrum based on the procedure reported by Peng and Izumitani.44 The 5D0 → 7F1 transition is the magnetic dipole transition and hence its intensity is independent of the host matrix, whereas the 5D0 → 7F2, 5D0 → 7F4 and 5D0 → 7F6 transitions are electric dipole transitions and depends upon Ω2, Ω4 and Ω6 values respectively. Among these electric dipole transitions, 5D0 → 7F6 transition is weak and falls in the infrared region (∼800 nm). Hence this transition is not observed properly due to instrumental limitations. Thus the Ω6 intensity parameter is taken as zero for determining the radiative parameters of Eu3+ ion.3 The following equation is used for calculating the radiative intensity parameters45,46where ν1 and νJ are the wavenumbers in cm−1 for the 5D0 → 7F1 and 5D0 → 7FJ (J = 2, 4, 6) transitions respectively. Smd gives the magnetic dipole line strength of the 5D0 → 7F1 transition of Eu3+ ions and n is the refractive index of the glass and for Eu2 glass its value is 1.562. The calculated JO parameters of Eu2 sample are listed in Table 4 and are compared with previous works.
3.7 Radiative parameters
Radiative parameters such as total radiative transition probability (AT), radiative life time (τR) and branching ratios (βR) for the 5D0 excited state of Eu2 sample have been evaluated from the JO intensity parameters3,4 and the equations used for calculation are given in Section S2 of the ESI.† The results obtained are tabulated in Table 5. The branching ratio βR represents the amplification of emission and it is considered that the energy level transition having βR value greater than 0.50 is good for optical amplification.3 Here, the transition 5D0 → 7F2 (red emission) of the Eu2 sample has relatively high transition probability and branching ratio which confirms that the sample provides good optical amplification to the radiation emitted by the Eu3+ ions.| Sl no. | 5D0 → | Energy (cm−1) | Sed (×10−22) | Smd (×10−22) | Aed (s−1) | Amd (s−1) | A (s−1) | βR |
|---|---|---|---|---|---|---|---|---|
| a Total radiative transition probability, AT = 341.37 s−1; radiative lifetime, τR = 2.929 ms. | ||||||||
| 1 | 7F4 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 463 463 |
1.41 | 0.00 | 105.74 | 0.00 | 105.74 | 0.3098 |
| 2 | 7F3 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 433 433 |
0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.0000 |
| 3 | 7F2 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 283 283 |
1.70 | 0.00 | 181.37 | 0.00 | 181.37 | 0.5313 |
| 4 | 7F1 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943 943 |
0.00 | 0.40 | 0.00 | 54.26 | 54.26 | 0.1590 |
| 5 | 7F0 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 320 |
0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.0000 |
The experimental lifetime (τ) of Eu2 sample obtained by monitoring the 613 nm emission (λex = 394 nm) is 0.90 ms and the total calculated radiative life time (τR) is 2.929 ms. The difference in the lifetimes of the Eu2 sample may be due to the non-radiative decays which is not considered while calculating total radiative lifetime. The ratio between the experimental lifetime and calculated lifetime of the particular excited level gives the luminescence quantum efficiency (η).2 Thus the quantum efficiency of the Eu2 sample is estimated to be 30.7% with a relative accuracy of 86.7%. The quantum efficiency of the Eu2 sample is also compared with various similar host matrices and are summerized in Table 6.
Also, the effective band-width (Δλeff), experimental branching ratio (βexp), peak stimulated emission cross-sections (σe) and optical gain (ΔG) are estimated from the emission spectrum of Eu2 sample for the 5D0 → 7F1,2,4 transitions and are represented in Table 7. The branching ratio gives information about the possibility of attaining stimulated emission from any particular transition. Also, the value of the stimulated emission cross-section signifies the gain provided to the emission of the doped rare-earth by the host material. The gain-bandwidth (σe × Δλeff) and optical gain, ΔG (σe × τR) are also critical parameters which predicts the optical amplification of the host medium in which the rare-earth ions are embedded.48
| Sl no. | 5D0 → | Δλeff (nm) | σe (×10−22) cm2 | Gain-bandwidth (×10−28) cm3 | ΔG (×10−25) cm2 s | βexp |
|---|---|---|---|---|---|---|
| 1 | 7F4 | 13.463 | 3.190 | 4.300 | 9.350 | 0.3000 |
| 2 | 7F2 | 9.450 | 4.760 | 4.490 | 13.929 | 0.5380 |
| 3 | 7F1 | 8.645 | 1.320 | 1.140 | 3.858 | 0.1610 |
It is clear from Table 7 that the experimental branching ratio agrees well with the calculated values. Among the transitions, 5D0 → 7F2 has a relatively higher branching ratio of 53.8% and an emission cross-section of 4.76 × 10−22 cm2, which proposes that the Eu2 sample can be used for photonic applications. Also, its gain-bandwidth and optical gain values show that the sample can be employed as a good optical amplifier.
3.8 Colorimetric analysis
The color luminescence emission intensities of the samples has been evaluated with Commission International d' Eclairage (CIE) 1931 system and also the dominant emission wavelength of the sample has been identified for evaluating its performance. The CIE co-ordinates calculated are (0.142, 0.192) and (0.411, 0.220) respectively for Eu0 and Eu2 samples and are marked in Fig. 9.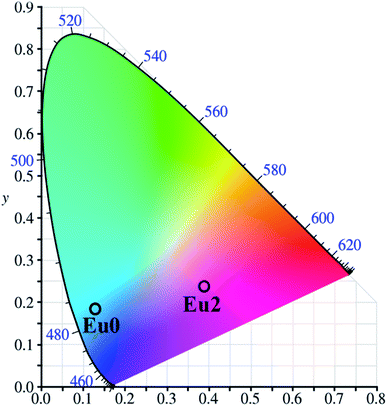 | ||
| Fig. 9 CIE chromaticity diagram of pure (Eu0) and 2 wt% Eu3+ doped SiO2–TiO2–PDMS ORMOSIL (Eu2) samples. | ||
4 Conclusion
XRD, FTIR and TG analyses of Eu3+ doped SiO2–TiO2–PDMS ORMOSILs confirmed that their structure remain unaltered with Eu3+ doping and these samples have appreciable thermal stability. The photoluminescence (PL) analysis by both direct excitation and CT band exhibits similar pattern and SiO2–TiO2–PDMS ORMOSIL doped with 2 wt% Eu3+ (Eu2) has maximum PL intensity and quenches above that concentration. The JO intensity parameters were determined for Eu2 sample and it was found that Ω2 > Ω4 which is an indication of the asymmetry in sites occupied by the Eu3+ ions in the host matrix. The phonon energy (ħω) associated with the non-radiative decay of the host matrix, obtained by Raman analysis (672 cm−1) agrees well with that determined by PSB analysis (670 cm−1). The radiative parameters of 5D0 → 7F1,2,4 transitions of the Eu2 sample were predicted using JO intensity parameters and the values imply that the Eu2 sample can be used for optical amplification applications. The decay lifetime of the Eu2 sample has been obtained as 0.9 ms which is fairly greater than that of various similar host matrices. The resultant pinkish red emission of the sample having a quantum efficiency of 30.7% confirmed that it can be effectively used in the WLED (RGB) fabrication.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to the University Grants Commission, Govt. of India and to the Department of Science and Technology, Govt. of India for the support in establishing experimental facilities at the School of Pure and Applied Physics, Mahatma Gandhi University, Kottayam, India through SAP-DRS programme No. F.530/12/DRS/2009 (SAP-1) and DST-PURSE programme No.SR/417&418/2017 respectively.References
- S. K. Gupta, M. Sahu, K. Krishnan, M. K. Saxena, V. Natarajan and S. V. Godbole, Bluish White Emitting Sr2CeO4 and Red Emitting Sr2CeO4:Eu3+ Nanoparticles: Optimization of Synthesis Parameters, Characterization, Energy Transfer and Photoluminescence, J. Mater. Chem. C, 2013, 1(42), 7054–7063 RSC.
- K. Binnemans, Interpretation of europium(III) spectra, Coord. Chem. Rev., 2015, 295, 1–45, DOI:10.1016/j.ccr.2015.02.015.
- M. S. Sajna, S. Gopi, V. P. Prakashan, M. S. Sanu, C. Joseph and P. R. Biju, et al., Spectroscopic Investigations and Phonon Side Band Analysis of Eu3+-Doped Multicomponent Tellurite Glasses, Opt. Mater., 2017, 70, 31–40, DOI:10.1016/j.optmat.2017.04.064.
- V. P. Prakashan, M. S. Sajna, G. Gejo, M. S. Sanu, P. R. Biju and J. Cyriac, et al., Perceiving Impressive Optical Properties of Ternary SiO2–TiO2–ZrO2:Eu3+ Sol–Gel Glasses with High Reluctance for Concentration Quenching: An Experimental Approach, J. Non-Cryst. Solids, 2018, 482, 116–125 CrossRef CAS.
- J. Reyes, D. Y. Medina, M. Aguilar, M. A. Barron, E. Garfias and A. d. J. Morales, Red, White and Blue Light Emission from Europium Doped Al2O3 Confined into a Silica Matrix, Open J. Appl. Sci., 2018, 08(08), 338–345 CrossRef CAS.
- M. Chang, Y. Song, Y. Sheng, J. Chen and H. Zou, Understanding the Remarkable Luminescence Enhancement: Via SiO2 Coating on TiO2:Eu3+ Nanofibers, Phys. Chem. Chem. Phys., 2017, 19(26), 17063–17074 RSC.
- B. Julián, R. Corberán, E. Cordoncillo, P. Escribano, B. Viana and C. Sanchez, Synthesis and Optical Properties of Eu3+-Doped Inorganic–Organic Hybrid Materials Based on Siloxane Networks, J. Mater. Chem., 2004, 14(22), 3337–3343 RSC.
- L. D. Carlos, Y. Messaddeq, H. F. Brito, R. A. Sá Ferreira, V. De Zea Bermudez and S. J. L. Ribeiro, Full-Color Phosphors from Europium(III)-Based Organosilicates, Adv. Mater., 2000, 12(8), 594–598 CrossRef CAS.
- F. Del Monte, P. Cheben, C. P. Grover and J. D. Mackenzie, Preparation and Optical Characterization of Thick-Film Zirconia and Titania Ormosils, J. Sol-Gel Sci. Technol., 1999, 15(1), 73–85 CrossRef CAS.
- H. Schmidt and W. Herbert, Organically Modified Ceramics And Their Applications, J. Non-Cryst. Solids, 1990, 121, 428–435 CrossRef CAS.
- K. Chakrabarti and C. M. Whang, Silver Doped ORMOSIL – An Investigation on Structural and Physical Properties, Mater. Sci. Eng., B, 2002, 88(1), 26–34 CrossRef.
- J. Wen and G. L. Wilkes, Organic/Inorganic Hybrid Network Materials by the Sol–Gel Approach, Chem. Mater., 1996, 8(8), 1667–1681 CrossRef CAS.
- J. N. Hay and H. M. Raval, Synthesis of Organic–Inorganic Hybrids via the Non-hydrolytic Sol–Gel Process, Chem. Mater., 2001, 13, 3396–3403 CrossRef CAS.
- R. J. M. Gopinath, V. Vidyadharan, S. M. Simon, A. C. Saritha, P. R. Biju and C. Joseph, et al., Investigations on the Blue Luminescence Enhancement of Organically Modified SiO2–TiO2–PDMS Glass Matrix, Nano-Struct. Nano-Objects, 2019, 20, 100377 CrossRef , available from: https://linkinghub.elsevier.com/retrieve/pii/S2352507X19302999.
- V. Vidyadharan, P. Vasudevan, S. Karthika, C. Joseph, N. V. Unnikrishnan and P. R. Biju, Structural , Optical and AC Electrical Properties of Ce3+-Doped TiO2–SiO2 Matrices, J. Electron. Mater., 2015, 44(8), 2754–2761 CrossRef CAS.
- M. Shwetha and B. Eraiah, Influence of Europium (Eu3+) Ions on the Optical Properties of Lithium Zinc Phosphate Glasses, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 310, 1–7 Search PubMed.
- L. Tellez, J. Rubio, F. Rubio, E. Morales and J. L. Oteo, Synthesis of Inorganic-Organic Hybrid Materials from TEOS, TBT and PDMS, J. Mater. Sci., 2003, 38(8), 1773–1780 CrossRef CAS.
- X. Zhang, H. Ye, B. Xiao, L. Yan, H. Lv and B. Jiang, Sol–Gel Preparation of PDMS/Silica Hybrid Antireflective Coatings with Controlled Thickness and Durable Antireflective Performance, J. Phys. Chem. C, 2010, 114(47), 19979–19983 CrossRef CAS.
- J. Lin and K. Baerner, Tunable Photoluminescence in Sol–Gel Derived Silica Xerogels, Mater. Lett., 2000, 46(2–3), 86–92 CrossRef CAS.
- J. D. Mackenzie, Q. Huang and T. Iwamoto, Mechanical Properties of Ormosils, J. Sol-Gel Sci. Technol., 1996, 7(3), 151–161 CrossRef CAS.
- C. F. Song, M. K. Lü, P. Yang, F. Gu, D. Xu and D. R. Yuan, A Potential Blue Photoluminescence Material: ZrO2–SiO2 Glasses, Mater. Sci. Eng., B, 2002, 94(2–3), 181–185 CrossRef.
- J. J. Velázquez, J. Mosa, G. Gorni, R. Balda, J. Fernandez, L. Pascual, A. Dwan and Y. Castro, Transparent SiO2-GdF3 Sol–Gel Nano-Glass Ceramics for Optical Applications, J. Sol-Gel Sci. Technol., 2019, 89, 322–332 CrossRef.
- L. Zhang, Y. Yao, X. Ye and Q. Wu, Luminescence Behavior of Eu3+ in Polytitanasiloxane Solution, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 1357–1363 CrossRef CAS.
- S. Okuzaki, K. Okude and T. Ohishi, Photoluminescence Behavior of SiO2 Prepared by Sol–Gel Processing, J. Non-Cryst. Solids, 2000, 265(1), 61–67 CrossRef CAS.
- A. Sarkar and G. Gopal Khan, The formation and detection techniques of oxygen vacancies in Titanium oxide-based Nano-structures, Nanoscale, 2019, 1–32, 10.1039/C8NR09666J.
- F. A. De Jesus, B. V. Santana, J. M. A. Caiut and V. H. V. Sarmento, Local Coordination, Influence on Synthesis and Luminescent Features of Eu3+ Ions in SiO2-Poly(methyl methacrylate) Hybrid Materials, Ind. Eng. Chem. Res., 2018, 57(11), 3941–3949 CrossRef.
- S. Kasturi and V. Sivakumar, Luminescence Properties of La2W2-xMoxO9 (x = 0–2): Eu3+ Materials and Their Judd–Ofelt Analysis: Novel Red Line Emitting Phosphors for pcLEDs, Mater. Chem. Front., 2017, 1(3), 550–561 RSC.
- S. Selvi, K. Marimuthu, N. Suriya Murthy and G. Muralidharan, Red Light Generation Through the Lead Boro-telluro-phosphate Glasses Activated by Eu3+ Ions, J. Mol. Struct., 2016, 1119, 276–285, DOI:10.1016/j.molstruc.2016.04.073.
- H. You and M. Nogami, Optical Properties and Local Structure of Eu3+ Ions in Sol–Gel TiO2–SiO2 Glasses, J. Phys. Chem. B, 2004, 108(32), 12003–12008 CrossRef CAS.
- Y. Tian, B. Chen, R. Hua, J. Sun, L. Cheng and H. Zhong, et al., Optical Transition, Electron-Phonon Coupling and Fluorescent Quenching of La2(MoO4)3:Eu3+ phosphor, J. Appl. Phys., 2011, 109(5), 1–7 CrossRef.
- M. A. Bizeto, V. R. L. Constantino and H. F. Brito, Luminescence properties of the layered niobate KCa2Nb3O10 doped with Eu3+ and La3+ ions, J. Alloys Compd., 2000, 311, 159–168 CrossRef CAS.
- B. B. J. Basu and N. Vasantharajan, Temperature Dependence of the Luminescence Lifetime of a Europium Complex Immobilized in Different Polymer Matrices, J. Lumin., 2008, 128(10), 1701–1708 CrossRef CAS.
- J. M. M. Buarque, D. Manzani, S. L. Scarpari, M. Nalin, S. J. L. Ribeiro and J. Esbenshade, et al., SiO2–TiO2 doped with Er3+/Yb3+/Eu3+ Photoluminescent Material: A Spectroscopy and Structural Study about Potential Application for Improvement of the Efficiency on Solar Cells, Mater. Res. Bull., 2018, 107, 295–307, DOI:10.1016/j.materresbull.2018.07.007.
- B. Julian, H. Beltrán, E. Cordoncillo, P. Escribano, B. Viana and C. Sanchez, Influence of the Matrix in the Optical Response of Organic-Inorganic Hybrid Materials Doped with Europium(III), J. Sol-Gel Sci. Technol., 2003, 26(1–3), 977–980 CrossRef CAS.
- S. K. Jose, S. Gopi, S. M. Simon, P. R. Mohan, C. Joseph and N. V. Unnikrishnan, et al., Structural and Optical Characterization of Eu3+ Doped Polymer-Zirconia Composites, J. Non-Cryst. Solids, 2016, 452, 245–252, DOI:10.1016/j.jnoncrysol.2016.08.041.
- L. Skuja, Optically Active Oxygen-Deficiency-Related Centers in Amorphous Silicon dioxide, J. Non-Cryst. Solids, 1998, 239, 16–48 CrossRef CAS.
- T. Miyakawa and D. L. Phonon Sidebands, Multiphonon relaxation of Excited states, and phonon – assisted energy transfer between ions in solids, Phys. Rev. B: Solid State, 1970, 1(7), 2961–2969 CrossRef.
- J. Dhanalakshmi, S. Iyyapushpam, S. T. Nishanthi, M. Malligavathy and D. P. Padiyan, Investigation of Oxygen Vacancies in Ce Coupled TiO2 Nanocomposites by Raman and PL Spectra, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2017, 8(1), aa5984, DOI:10.1088/2043-6254/aa5984.
- M. F. Best and R. A. Condrate, A Raman study of TiO2–SiO2 glasses prepared by sol–gel processes, J. Mater. Sci. Lett., 1985, 4, 994–998 CrossRef CAS.
- B. Karmakar, Glass Nanocomposites, Elsevier Inc., 2016, pp. 3–53, DOI:10.1016/B978-0-323-39309-6.00001-8.
- L. Marchese, E. Gianotti, V. Dellarocca, T. Maschmeyer, F. Rey and J. M. Thomas, et al., Structure – functionality relationships of grafted Ti-MCM41 silicas. Spectroscopic and catalytic studies, Phys. Chem. Chem. Phys., 1999, 1, 585–592 RSC.
- B. Deva Prasad Raju and C. Madhukar Reddy, Structural and Optical Investigations of Eu3+ Ions in Lead Containing Alkali Fluoroborate Glasses, Opt. Mater., 2012, 34(8), 1251–1260, DOI:10.1016/j.optmat.2012.01.027.
- W. Stambouli, H. Elhouichet, B. Gelloz and M. Férid, Optical and Spectroscopic Properties of Eu-Doped Tellurite Glasses and Glass Ceramics, J. Lumin., 2013, 138, 201–208 CrossRef CAS.
- B. Peng and T. Izumitani, The Fluorescence Properties of Eu3+ in Various Glasses and the Energy Transfer Between Eu3+ and Sm3+ in Borosilico-phosphate Glass, Rev. Laser Eng., 1994, 22(1), 16–27 CrossRef CAS.
- B. R. Judd, Optical Absorption Intensities of Rare-Earth Ions, Phys. Rev., 1962, 127(3), 750–761, DOI:10.1103/PhysRev.127.750.
- G. S. Ofelt, Intensities of Crystal Spectra of Rare-Earth Ions, J. Chem. Phys., 1962, 37(3), 511–520 CrossRef CAS.
- Y. Zheng, Y. Chen, C. Yang, Q. Wang, J. Lin and L. Zhang, Design of Europium doped SiO2–TiO2 Hybrids as Novel Luminescent Photocatalyst, J. Lumin., 2012, 132(7), 1639–1641 CrossRef CAS.
- C. Manjunath, M. S. Rudresha, B. M. Walsh, R. Hari Krishna, B. S. Panigrahi and B. M. Nagabhushana, Optical Absorption Intensity Analysis Using Judd–Ofelt Theory and Photoluminescence Investigation of Orange-Red Sr2SiO4:Sm3+ Nanopigments, Dyes Pigm., 2018, 148, 118–129 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra03073b |
| This journal is © The Royal Society of Chemistry 2020 |