Open Access Article
Open Access ArticleHierarchical porous LiNi1/3Co1/3Mn1/3O2 with yolk–shell-like architecture as stable cathode material for lithium-ion batteries†
Zhen Chen ab,
Dongliang Chao
ab,
Dongliang Chao b,
Minghua Chen
b,
Minghua Chen *a and
Zexiang Shen
*a and
Zexiang Shen *b
*b
aKey Laboratory of Engineering Dielectric and Applications (Ministry of Education), Harbin University of Science and Technology, Harbin 150080, P. R. China. E-mail: mhchen@hrbust.edu.cn
bDivision of Physics and Applied Physics, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, 637371, Singapore. E-mail: zexiang@ntu.edu.sg
First published on 18th May 2020
Abstract
The relatively sluggish lithium ion diffusion of LiNi1/3Co1/3Mn1/3O2 (NCM) is one of the fatal factors which can significantly prevent its widespread usage in high-power applications. In this work, the monodispersed hierarchical porous yolk–shell-like LiNi1/3Co1/3Mn1/3O2 (YS-NCM) with exposure to {010} electrochemical active facets was successfully synthesized, aiming to elevate the lithium ion diffusion ability and thus to enhance the electrochemical performance. The hierarchical porous nano-/microsphere morphology as well as the voids between the yolk and the shell allow for shortened Li+ diffusion pathways, leading to improved Li+ diffusion capability. These voids are also beneficial for providing more buffers for the volume changes during repeated charge and discharge. Additionally, the exposure of {010} electrochemical active facets provides more open structure for unimpeded Li+ migration. Therefore, by this design strategy, the lithium ion transport kinetics is greatly improved, yielding superior electrochemical performances. When examined as the cathode material for lithium-ion batteries (LIBs), the YS-NCM-based cells have achieved superior rate capability and stable cycling performance, rendering it as a promising cathode candidate for practical lithium-ion battery applications.
1. Introduction
Over the past decades, impelled by the exhaustion of fossil fuels and the increasingly prominent environmental problems, the significance of developing efficient and green energy storage devices has grown tremendously.1,2 Lithium-ion batteries (LIBs) featuring high energy density, long cycle life, superior safety and low cost have successfully conquered the market of portable consumer devices, and have been drawing tremendous attention for large-scale applications such as electric vehicles (EVs) and hybrid electric vehicles (HEVs).3–6 As a derivative of LiCoO2, the symmetrical ternary layered LiNi1/3Co1/3Mn1/3O2 (NCM), which exhibits much higher deliverable capacity with enhanced safety, was firstly reported by Ohzuku and Makimura in 2001.7–9 This finding later has evoked enormous academic and industrial interest in seeking derivatives with a combination of higher energy density, lower cost, better thermal and structural stability and improved safety properties.7,8,10–16 However, these targets are difficult to meet at the same time. In fact, recently more and more interest has been focusing on increasing the nickel content (i.e., Ni-rich NCM), in order to achieve higher capacity and energy density with less cost. Nonetheless, this usually is realized at the cost of not only reduced chemical and structural stability and thus shortened cycling lifetime, but moreover reduced thermal stability and safety as well. These are primarily attributed to (1) more severe Li+/Ni2+ cation mixing that can trigger phase transitions; (2) the formation of surface lithium-containing residuals (e.g., LiOH, Li2CO3) that can not only build accumulative resistance hindering the charge transfer at the interface of electrode/electrolyte but also generate gas (O2, CO, CO2) during cycling; (3) the formation of oxygen species due to the reactions of highly reactive Ni4+ at delithiation status with electrolyte, concomitantly with structural evolutions.17–19 By far, LiNi1/3Co1/3Mn1/3O2 which shows advantages of better structural and thermal stability and more stable cycling stability over those Ni-rich NCM cathode materials, has been deemed as the most ideal cathode material because of its overall modest performance.20 However, drawbacks including the fatal capacity degradation in terms of long cycles and inferior rate capability especially at high C-rates as a result of sluggish lithium ions diffusion (∼10−11 cm2 s−1)21,22 have impeded its wide spread usage in high-power applicants.Targeting to solve this issue, it is essentially important to improve the diffusivity of lithium ions to achieve desirable electrochemical performance, in particularly high rate capability.22 Tremendous efforts have been devoted to addressing this issue, among which the morphology design of hierarchical nano-/micro-architecture of NCM has been revealed as an effective solution.23–28 In our previous study,25 we have already demonstrated that the diffusion length for Li+ is effectively reduced through synthesizing hierarchical porous LiNi1/3Co1/3Mn1/3O2 with nano-/micro-architecture, in which the nano-sized primary particles contribute to excellent rate capacity while the micro-sized secondary particles stabilize the structure to ensure a good cycle life. The voids between the primary particles facilitate the penetration of electrolyte and thus allow for enhanced lithium ions diffusivity. Furthermore, the micro-sized secondary particles can increase the initial coulombic efficiency and are more suitable for commercial fabrication.15
Herein, we report an effective and efficient strategy to further boost the Li+ diffusion ability via the synthesis of hierarchical porous LiNi1/3Co1/3Mn1/3O2 nano-/microspheres with yolk–shell-like architecture (hereafter denoted as YS-NCM). Besides of the above-mentioned advantages of the hierarchical nano-/micro-structure, the yolk–shell-like architecture of YS-NCM we designed here brings some additional benefits. The voids between the yolk and the shell can not only provide more buffers for the volume changes during repeated charge and discharge, but moreover enable more electrolyte penetration to further boost the Li+ diffusions. Thereby, when revealed as the cathode material for lithium-ion batteries, the YS-NCM-based cells achieve superior capacity retention and excellent rate capability. Specifically, the capacity retention ratios as high as 85.99% (0.1C), 91.08% (1C) and 93.23% (2C) after 100 cycles have been achieved, respectively. In terms of the rate capability, the YS-NCM-based cells deliver discharge capacities of 145.93, 126.01, 109.58, 93.61, 79.16, 69.22 and 64.50 mA h g−1 at 1, 2, 5, 10, 15, 20 and 30C respectively. The design strategy presented in our work is expected to provide a useful idea for the synthesis of cathode materials with high long-term cycling stability and in particularly with high rate capability for lithium-ion batteries.
2. Experimental section
2.1 Preparation of Ni1/3Co1/3Mn1/3CO3 precursor
Firstly, stoichiometric amounts of Ni(Ac)2·4H2O, Co(Ac)2·4H2O and Mn(Ac)2·4H2O were dissolved in ethylene glycol (40 mL) under rigorous stirring until a transparent solution was obtained. Then, 2.37 g of (NH4)HCO3 was added into the above solution which was kept under stirring till the salt was completely dissolved. In a next step, the mixed transparent solution was transferred into a Teflon-lined stainless steel autoclave with a volume of 50 mL, and then sealed tightly before transferred to an oven, in which the autoclave was quickly heated up to 200 °C and kept at such temperature for 20 h. The precursor product was collected and washed by centrifugation using deionized water (DIW) and absolute ethanol until the pH of the supernatant became neutral. The as-collected Ni1/3Co1/3Mn1/3CO3 was then dried in a vacuum oven at 80 °C overnight.2.2 Preparation of LiNi1/3Co1/3Mn1/3O2 (YS-NCM)
The as-prepared Ni1/3Co1/3Mn1/3CO3 precursor was dispersed in ethanol (40 mL), together with LiOH·H2O (6% excess to compensate the lithium evaporation during calcination and suppress the Li+/Ni2+ cation mixing). The suspension was kept at 60 °C under continuous stirring overnight to get completely dried. The pre-dried powder was then collected and calcined in a tube furnace at 850 °C for 10 h in air atmosphere.2.3 Material characterizations
X-ray diffraction (XRD) patterns were recorded using Bruker D8 with Cu Kα radiation (λ = 0.15406 nm). The thermal Brunauer–Emmett–Teller (BET) surface area and porosity were determined by nitrogen-sorption using a Micromeritics ASAP 2020 analyzer. Field-emission scanning electron microscopy (FESEM, JSM-7600F, JEOL) was used to investigate the morphology of both Ni1/3Co1/3Mn1/3CO3 and YS-NCM samples. Energy dispersive X-ray spectroscopy (EDS) (Oxford INCA, England) was used to characterize the elemental compositions of the YS-NCM sample. High resolution transmission electron microscope (HRTEM, JEOL JEM-2010F, 200 kV) was used to investigate the nanostructure of the Ni1/3Co1/3Mn1/3CO3 and YS-NCM samples.2.4 Electrochemical measurements
All the electrochemical performance tests were carried out in CR2032 coin cells assembled in an argon-filled glove box (O2 < 0.1 ppm, H2O < 0.1 ppm) using metallic lithium as the counter electrode, Celgard 2400 films as the separator and 1 M LiPF6 dissolved in ethyl carbonate–dimethyl carbonate (EC–DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as the electrolyte. The electrodes were prepared by casting slurries (80 wt% of YS-NCM, 10 wt% of carbon black and 10 wt% of poly-(vinylidene fluoride) (PVDF) binder dissolved in N-methyl-2-pyrrolidone (NMP)) on aluminum foils using doctor blade technique. The wet electrodes were first pre-dried in an oven (60 °C, 1 h) and then vacuum dried in a vacuum chamber at 100 °C overnight. The average active material mass loading density of working electrodes is ∼2.0 ± 0.2 mg cm−2. All the electrochemical performance tests were conducted on a Neware battery tester. The cells were cycled in CCCV mode, and the termination condition of the constant voltage (CV) charging mode is the decreased current flow to one tenth of the given applied current. Cyclic voltammetry (CV) curves (five cycles at a scan rate of 0.1 mV s−1) as well as the subsequent CV curves measured with increasing scan rates spanning from 0.1 mV s−1 to 1.5 mV s−1 were conducted on a CHI750d electrochemical workstation between the potential range of 2.5 and 4.5 V (vs. Li/Li+). Electrochemical impedance spectroscopy (EIS) measurements were performed in two-electrode coin cells at room temperature with CHI760d electrochemical workstation over the frequency range between 0.1 Hz and 100 kHz.
1 v/v) as the electrolyte. The electrodes were prepared by casting slurries (80 wt% of YS-NCM, 10 wt% of carbon black and 10 wt% of poly-(vinylidene fluoride) (PVDF) binder dissolved in N-methyl-2-pyrrolidone (NMP)) on aluminum foils using doctor blade technique. The wet electrodes were first pre-dried in an oven (60 °C, 1 h) and then vacuum dried in a vacuum chamber at 100 °C overnight. The average active material mass loading density of working electrodes is ∼2.0 ± 0.2 mg cm−2. All the electrochemical performance tests were conducted on a Neware battery tester. The cells were cycled in CCCV mode, and the termination condition of the constant voltage (CV) charging mode is the decreased current flow to one tenth of the given applied current. Cyclic voltammetry (CV) curves (five cycles at a scan rate of 0.1 mV s−1) as well as the subsequent CV curves measured with increasing scan rates spanning from 0.1 mV s−1 to 1.5 mV s−1 were conducted on a CHI750d electrochemical workstation between the potential range of 2.5 and 4.5 V (vs. Li/Li+). Electrochemical impedance spectroscopy (EIS) measurements were performed in two-electrode coin cells at room temperature with CHI760d electrochemical workstation over the frequency range between 0.1 Hz and 100 kHz.
3. Results and discussion
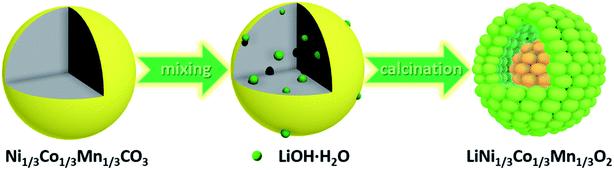
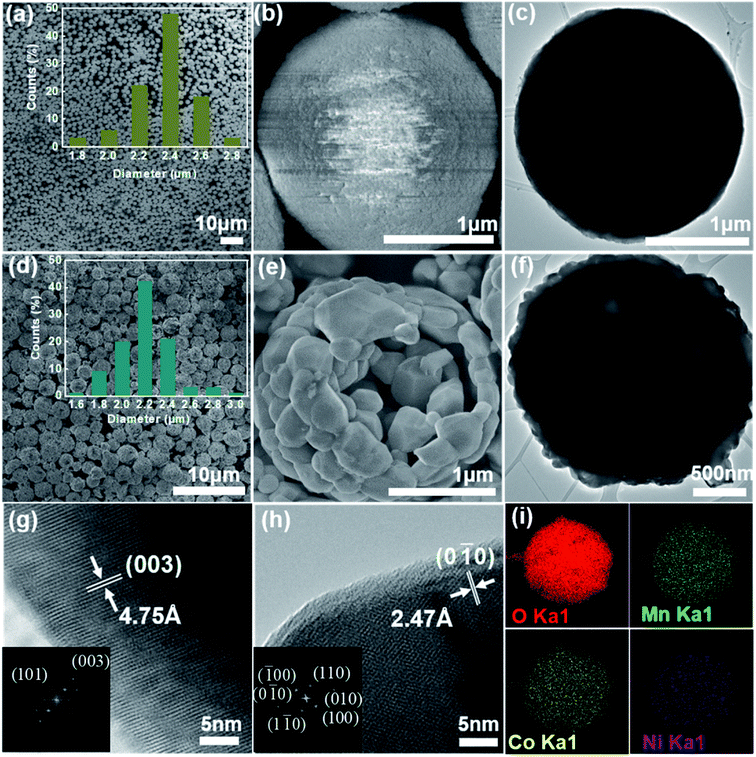
The synthesis route of the hierarchical porous yolk–shell-like LiNi1/3Co1/3Mn1/3O2 (YS-NCM) is illustrated in Fig. 1. The Ni1/3Co1/3Mn1/3CO3 microspheres, which were obtained via a hydrothermal method, serve as both the self-template and the precursor. After mixing homogeneously with LiOH·H2O, the powder mixture was then calcined in a tube furnace (850 °C for 10 h) to obtain the final product (YS-NCM).The morphology of the Ni1/3Co1/3Mn1/3CO3 precursor at different magnifications is characterized by field-emission scanning electron microscopy (FESEM, Fig. 2a and b) and transmission electron microscopy (TEM, Fig. 2c). The monodisperse feature of the precursor with spherical morphology is clearly revealed from the panoramic view (Fig. 2a). The average diameter of Ni1/3Co1/3Mn1/3CO3 microspheres is around 2.4 μm according to the statistical datum of one hundred particles (inset in Fig. 2a). The Ni1/3Co1/3Mn1/3CO3 microspheres are porous with a specific surface area of 73.24 m2 g−1 (see ESI, inset in Fig. S1a†), determined by the nitrogen adsorption–desorption isotherm. According to the pore size distribution plot, the average pore size is ∼2 nm. The monodispersed porous Ni1/3Co1/3Mn1/3CO3 particles are favorable for the homogeneous mixing with Li+ source and thus allow for a uniform solid state reaction during the subsequent calcination step.
From the panoramic view (Fig. 2d), it is obvious to see that the YS-NCM perfectly inherits the microspheres with rather intact morphology after calcination. The diameter of YS-NCM secondary particles, which are composed of many nano-sized primary particles, is around 2.2 μm (inset in Fig. 2d), slightly smaller than that of the precursor which can be due to the densification during calcination process. From our BET results (Fig. S1b†), the specific surface area of YS-NCM is 19.19 m2 g−1, which is at least comparable and even higher than those hierarchical porous structured LiNi1/3Co1/3Mn1/3O2.29–31 According to the pore size distribution plot, two types of pore sized are observed, which are ∼1.2 nm and ∼2.5 nm, that could be resulted from the different densification level of the yolk and the shell parts during calcination step. A closer look of one representative broken microsphere of YS-NCM shown in Fig. 2e reveals the yolk–shell-like structure of YS-NCM with hierarchical nano-/micro-architecture. The thickness of the shell of YS-NCM is about 200 nm. Such a yolk–shell-like structure is further verified by a TEM image shown in Fig. 2f, in which the dark regions correspond to the yolk and shell parts with high thickness that blocks the transmission of electrons whereas the bright regions between the yolk and the shell correspond to the empty space. The formation of yolk–shell-like structure can be considered as a result of heterogeneous contraction caused by non-equilibrium heating.32,33 Initially, a dense rigid shell is formed due to the large temperature gradient (ΔT) along the radical direction of the particles.34 Between the dense shell and inner core there exist two forces, i.e. the cohesive force (σco) and the adhesive force (σad) with opposite directions. The σad force resists the inward shrinkage of the inner core while the σco causes the inner core shrinks inwards with occurrence of mass loss during heating. When σco > σad, the inner core contracts inward and detaches from the dense shell, forming the yolk–shell-like structure eventually.
The high resolution TEM images and the corresponding fast Fourier transform (FFT) patterns (inset images) of the lateral plane and frontal plane of YS-NCM are shown in Fig. 2g and h, where two sets of clear lattice fringes are observed. One lattice fringe with an inter-planar spacing of 4.75 Å depicted in Fig. 2g corresponds to the (003) crystal plane of NCM, which indicates that the lateral plane is (0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) plane belonging to {010} active facet which provides unimpeded pathway for Li+ insertion and extraction.22,35 The other lattice fringe (Fig. 2h) can be assigned to (0
0) plane belonging to {010} active facet which provides unimpeded pathway for Li+ insertion and extraction.22,35 The other lattice fringe (Fig. 2h) can be assigned to (0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) plane with a lattice spacing of 2.47 Å. The FFT patterns indicate the single crystallinity of YS-NCM with a hexagonal symmetry, that is in consistence with a typical hexagonal structure of NCM. The energy dispersive X-ray spectroscopy (EDS) mapping analysis was performed (Fig. 2i), showing the homogeneous elemental distributions of O, Mn, Co and Ni of YS-NCM. The molar ratio of Ni, Co and Mn is measured to be 1
0) plane with a lattice spacing of 2.47 Å. The FFT patterns indicate the single crystallinity of YS-NCM with a hexagonal symmetry, that is in consistence with a typical hexagonal structure of NCM. The energy dispersive X-ray spectroscopy (EDS) mapping analysis was performed (Fig. 2i), showing the homogeneous elemental distributions of O, Mn, Co and Ni of YS-NCM. The molar ratio of Ni, Co and Mn is measured to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S2†), which is in a good agreement with the theoretical ratio.
1 (Fig. S2†), which is in a good agreement with the theoretical ratio.
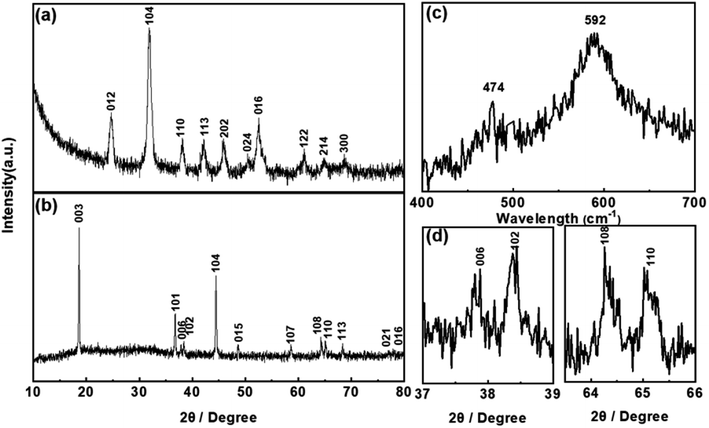
The structural transformation process, i.e. from the precursor (Ni1/3Co1/3Mn1/3CO3) to final product (LiNi1/3Co1/3Mn1/3O2), is studied by X-ray power diffraction (XRD). The diffraction peaks presented in Fig. 3a can be indexed to a typical hexagonal structure with a space group of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) C corresponding to MnCO3 (JCPDS no. 44-1472).36 The diffraction pattern of YS-NCM displayed in Fig. 3b is well assigned to the α-NaFeO2 structure (R
C corresponding to MnCO3 (JCPDS no. 44-1472).36 The diffraction pattern of YS-NCM displayed in Fig. 3b is well assigned to the α-NaFeO2 structure (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group) without any impurity phase. Highly ordered hexagonal layered structure is discerned by the sharp reflections in the diffraction patterns with clear separations of the (006)/(102) and the (108)/(110) doublets (Fig. 3d). The peak intensity ratio of the (003) and (104) peaks is calculated to be 1.58 (>1.2), indicating a low Li+/Ni2+ cation mixing. To further study the structure of YS-NCM, Raman spectroscopy, which is known to be quite sensitive in differentiating various structures of different atomic orderings, was carried out.37,38 The Raman spectra of YS-NCM (Fig. 3c) show a broad band comprising of two bands at 474 and 592 cm−1, assigning to the Eg (M–O–M bending) and the A1g (M–O stretching) vibrations within a hexagonal lattice belonging to the same space symmetry group (R
m space group) without any impurity phase. Highly ordered hexagonal layered structure is discerned by the sharp reflections in the diffraction patterns with clear separations of the (006)/(102) and the (108)/(110) doublets (Fig. 3d). The peak intensity ratio of the (003) and (104) peaks is calculated to be 1.58 (>1.2), indicating a low Li+/Ni2+ cation mixing. To further study the structure of YS-NCM, Raman spectroscopy, which is known to be quite sensitive in differentiating various structures of different atomic orderings, was carried out.37,38 The Raman spectra of YS-NCM (Fig. 3c) show a broad band comprising of two bands at 474 and 592 cm−1, assigning to the Eg (M–O–M bending) and the A1g (M–O stretching) vibrations within a hexagonal lattice belonging to the same space symmetry group (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) respectively.39–42
m) respectively.39–42
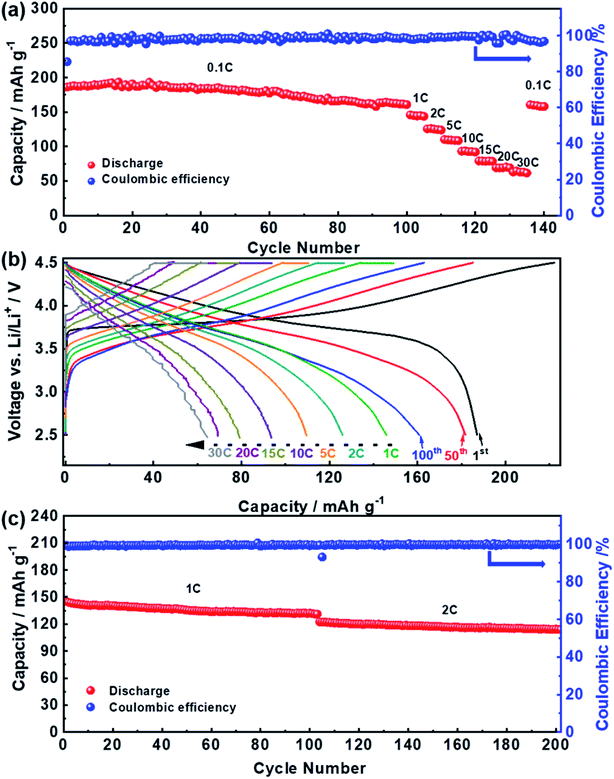
The electrochemical performance of the YS-NCM-based cells is demonstrated in Fig. 4. A fresh cell was firstly subjected to a long-term cycling at 0.1C for 100 cycles, followed by a rate capability test at various C-rates (1C–30C, 1C = 200 mA g−1, voltage range: 2.5–4.5 V). The initial charge and discharge capacities at 0.1C are 222.31 and 187.12 mA h g−1 respectively, yielding an initial coulombic efficiency of 84.17% (Fig. 4a). The cell can still deliver a capacity of 181.84 mA h g−1 after 50 cycles (97.18%) and 160.90 mA h g−1 after 100 cycles (85.99%). When subjecting to subsequent rate performance test at various C-rates (Fig. 4a), the YS-NCM-based cell can still retain reversible capacities of, ca., 145.93 (1C), 126.01 (2C), 109.58 (5C), 93.61 (10C), 79.16 (15C), 69.22 (20C) and 64.50 (30C) mA h g−1, respectively. Fig. 4b depicts some selected charge/discharge profiles of YS-NCM-based cell from Fig. 4a. It is notable that the initial charge/discharge profiles at a low C-rate of 0.1C are almost ideally symmetric, implying that kinetic difference upon charging and discharging is tiny. However, along with further consecutive cycling, these profiles become less symmetric, indicating higher polarization accumulates at discharge process. The long-term cycling performance at higher C-rates is revealed in Fig. 4c (after three formation cycles at 0.1C). The YS-NCM-based cells retain capacities of 132.13 mA h g−1 (1C) after 100 cycles and 114.11 mA h g−1 after another 100 cycles (2C), achieving capacity retention ratios of 91.08% (1C) and 93.23% (2C), respectively. All these results indicate that our YS-NCM-based cells exhibit superior long-term cycling stability and excellent high C-rates capability, which are at least comparable to or even better than the literature (see Table S1†). This is primarily owing to our design strategy that the hierarchical porous LiNi1/3Co1/3Mn1/3O2 nano-/microspheres with yolk–shell-like architecture and with exposed {010} active facets improve the Li+ diffusion ability and meanwhile maintain the structure by providing sufficient buffers for volume changes upon charging/discharging.
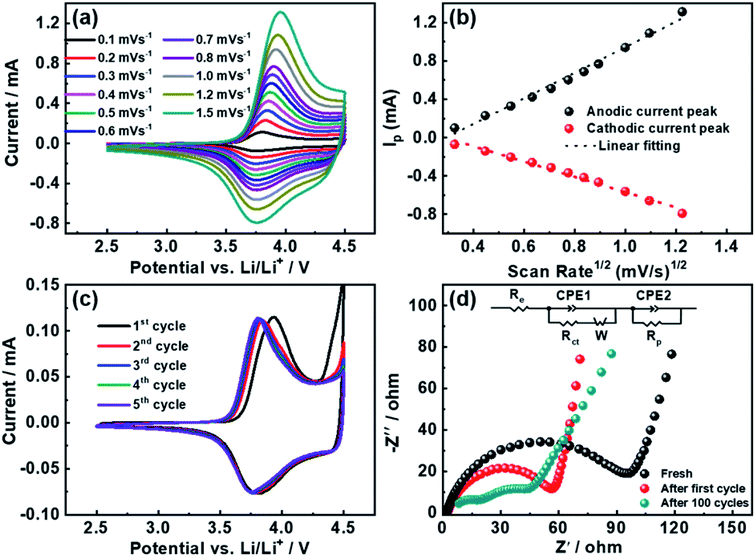
To investigate the lithium lithiation/delithiation and charge-transfer kinetics, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements were performed as shown in Fig. 5. Fig. 5a depicts the cyclic voltammetry at increasing scan rates from 0.1 to 1.5 mV s−1. According to Randles–Sevcik equation, the determination of lithium ion apparent diffusion coefficient can be achieved based on the plot of the peak intensity versus the square root of scan rates (derived from Fig. 5a).43 The lithium ion diffusion coefficients are calculated to be 4.5 × 10−9 and 1.6 × 10−9 cm2 s−1 for delithiation and lithiation processes respectively, which is within the range of reported values.16,25,30,44
Fig. 5c shows the first five CV curves of YS-NCM swept in the voltage range of 2.5–4.5 V at 0.1 mV s−1. A typical pair of redox peaks for NCM system was evidenced between 3.7 and 4.0 V with very small potential interval of the anodic and cathodic peaks, ca., ∼0.127 V for the initial cycle. For the following four cycles, the greatly reduced redox peak potential intervals and the perfectly overlapped curves suggest that the YS-NCM-based cell exhibits excellent reversibility and very small electrode polarization. Fig. 5d demonstrates the Nyquist plots (fresh, after one cycle, and after 100 cycles (0.1C)) of the YS-NCM-based cell, recorded over the frequency range from 0.1 Hz to 100 kHz. Typically, a depressed semicircle with a low-frequency oblique line is observed (Fig. 5d).45 The intercept of the semicircle at high frequency region is related to the equivalent internal resistance (Re), including a combination of total resistance from electrolyte, electrodes, separator. The semicircle in high-frequency region can be assigned to the resistance of the formation of a passivation layer (Rp) and the corresponding constant phase element (CPE). The middle-frequency semicircle is attributed to the charge transfer resistance (Rct) together with its corresponding capacitance. The straight line in the low-frequency region is related to the Warburg diffusion process. After first electrochemical cycling, a greatly reduced Rct and Rp is evidenced, primarily due to the reorganization of the lithium metal surface,46 which usually takes a big share of the total resistance in half-cells. The YS-NCM-based cell shows even smaller resistance of Rct and Rp after 100 cycles, implying that no severe resistance accumulation built upon cycling. Taken together the CV and EIS results, the fast Li+ diffusion and charge transfer kinetics explain the superior C-rate capability and stable cycling performance we achieved here.
4. Conclusions
To sum up, in this work we designed and successfully synthesized the monodispersed hierarchical porous LiNi1/3Co1/3Mn1/3O2 nano-/microspheres with yolk–shell-like architecture (YS-NCM) and with exposed {010} electrochemical active facets. The morphology we designed here allows for fast Li+ transport kinetics owing to both the shortened lithium-ion diffusion paths and the open structure of {010} facets. This is corroborated by the rather high lithium ion apparent diffusion coefficient derived from the cyclic voltammetry analysis. Furthermore, the yolk–shell-like structure is capable of buffering the volume changes upon charging/discharging. Benefiting from these, the YS-NCM-based cells demonstrate stable long-term cycling performance and outstanding high C-rates capability. Specifically, when examined as the cathode material for LIBs, the YS-NCM-based cells can maintain capacity retention ratios as high as 85.99% (0.1C), 91.08% (1C) and 93.23% (2C) after 100 cycles, respectively. Even being tested at 30C, our YS-NCM-based cells can still deliver a reversible capacity of 64.50 mA h g−1, making our sample a highly promising candidate for practical lithium-ion battery applications with fast charge/discharge ability and superior long-term stability.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by Ministry of Education (MOE) of Singapore for the research funding through the following grants, AcRF Tier 1 (Reference No: RG103/16); AcRF Tier 1 (RG195/17); AcRF Tier 3 (MOE2016-T3-1-006 (S)).Notes and references
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- M. S. Whittingham, MRS Bull., 2008, 33, 411–419 CrossRef CAS.
- B. Scrosati and J. Garche, J. Power Sources, 2010, 195, 2419–2430 CrossRef CAS.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2001, 4, 3243–3262 RSC.
- R. Marom, S. F. Amalraj, N. Leifer, D. Jacob and D. Aurbach, J. Mater. Chem., 2011, 21, 9938–9954 RSC.
- D. Bresser, K. Hosoi, D. Howell, H. Li, H. Zeisel, K. Amine and S. Passerini, J. Power Sources, 2018, 382, 176–178 CrossRef CAS.
- T. Ohzuku and Y. Makimura, Chem. Lett., 2001, 30, 642–643 CrossRef.
- N. Yabuuchi and T. Ohzuku, J. Power Sources, 2003, 119–121, 171–174 CrossRef CAS.
- Y. Koyama, I. Tanaka, H. Adachi, Y. Makimura and T. Ohzuku, J. Power Sources, 2003, 119–121, 644–648 CrossRef CAS.
- K. Dokko, M. Mohamedi, Y. Fujita, T. Itoh, M. Nishizawa, M. Umeda and I. Uchida, J. Electrochem. Soc., 2001, 148, A422–A426 CrossRef CAS.
- K. M. Shaju, G. V. Subba Rao and B. V. R. Chowdari, Electrochim. Acta, 2002, 48, 145–151 CrossRef CAS.
- I. Belharouak, Y. K. Sun, J. Liu and K. Amine, J. Power Sources, 2003, 123, 247–252 CrossRef CAS.
- K. M. Shaju and P. G. Bruce, Adv. Mater., 2006, 18, 2330–2334 CrossRef CAS.
- J. Zhu, T. Vo, D. Li, R. Lu, N. M. Kinsinger, L. Xiong, Y. Yan and D. Kisailus, Cryst. Growth Des., 2012, 12, 1118–1123 CrossRef CAS.
- J. Li, C. Cao, X. Xu, Y. Zhu and R. Yao, J. Mater. Chem. A, 2013, 1, 11848–11852 RSC.
- J. Li, S. Xiong, Y. Liu, Z. Ju and Y. Qian, Nano Energy, 2013, 2, 1249–1260 CrossRef CAS.
- D. Becker, M. Börner, R. Nölle, M. Diehl, S. Klein, U. Rodehorst, R. Schmuch, M. Winter and T. Placke, ACS Appl. Mater. Interfaces, 2019, 11, 18404–18414 CrossRef CAS PubMed.
- Z. Chen, G.-T. Kim, Y. Guang, D. Bresser, T. Diemant, Y. Huang, M. Copley, R. J. Behm, S. Passerini and Z. Shen, J. Power Sources, 2018, 402, 263–271 CrossRef CAS.
- Z. Chen, D. Chao, J. Lin and Z. Shen, Mater. Res. Bull., 2017, 96, 491–502 CrossRef CAS.
- Y. Shao, B. Huang, Z. Lu, Y. Liu, X. Meng, L. Du, H. Song and S. Liao, Energy Technol., 2019, 7, 1800769–1800776 CrossRef.
- M. Park, X. Zhang, M. Chung, G. B. Less and A. M. Sastry, J. Power Sources, 2010, 195, 7904–7929 CrossRef CAS.
- Z. Chen, D. Chao, J. Liu, M. Copley, J. Lin, Z. Shen, G.-T. Kim and S. Passerini, J. Mater. Chem. A, 2017, 5, 15669–15675 RSC.
- B. Kang and G. Ceder, Nature, 2009, 458, 190–193 CrossRef CAS PubMed.
- K. Kang, D. Morgan and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 014305–014308 CrossRef.
- Z. Chen, J. Wang, D. Chao, T. Baikie, L. Bai, S. Chen, Y. Zhao, T. C. Sum, J. Lin and Z. Shen, Sci. Rep., 2016, 6, 25771–25781 CrossRef CAS PubMed.
- Z. Zhu, D. Zhang, H. Yan and W. Li, J. Mater. Chem. A, 2013, 1, 5492–5496 RSC.
- W. Xiong, Y. Jiang, Z. Yang, D. Li and Y. Huang, J. Alloys Compd., 2014, 589, 615–621 CrossRef CAS.
- Y. Wu, C. Cao, Y. Zhu, J. Li and L. Wang, J. Mater. Chem. A, 2015, 3, 15523–15528 RSC.
- J. Li, X. Wang, J. Zhao, J. Chen, T. Jia and C. Cao, J. Power Sources, 2016, 307, 731–737 CrossRef CAS.
- L. Peng, Y. Zhu, U. Khakoo, D. Chen and G. Yu, Nano Energy, 2015, 17, 36–42 CrossRef CAS.
- Z. Yang, J. Lu, D. Bian, W. Zhang, X. Yang, J. Xia, G. Chen, H. Gu and G. Ma, J. Power Sources, 2014, 272, 144–151 CrossRef CAS.
- J. Guan, F. Mou, Z. Sun and W. Shi, Chem. Commun., 2010, 46, 6605–6607 RSC.
- L. Zhou, D. Zhao and X. W. Lou, Adv. Mater., 2012, 24, 745–748 CrossRef CAS PubMed.
- F. Mou, J. Guan, Z. Sun, X. Fan and G. Tong, J. Solid State Chem., 2010, 183, 736–743 CrossRef CAS.
- N. Wu, Y. Zhang, Y. Guo, S. Liu, H. Liu and H. Wu, ACS Appl. Mater. Interfaces, 2016, 8, 2723–2731 CrossRef CAS PubMed.
- Y. Jiang, Z. Yang, W. Luo, X. Hu and Y. Huang, Phys. Chem. Chem. Phys., 2013, 15, 2954–2960 RSC.
- M. Delhaye and P. Dhamelincourt, J. Raman Spectrosc., 1975, 3, 33–43 CrossRef CAS.
- M. Delhaye, M. Bridoux and F. Wallart, J. Mol. Struct., 1982, 79, 51–66 CrossRef CAS.
- T. Mei, Y. Zhu, K. Tang and Y. Qian, RSC Adv., 2012, 2, 12886–12891 RSC.
- C. Julien and M. Massot, Solid State Ionics, 2002, 148, 53–59 CrossRef CAS.
- C. Julien, C. Letranchant, S. Rangan, M. Lemal, S. Ziolkiewicz, S. Castro-Garcia, L. El-Farh and M. Benkaddour, Mater. Sci. Eng., B, 2000, 76, 145–155 CrossRef.
- Z. Chen, Z. Wang, G.-T. Kim, G. Yang, H. Wang, X. Wang, Y. Huang, S. Passerini and Z. Shen, ACS Appl. Mater. Interfaces, 2019, 11, 26994–27003 CrossRef CAS PubMed.
- Z. Chen, G.-T. Kim, D. Bresser, T. Diemant, J. Asenbauer, S. Jeong, M. Copley, R. J. Behm, J. Lin, Z. Shen and S. Passerini, Adv. Energy Mater., 2018, 8, 1801573–1801585 CrossRef.
- B. Luo, B. Jiang, P. Peng, J. Huang, J. Chen, M. Li, L. Chu and Y. Li, Electrochim. Acta, 2019, 297, 398–405 CrossRef CAS.
- K. M. Shaju, G. V. Subba Rao and B. V. R. Chowdari, J. Electrochem. Soc., 2004, 151, A1324–A1332 CrossRef CAS.
- G. B. Appetecchi, G.-T. Kim, M. Montanino, F. Alessandrini and S. Passerini, J. Power Sources, 2011, 19, 6703–6709 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra03022h |
| This journal is © The Royal Society of Chemistry 2020 |