Open Access Article
Open Access ArticleMass spectrometric analysis of acid-assisted photochemical release of the trimethyl lock system on the monolayers on gold†
Geunhyeok Yu and
Woon-Seok Yeo *
*
Department of Bioscience and Biotechnology, Bio/Molecular Informatics Center, Konkuk University, Seoul 05029, Korea. E-mail: wsyeo@konkuk.ac.kr
First published on 11th May 2020
Abstract
We report the acid-assisted photolysis of the trimethyl lock system which has long been harnessed for a variety of applications such as drug delivery, cellular imaging, enzyme activity assays, and surface patterning. By mass spectrometric analysis, we found that photoinduced intramolecular cyclization and the ensuing release of the pendant groups of the trimethyl lock on the self-assembled monolayers proceeded cleanly in the presence of HCl, to give a high yield.
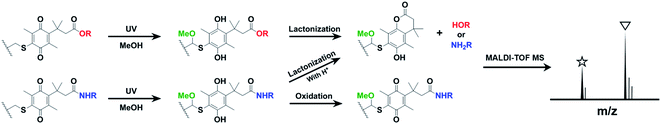
The release of a specific substance under certain conditions is advantageous for modulating the activities and properties of biological/chemical molecules.1,2 In this regard, the trimethyl lock, o-hydroxydihydrocinnamic acid with three methyl substituents, has long been harnessed for drug delivery, cellular imaging, enzyme activity assays, ligand/surface patterning, and development of gradient surfaces.3–6 The trimethyl lock is initiated by demasking phenolic oxygen, which then undergoes intramolecular cyclization (lactonization), resulting in the release of pendant groups. A number of methods have been used for trimethyl lock initiation, including chemical/electrochemical reduction, photochemical induction, and enzyme reactions.2,7–10 Photochemical reduction appears to be advantageous over other initiation methods, as it does not require any complicated reagents or instruments and allows spatiotemporal control of on-demand activation.
Recently, the Dougherty's group reported photodecaging trimethyl lock systems based on a quinone redox reaction in which a photoinduction leads to the cleavage of amine/alcohol groups.11,12 Inspired by this photocleavable molecule, we have designed a photoresponsive dynamic surface for the photochemical release of the pendant groups of trimethyl locks, which has not yet been reported, to the best of our knowledge. The complete cleavage of target bonds without any significant side reactions upon activation is of utmost importance in surface releasing systems. Thus, photoinduced cleavage of the trimethyl lock on surfaces must be investigated prior to the use of this lock in various applications. In this study, we report on the preparation of the surface releasing system using quinone-based photocleavable trimethyl lock (Q-PTL) derivatives with ester or amide functionality and molecular-level characterization of photochemical reactions. Our system relies on self-assembled monolayers (SAMs) on gold and matrix-assisted laser desorption ionization time-of flight mass spectrometry (MALDI-TOF MS), known as SAMDI, for characterization.13–15 This is a powerful and efficient tool for analysis of the SAMs on gold because of its short timeframes, simple sampling process, and high degree of discrimination of multiple analytes despite structural similarities. This paper will also discuss the findings of our study. A key finding is that the photochemical release of Q-PTL on the surface is strongly enhanced under acidic conditions, particularly in the Q-PTL-amide (Fig. 1).
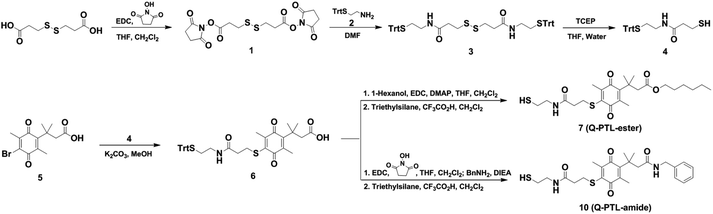
Scheme 1 illustrates the synthesis of Q-PTL derivatives used in this study. Briefly, carboxylic acid containing trimethyl lock quinone with protected thiol (6) was prepared by a conjugation reaction between a thiol molecule (4) and brominated trimethyl-lock quinone (5). Subsequent tethering to an alcohol (1-hexanol) or an amine (benzyl amine) followed by deprotecting the thiol group afforded Q-PTL-ester (7) or Q-PTL-amide (10). For the preparation of 2 and 5, see Fig. S1.† Note that 5 was synthesized using a method previously reported by the Dougherty's group.11
First, we analyzed the photochemical reaction of Q-PTL derivatives in solution for a better understanding of the photolysis characteristics of Q-PTL. It was known that a quinone with a sulfide as an electron door, such as Q-PTL, shows broad absorbance near 400 nm, and the photolysis and release of caged molecules in methanol proceed cleanly including a solvent capture step through zwitterion intermediates without a complex mixture of byproducts.12 Thus, we irradiated the Q-PTL solution in MeOH or EtOH by 395 nm light for photoinduction. The Q-PTL-ester or amide solution in MeOH or EtOH (1 mM) was irradiated at 395 nm for 5 min, incubated for an additional 5 min, and analyzed by MALDI-TOF MS. The observed mass peaks for the photochemical reaction were in very good accordance with the previous report by the Dougherty's group,12 and we elucidated the photochemical products on the basis of mass data. As seen in mass spectra, the peak corresponding to the Q-PTL-ester (△) decreased significantly after irradiation, and gave rise to the peaks due to the solvent captured lactonized products (□ in MeOH and ◇ in EtOH) (Fig. 2(a)). Unlike Q-PTL-ester, Q-PTL-amide (▲) gave rise to solvent captured, non-lactonized quinone products (■ in MeOH and ◆ in EtOH) with relatively high intensity compared to that for solvent captured lactonized products (□ and ◇) (Fig. 2(b)). This difference may be attributed to the bond strength difference between the amide and the ester. The amide nitrogen forms a conjugate system by delocalization of the lone pair electrons into the carbonyl group, resulting in a partial double bond character between nitrogen and carbon, and thus, a stronger bond than the ester bond. Therefore, the hydroquinone intermediate produced by photoreduction and subsequent solvent capture may have sufficient time to be oxidized to the corresponding quinone rather than to lactonize, as shown in Fig. 1. For this reason, we harnessed acid-catalyzed amide bond cleavage to promote the lactonization. Fig. 2(c) clearly shows that when Q-PTL-amide was irradiated in the presence of HCl, the solvent captured quinone products (■ and ◆) completely disappeared and the solvent captured lactonized products (□ and ◇) were formed with high peak intensity. Note that the treatment of Q-PTL-amide with HCl for 10 min in the absence of irradiation did not affect the stability of Q-PTL-amide (Fig. 2(c), first panel).
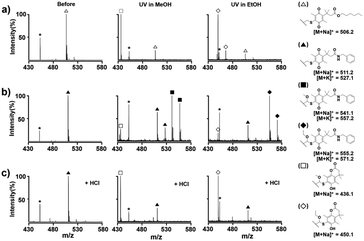 | ||
| Fig. 2 Mass spectrometric analysis and peak assignments for photochemical reactions of (a) Q-PTL-ester, (b) Q-PTL-amide, and (c) Q-PTL-amide in the presence of HCl (* matrix peak). | ||
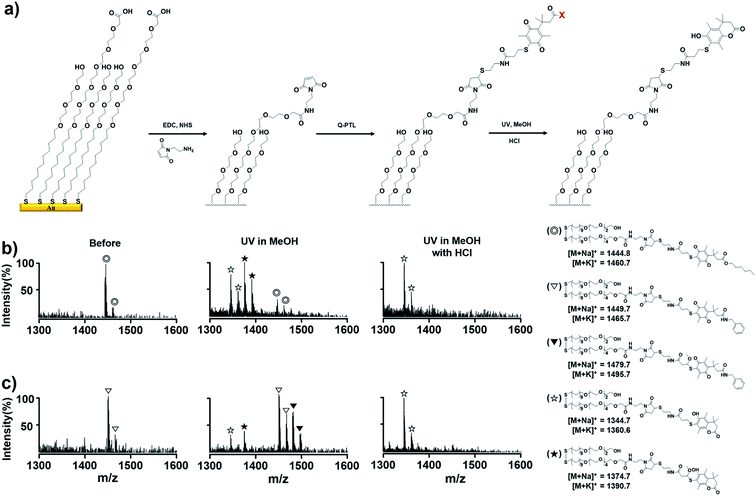
Second, we applied acid-assisted photolysis of Q-PTL to the photo-triggered release system on surfaces. Accordingly, we utilized SAMs on a gold platform, where chemical/biochemical conversion could be easily analyzed by MALDI-TOF MS. Fig. 3(a) illustrates the preparation of Q-PTL-presenting SAMs and the acid-assisted photochemical release process. Carboxylic acid groups amongst a tri(ethyleneglycol) background on gold were coupled with N-(2-aminoethyl)maleimide, and subsequent thiol-Michael addition of Q-PTL derivatives to maleimide led to Q-PTL-presenting SAMs. We then verified the acid-assisted photochemical release of Q-PTL on the surface by mass spectrometry. In general, disulfides of alkanethiolates were the dominant species observed in MALDI-TOF MS profile of the SAMs on gold. The Q-PTL-ester presenting monolayer gave rise to peaks for Q-PTL-ester-containing disulfide (◎) (Fig. 3(b), first panel). Following irradiation, the mass spectrometric analysis revealed peaks due to photochemical lactonized products (lactone disulfide ☆ and solvent captured lactone disulfide ★) with traces of the starting material (◎) (Fig. 3(b), second panel). When an identical monolayer was irradiated in the presence of HCl (5 mM), only lactone disulfide (☆) was observed, indicating reaction completion (Fig. 3(b), third panel). The same experiment was performed for the Q-PTL-amide presenting monolayer (Fig. 3(c)). Following irradiation, high-density peaks for Q-PTL-amide-containing disulfide (▽) still appeared, whereas peaks for photochemical lactonized products (☆ and ★) had low density. In addition, considerable amount of the solvent captured non-lactonized quinone disulfide (▼) was observed, unlike the case for the Q-PTL-ester surface. When the monolayer was irradiated in the presence of HCl (5 mM), only lactone disulfide (☆) was observed, similar to the case of the Q-PTL-ester monolayer, clearly implying that the lactonization and ensuing release of alcohol or amine pendant are facilitated by HCl. These results are significant because surface releasing systems require the complete cleavage of target bonds without any significant side reactions upon activation as we discussed above. For the optimization of acid-assisted photochemical release on the surface, the Q-PTL-presenting monolayers were treated with 1 mM HCl, resulting in a higher yield of photochemical lactonized products (☆ and ★) compared to that for the untreated surfaces (Fig. S2†). Interestingly, unlike in solution, the solvent capture process was not dominant on the surface, and even was not observed with HCl treatment. Furthermore, upon irradiation without HCl treatment, a trace amount of non-lactonized solvent captured quinone disulfide was observed for the Q-PTL-ester, whereas the Q-PTL-amide surface yielded high-density peaks for solvent captured non-lactonized quinone disulfide (▼). Although we cannot confirm this observation at present, we presume that the solvent molecules have reduced accessibility to the photoinduced zwitterion intermediates on the surface than in solution, leading to lactonization without solvent capture.
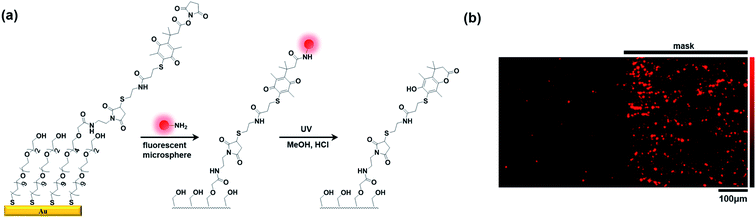
Finally, we verified the fidelity of our acid-assisted photochemical cleavage of the trimethyl lock for practical surface release systems via selective photoactivation. The prepared maleimide-presenting monolayers were immersed into a solution of Q-PTL-NHS which was prepared from 6 and N-hydroxysuccinimide, and subsequently treated with amine-modified red fluorescent microspheres (Fig. 4(a)). Half of the resulting Q-PTL-microsphere-presenting monolayers were irradiated at 395 nm in the presence of HCl (5 mM) in MeOH. Fluorescence microscopy analysis using a Texas Red filter showed that the fluorescence from the unmasked region of the monolayer had disappeared almost completely, whereas the masked region of the monolayer was intact. This indicated the selective release of fluorescent microspheres in the photoinduced region of the Q-PTL monolayers (Fig. 4(b)).
A variety of strategies for photochemical removal of protecting groups and surface releasing systems with photochemical initiation have been reported. For example, Wang et al. introduced photochemical cleavage of benzylic C–N and C–O bonds for the release of amines, alcohols, and carboxylic acids.16–18 Kikuchi et al. demonstrated a dynamic surface for light-induced cell migrations, and Miguel et al. realized caged surfaces for wavelength-selective release of chromophores by harnessing photo-labile linkers.19,20 In most cases, the photoinduction was performed by irradiating 250–350 nm UV light which sometimes can be invasive to biological and biochemical systems, while photoinductions at longer wavelengths are not common. On the other hand, the trimethyl lock system described here can operate into the visible range by synthetic modifications.11 In addition, our surface releasing system relies on SAMs on gold, which provide various advantages over other types of scaffolds. For example, SAMs on gold are stable in contact with air and water and suitable for biological applications. In addition, they can be characterized with MALDI-TOF MS, and therefore, chemical or biochemical conversions on the surface along with structural changes can be easily trackable.
In summary, we synthesized quinone-based photocleavable trimethyl lock (Q-PTL) derivatives with ester of amide functionality. MALDI-TOF MS analysis revealed that Q-PTL-amide showed inefficient photochemical cleavage of the tethered molecular moiety upon irradiation, unlike the case of the Q-PTL-ester. However, the degree of photolysis significantly improved under acidic conditions. We also confirmed that the photochemical release of Q-PTL on the surface was greatly enhanced under acidic conditions. The feasibility of the Q-PTL releasing system from the surface was verified by fluorescence imaging. We believe that our strategy will be helpful for various applications utilizing photoinduced trimethyl locks.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This paper was supported by Konkuk University in 2018.Notes and references
- C.-C. Lin and K. S. Anseth, Pharm. Res., 2009, 26, 631 CrossRef CAS PubMed.
- I. Choi and W. S. Yeo, ChemPhysChem, 2013, 14, 55 CrossRef CAS PubMed.
- M. N. Levine and R. T. Raines, Chem. Sci., 2012, 3, 2412 RSC.
- G. G. Dias, A. King, F. de Moliner, M. Vendrell and E. N. da Silva Júnior, Chem. Soc. Rev., 2018, 47, 12 RSC.
- H. Seo, I. Choi, J. Lee, S. Kim, D. E. Kim, S. K. Kim and W. S. Yeo, Chem.–Eur. J., 2011, 17, 5804 CrossRef CAS PubMed.
- J. Lee, I. Choi and W. S. Yeo, Chem.–Eur. J., 2013, 19, 5609 CrossRef CAS PubMed.
- S. Ciampi, M. James, M. H. Choudhury, N. A. Darwish and J. J. Gooding, Phys. Chem. Chem. Phys., 2013, 15, 9879 RSC.
- X. Wang and J. A. Kalow, Org. Lett., 2018, 20, 1716 CrossRef CAS PubMed.
- D. P. Walton and D. A. Dougherty, Chem. Commun., 2019, 55, 4965 RSC.
- D. Pan, F. Luo, X. Liu, W. Liu, W. Chen, F. Liu, Y.-Q. Kuang and J.-H. Jiang, Analyst, 2017, 142, 2624 RSC.
- D. P. Walton and D. A. Dougherty, J. Am. Chem. Soc., 2017, 139, 4655 CrossRef CAS PubMed.
- C. J. Regan, D. P. Walton, O. S. Shafaat and D. A. Dougherty, J. Am. Chem. Soc., 2017, 139, 4729 CrossRef CAS PubMed.
- J. R. Lee, J. Lee, S. K. Kim, K. P. Kim, H. S. Park and W. S. Yeo, Angew. Chem., Int. Ed., 2008, 47, 9518 CrossRef CAS PubMed.
- Z. A. Gurard-Levin and M. Mrksich, Annu. Rev. Anal. Chem., 2008, 1, 767 CrossRef CAS PubMed.
- M. Mrksich, ACS Nano, 2008, 2, 7 CrossRef CAS PubMed.
- P. Wang, D. A. Devalankar and W. Lu, J. Org. Chem., 2016, 81, 6195–6200 CrossRef CAS PubMed.
- P. Wang, W. Lu, D. Devalankar and Z. Ding, Org. Lett., 2015, 17, 170 CrossRef CAS PubMed.
- P. Wang, W. Lu, D. A. Devalankar and Z. Ding, Org. Lett., 2015, 17, 2114 CrossRef CAS PubMed.
- Y. Kikuchi, J. Nakanishi, H. Nakayama, T. Shimizu, Y. Yoshino, K. Yamaguchi, Y. Yoshida and Y. Horiike, Chem. Lett., 2008, 37, 1062 CrossRef CAS.
- V. San Miguel, C. G. Bochet and A. del Campo, J. Am. Chem. Soc., 2011, 133, 5380 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and additional figures. See DOI: 10.1039/d0ra02110e |
| This journal is © The Royal Society of Chemistry 2020 |