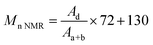Open Access Article
Open Access ArticleSynthesis and properties of a temperature-sensitive hydrogel based on physical crosslinking via stereocomplexation of PLLA-PDLA
Xiaoyu Shiab,
Jie Wuab,
Zhidan Wangab,
Fei Songab,
Wenli Gaoab and
Shouxin Liu *ab
*ab
aKey Laboratory of Applied Surface and Colloid Chemistry (Shaanxi Normal University), Ministry of Education, Xi'an, 710062, PR China. E-mail: liushx@snnu.edu.cn; Tel: +86-29-81530781
bSchool of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi'an 710062, PR China
First published on 26th May 2020
Abstract
A synthetic route to amphiphilic conetwork (APCN) gels was developed and involved (1) a ring-opening polymerization (ROP) synthesis of the macromonomer HEMA-PLLA/PDLA, and (2) a radical polymerization of a stereocomplex of the synthesized macromonomers with P(MEO2MA-co-OEGMA) to form the APCN gels. The structure of the gel was successfully verified using X-ray diffraction. Thermal analysis and differential scanning calorimetry data showed that the thermal behaviors of the gels were greatly improved compared with that of polylactic acid (PLA). The mechanical properties of the gels were measured by using a dynamic viscometer, and the results indicated a greater mechanical strength before swelling than afterwards, and an increasing strength of the gels with increasing amount of PLA stereocomplex. Gels placed in different aqueous phases at different temperatures showed different swelling ratio (SR) values. Specifically, the SR gradually decreased as the temperature was increased, indicating a temperature sensitivity of the gels. In addition, the gels placed in the aqueous and organic phases presented as hydrogels and hydrophobic gels, respectively, and their SR values were relatively low. These results indicated the amphiphilic nature of the gel, and indicated great application prospects for the gel in biomedicine.
1. Introduction
Polylactic acid (PLA) is an important degradable polymer material. Its synthetic raw materials are obtained from renewable resources. After being used, it can be degraded into H2O and CO2, and hence introduce no pollution to the environment.1–3 At present, polylactic has been widely used in controlled drug release, non-removable surgical sutures and microcapsules for injections, landfills, as support materials, and as repair materials in tissue engineering.4 PLA forms two enantiomers: poly (L-lactide) (PLLA) and poly (D-lactide) (PDLA), which can form a racemate by hydrogen bonding, i.e., the so-called stereocomplex.5–7 Compared with the individual enantiomers, PLA stereocomplexes exhibit better physical properties, such as higher melting points, higher mechanical strength levels, and improved thermal and hydrolytic stability levels.8–12 These unique characteristics are beneficial to the applications of PLA in biomedicine. Most importantly, using the PLA stereocomplex as a cross-linker has several advantages over other cross-linking methods, such as reaction under mild conditions and avoiding the use of catalysts, auxiliary crosslinking agents, and other active molecules.13Poly(N-isopropylacrylamide) (PNIPAM) has been by far the most-studied thermoresponsive polymer in materials science. Indeed, this synthetic polymer exhibits a low critical solution temperature (LCST) of about 32 °C in aqueous medium and is, therefore, very useful for preparing smart materials for biological applications. However, in recent years, polymer chemists reported very interesting alternatives to PNIPAM.14,15 Temperature-sensitive, water soluble biocompatible copolymers of 2-(2-methoxyethoxy) ethyl methacrylate (MEO2MA) and oligo(ethylene glycol) methacrylate (OEGMA) have been widely studied. LCST values of 26 °C and 90 °C have been measured for PMEO2MA and POEGMA, respectively—with the copolymers exhibiting LCSTs between these temperatures, and which can be precisely adjusted by varying the ratio of the amount of one co-monomer to that of the other.16–18 P(MEO2MA-co-OEGMA) has been extensively studied in drug delivery systems due to its excellent temperature response, and excellent hydrophilicity, biocompatibility and non-toxicity.
Amphiphilic conetwork (APCN) gels as a rapidly emerging new material, consist not only of hydrophilic polymer components, but also hydrophobic polymer components, which are covalently bonded together in a cross-linked macromolecular assembly.19–22 APCN gels are more mechanically stable than are their pure homopolymeric analogues. Importantly, APCN gels have some extraordinary properties, such as swelling independent of solvent polarity, formation of a nanophase structure, excellent mechanical strength, and good biocompatibility, which make them promising for applications in a wide variety of areas.23–30
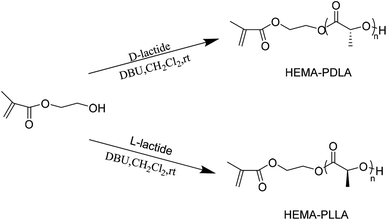
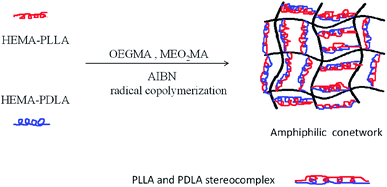
In this study, we successfully synthesized biocompatible APCN gels, namely the poly[2-(2-methoxyethoxy)ethylmethacrylate-co-oligo(ethylene glycol)methacrylate]-l-stereocomplex of poly(L-lactide) and poly(D-lactide) (P[MEO2MA-co-OEGMA]-l-S-(PLLA-PDLA)). First, physical cross-linking was accomplished via stereocomplexation of the macromonomers HEMA-PLLA and HEMA-PDLA, and then the hydrophilic monomers MEO2MA and OEGMA were added to form the APCN gels. Hydrogels formed by physical crosslinking display better mechanical strengths and higher melting points than do hydrogels formed by chemical crosslinking.31–34 Importantly, these materials were found to be biocompatible and degradable, which can promote their application in the field of biomedicine.
2. Experimental
2.1. Materials
2-Hydroxyethyl methacrylate (HEMA, Mn = 130.14 g mol−1), 2-(2-methoxyethoxy) ethyl methacrylate (MEO2MA, 95%, Mn = 188 g mol−1) and oligo (ethylene glycol) methacrylate (OEGMA, 98%, Mn = 475 g mol−1) were purchased from TCL (Shanghai Development Co., Ltd., Shanghai, China) and purified by passing them through a column with neutral alumina oxide to remove inhibitor. 2,2-Azoisobutyronitrile (AIBN) was purified by recrystallizing it from methanol. L-Lactide and D-lactide (Mn = 144.13 g mol−1) were purchased from TCL (Shanghai Development Co., Ltd., Shanghai, China) and were purified by recrystallizing them from ethyl acetate. Dichloromethane (DCM) was dried by performing distillation with calcium hydride before use. Tetrahydrofuran (THF) containing NaOH was dried by performing distillation with benzophenone before use. 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) (98%, Aldrich) was used as received.2.2. Preparation of the amphiphilic conetwork gels
2.3. Structural characterization
2.4. Property measurements
| SR = (Wt − Wd)/Wd, |
| SR = (Wt − Wd)/Ws, |
In addition, the P3 hydrogel after having reached swelling equilibrium was placed in a water bath at pH = 7, 42 °C. After 30 minutes, it was taken out, and the surface moisture was wiped prior to weighing the sample. The sample was then put into a water bath at pH = 7, 22 °C again, and taken out after 30 minutes, had its surface moisture wiped off, and was then weighed. The above operation was repeated multiple times over the course of about 5 hours. The reversible swelling of the gel was calculated by using the above formula.
3. Results and discussion
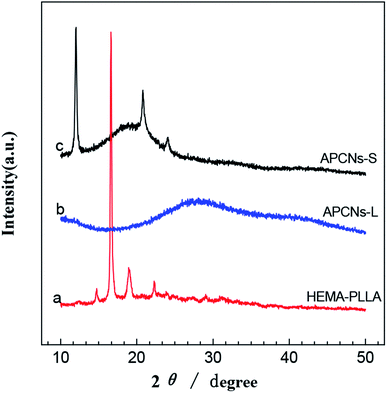
3.1. Characterization of polymers and gels
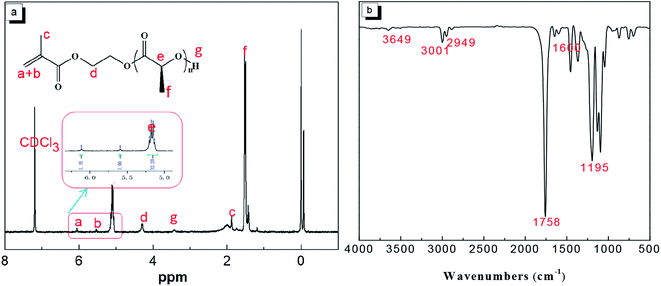
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH–(CH3). The methyl proton peak at 2.01 ppm can be assigned to CH3–. The active hydrogen peak at 3.3–3.4 ppm can be assigned to –CH–(CH3)–OH. The methylene proton peak at 4.3 ppm can be assigned to –O–CH2–. The methine proton peak at 5.1 ppm can be assigned to –(C
O)–CH–(CH3). The methyl proton peak at 2.01 ppm can be assigned to CH3–. The active hydrogen peak at 3.3–3.4 ppm can be assigned to –CH–(CH3)–OH. The methylene proton peak at 4.3 ppm can be assigned to –O–CH2–. The methine proton peak at 5.1 ppm can be assigned to –(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH–(CH3). The peaks at 5.5 ppm and 6.2 ppm are the absorption peaks of the –(CH3)C
O)–CH–(CH3). The peaks at 5.5 ppm and 6.2 ppm are the absorption peaks of the –(CH3)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 methylene proton. The molecular weight of HEMA-PLLA (HEMA-PDLA) was calculated from the 1H NMR spectra by using the formula13
CH2 methylene proton. The molecular weight of HEMA-PLLA (HEMA-PDLA) was calculated from the 1H NMR spectra by using the formula13where Aa+b and Ad stand for the integral areas of peaks (a+b) and (d), respectively. The values of 72 and 130 are the molecular weights of the repeat units of PLLA and the macroinitiator HEMA, respectively.
As shown in Fig. 1(b), the infrared absorption spectrum of HEMA-PLLA showed various absorption peaks: at 1195 cm−1, characteristic of –COC–; at 1600 cm−1, characteristic of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–; a characteristic peak of C
C–; a characteristic peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1758 cm−1; characteristic peaks of –CH2, –CH3 at 3001 cm−1 and 2949 cm−1; and a characteristic peak of –OH at 3649 cm−1. The nuclear magnetic resonance and infrared spectra of HEMA-PDLA were observed to be essentially identical to those of HEMA-PLLA.
O at 1758 cm−1; characteristic peaks of –CH2, –CH3 at 3001 cm−1 and 2949 cm−1; and a characteristic peak of –OH at 3649 cm−1. The nuclear magnetic resonance and infrared spectra of HEMA-PDLA were observed to be essentially identical to those of HEMA-PLLA.
3.2. Properties of the gels
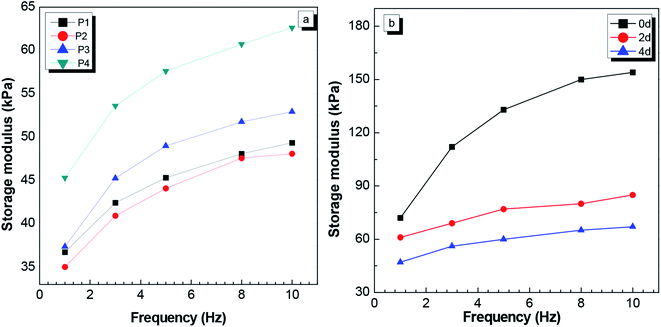 | ||
| Fig. 3 (a) Dynamic mechanical analysis (DMA) curves of the P1, P2, P3, P4 gels after swelling for 4 days. (b) The P3 DMA curves after swelling for 0 d, 2 d and 4 d in distilled water. | ||
At 25 °C, P3 was subjected to constant stresses of 6 kPa, 8 kPa and 10 kPa. Inspection of the corresponding creep curves (displayed in Fig. 4) showed the gel strain changing with time, reflecting the extensibility of the gels. When the applied stress was 10 kPa, the gels showed a great strain of 23%; and when the applied stresses were 8 kPa and 6 kPa, the strains were sequentially reduced to 19% and 16%, respectively. After the stress was removed, the deformation the gel was observed to diminish. Obviously, the greater the stress, the smaller was the recovered strain. The experimental results showed a certain level of elasticity displayed by the hydrogel, and as the stress was increased, the elasticity of the gel gradually decreased. (The experiment was repeated three times to ensure the accuracy of the data (Fig. 5).)
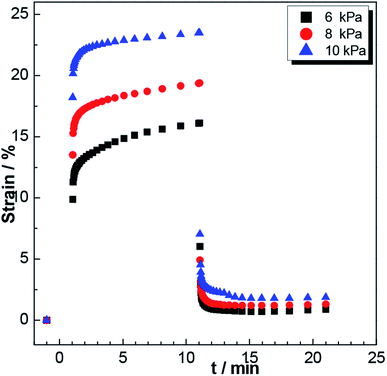 | ||
| Fig. 4 Curves of strain versus time for P3 gels subjected to constant stresses of 6 kPa, 8 kPa and 10 kPa. | ||
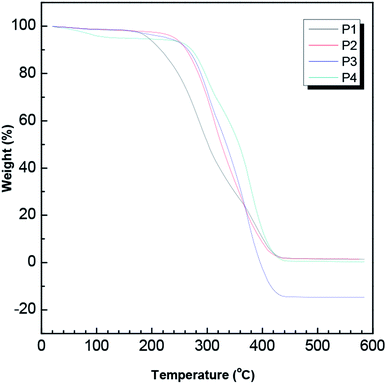
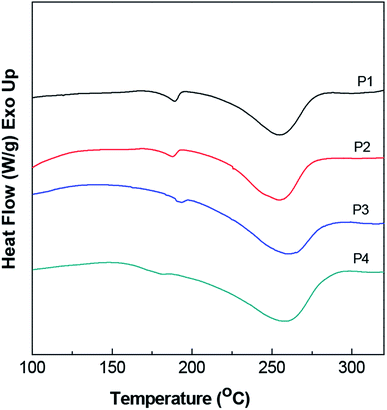
Further evidence for the relatively high thermal stability levels of our gels was provided by the results of DSC analysis. As shown in Fig. 7, the DSC results of the gels indicated two typical physical transitions: a glass transition at a temperature (Tg) close to 180 °C and crystal melting at a temperature (Tm) roughly in the vicinity of 280 °C. The crystal melting peak was due to the successful stereocomplexation of PLLA and PDLA and the presence of SC crystallites. Moreover, with the increase of the degree of PLA polymerization, the Tm value gradually increased, completely consistent with the results of the thermogravimetric analysis. The glass transition temperature (Tg) did not much vary from 180 °C as the PLA polymerization degree was increased, indicating little effect of the stereocomplex on the movement of the segments in the blend.
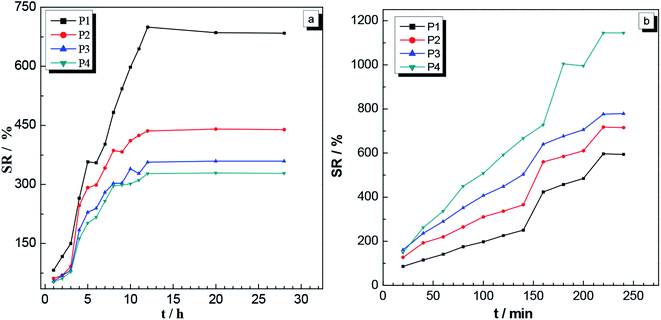 | ||
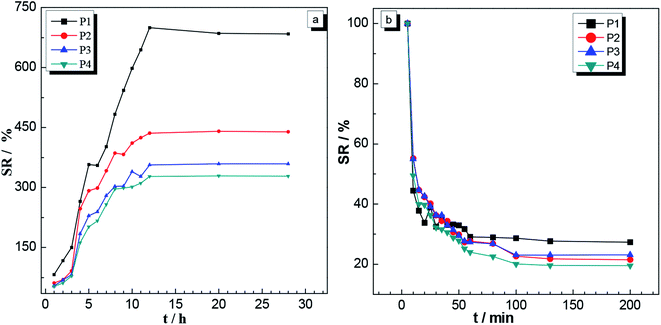
| Fig. 8 Swelling ratios as a function of time for the P1, P2, P3 and P4 gels placed in (a) deionized water at 25 °C and pH 7, and (b) THF. | ||
| Swelling degree (%) | ||||
|---|---|---|---|---|
| Sample | H2O | CH3OH | THF | DCM |
| P1 | 692 ± 12 | 212 ± 28 | 593 ± 25 | 928 ± 22 |
| P2 | 436 ± 15 | 253 ± 23 | 715 ± 36 | 1312 ± 24 |
| P3 | 357 ± 8 | 283 ± 20 | 778 ± 21 | 1307 ± 18 |
| P4 | 328 ± 10 | 318 ± 37 | 1144 ± 29 | 1533 ± 32 |
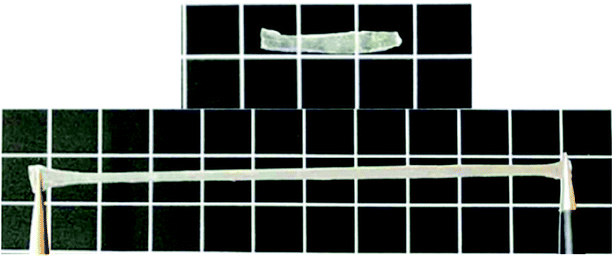
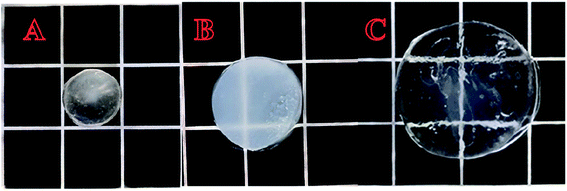
Fig. 9 shows pictures of the gel taken with a digital camera. Panel (A) shows a picture of the untreated gel; panel (B) shows a picture of the gel after it was completely swollen in a deionized water bath at pH 7, 25 °C; and panel (C) shows a picture of the gel after it was swollen in THF. Inspection of these photographs also showed that the gels swelled in both the aqueous phase and the organic phase, completely consistent with the results in Fig. 8(a) and (b), thus further illustrating the amphiphilic natures of the gels.
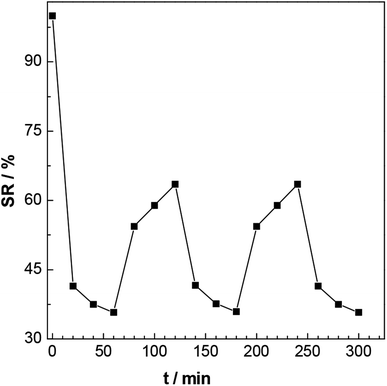 | ||
| Fig. 12 The swelling and de-swelling of the P3 gel in deionized water at 25 °C and 42 °C (see text). | ||
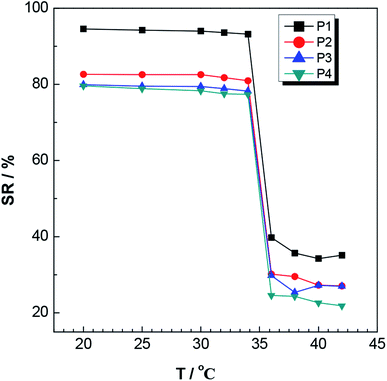
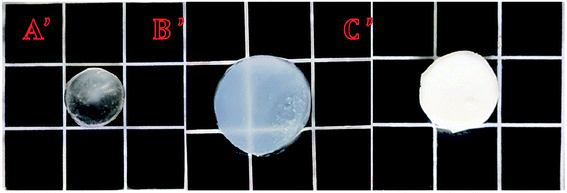
Fig. 13 shows photographs of this P3 gel taken with a digital camera. (A′) shows a photograph of the untreated gel; (B′) shows a picture of the gel completely swelled in a deionized water bath at pH = 7, 25 °C; and (C′) shows a photograph after the gel was placed in a 40 °C deionized water bath. These photographs showed that after the gel was completely swollen, it shrank and whitened with increasing temperature, completely consistent with the results of 3.6.2 (a) and (b). These results illustrated the temperature sensitive nature of the gels.
4. Conclusions
APCNs were successfully synthesized by carrying out free radical copolymerization of PLLA and PDLA stereocomplex with MEO2MA and OEGMA comonomers. A series of APCN gels with different compositions were obtained by tuning the ratio of the amount of hydrophobic macromonomer to that of the hydrophilic monomer. The appearance of characteristic XRD and DSC peaks indicated that the gel stereocomplexations were successful. The gels swelled in both the organic and aqueous phases, and the swelling behaviors of the gels were different in aqueous phases at different temperatures, indicative of the relatively high amphiphilicity and temperature sensitivity levels of the gels. DMA and thermal analysis system measurements showed that PLA was greatly improved by MEO2MA and OEGMA, and indicated the higher mechanical strengths and better thermal performances of the amphiphilic co-network gels. These APCNs and gels may, due to their unique structures and properties, find potential biomedical applications such as in encapsulating and delivering hydrophobic drug molecules.Conflicts of interest
There are no conflicts to declare. With the consent of all of the authors, we made corrections.Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 21773147).Notes and references
- S. M. Cannizzaro and R. S. Langer, Polymeric systems for controlled drug release, Chem. Rev., 1999, 99, 3181–3198 CrossRef PubMed.
- E. Chielhi and R. Solaro, Biodegradable polymeric materials, Adv. Mater., 1996, 8, 305–313 CrossRef.
- H. R. Kricheldorf, M. Berl and N. Scharnagl, Poly(lacto- -nes) polymerization mechanism of metal alkoxideinitiated polymerizations of lactide and various lactones, Macromolecules, 1988, 21, 286–293 CrossRef CAS.
- M. Jamshidian, E. A. Tehrany, M. Imran, M. Jacquot and S. Desobry, Poly-lacticacid: production, applications, nanocomposites, andreleasestudies, Compr. Rev. Food Sci. Food Saf., 2010, 9, 552–571 CrossRef CAS.
- P. J. Pan, L. L. Han, J. N. Bao, Q. Xie, G. R. Shan and Y. Z. Bao, Competitive stereocomplexation, homocrystallization, and polymorphic crystalline transition in poly(L-lactic acid)/poly(D-lactic acid) racemic blends: molecular weight effects, J. Phys. Chem. B, 2015, 119, 6462–6470 CrossRef CAS PubMed.
- H. L. Mao, P. J. Pan, G. R. Shan and Y. Z. Bao, In situ formation and gelation mechanism of thermoresponsive stereocomplexed hydrogels upon mixing diblock and triblockpoly(lactic acid)/poly(ethylene glycol) copolymers, J. Phys. Chem. B, 2017, 119, 6471–6480 CrossRef PubMed.
- J. M. Zhang, H. Sato, H. Tsuji, I. Noda and Y. Ozaki, Infrared spectroscopic study of CH3···O=C interaction during poly(L-lactide)/poly(D-lactide) stereocomplex formation, Macromolecules, 2005, 38, 1822–1828 CrossRef CAS.
- Z. W. Zhao, Z. Zhang, L. Chen, Y. Cao, C. L. He and X. S. Chen, Biodegradable stereocomplex micelles based on dextran-block-polylactide as effcient drug deliveries, Langmuir, 2013, 29, 13072–13080 CrossRef CAS PubMed.
- E. Gisha and C. K. S. P. Luckachan, Biodegradable polymers-areview on recent trends and emerging perspectives, J. Polym. Environ., 2011, 19, 637–676 CrossRef.
- J. Slager and A. J. Domb, Biopolymer stereocomplexes, Adv. Drug Delivery Rev., 2003, 55, 549–583 CrossRef CAS PubMed.
- M. Kakuta, M. Hirata and Y. Kimura, Stereoblock polylactides as high-performance bio-based polymers, J. Macromol. Sci., Polym. Rev., 2009, 49, 107–140 CAS.
- P. J. Pan, J. J. Yang, G. R. Shan, Y. Zh. Bao, Z. X. Weng, A. Cao, K. Yazawa and Y. Inoue, Temperature-variable FTIR and solid-state 13C NMR investigations on crystalline structure and molecular dynamics of polymorphic poly(L-lactide) and poly(Llactide)/poly(D-lactide) stereocomplex, Macromolecules, 2012, 45, 189–197 CrossRef CAS.
- X. S. Fan, M. Wang, D. Yuan and C. B. He, Amphiphilic conetworks and gels physically cross-linked via stereocomplexation of polylactide, Langmuir, 2013, 29, 14307–14313 CrossRef CAS.
- G. Kali, S. l. Vavra, K. László and B. Iván, Thermally responsive amphiphilic conetworks and gels based on poly(N-isopropylacrylamide) and polyisobutylene, Macromolecules, 2013, 46, 5337–5344 CrossRef CAS.
- J. F. Lutz, Thermo-switchable materials prepared using the OEGMA-platform, Adv. Mater., 2011, 23, 2237–2243 CrossRef CAS.
- J. Franc, O. Lutz and A. Hoth, Preparation of ideal PEG analogues with a tunable thermosensitivity by controlled radical copolymerization of 2-(2-methoxyethoxy)ethyl methacrylate and oligo(ethylene glycol) methacrylate, Macromolecules, 2006, 39, 893–896 CrossRef.
- S. T. Sun and P. Y. Wu, On the thermally reversible dynamic hydration behavior of oligo(ethylene glycol) methacrylate-based polymers in water, Macromolecules, 2013, 46, 236–246 CrossRef CAS.
- J. Wu, X. Y. Shi, Z. D. Wang, F. Song, W. L. Gao and S. X. Liu, Stereocomplex Poly(Lactic Acid) Amphi- -philic Conetwork Gel with Temperature and pH Dual Sensitivity, Polymers, 2019, 11, 1940 CrossRef CAS PubMed.
- M. R. Kalourkoti, E. N. Kitiri, C. S. Patrickios, E. Leontidis, M. Constantinou, G. Constantinides, X. h. Zhou and C. M. Papadakis, Double networks based on amphiphilic cross-linked star block copolymer first conetworks and randomly cross-linked hydrophilic second networks, Macromolecules, 2016, 49, 1731–1742 CrossRef.
- C. Lin and I. Gitsov, Preparation and characterization of novel amphiphilic hydrogels with covalently attached drugs and fluorescent markers, Macromolecules, 2010, 43, 10017–10030 CrossRef CAS.
- D. Kafouris, M. Gradzielski and C. S. Patrickios, Semisegmented amphiphilic polymer conetworks: synthesis and characterization, Macromolecules, 2009, 42, 2972–2980 CrossRef CAS.
- M. D. Rikkou, E. Loizou, L. Porcar, P. Butler and C. S. Patrickios, Degradable amphiphilic end-linked conetworks with aqueous degradation rates determined by polymer topology, Macromolecules, 2009, 42, 9412–9421 CrossRef CAS.
- C. A. K. Singh, B. Nutan, I. H. Raval and S. K. Jewrajka, Self-assembly of partially alkylated dextran-graft-poly[(2dimethylamino)ethyl methacrylate] copolymer facilitating hydrophobic/hydrophilic drug delivery and improving conetwork hydrogel properties, Biomacromolecules, 2018, 19, 1142–1153 CrossRef PubMed.
- G. Guzman, T. Nugay, I. Nugay, N. Nugay, J. Kennedy and M. Cakmak, High strength bimodal amphiphilic conetworks for immunoisolation membranes: synthesis, characterization, and properties, Macromolecules, 2015, 48, 6251–6262 CrossRef CAS.
- T. Hiroi, S. Kondo, T. Sakai, E. P. Gilbert, Y. S. Han, T. H. Kim and M. Shibayama, Fabrication and structural characterization of module-assembled amphiphilic conetwork gels, Macromolecules, 2016, 49, 4940–4947 CrossRef CAS.
- A. Domján, C. Fodor, S. Kovács, T. Marek, B. Iván and K. Süvegh, Anomalous swelling behavior of poly(N-vinylimidazole)-l-poly(tetrahydrofuran) amphiphilic conetwork in water studied by solid-state NMR and positron annihilation lifetime spectroscopy, Macromolecules, 2012, 45, 7557–7565 CrossRef.
- A. Domján, P. Mezey and J. Varga, Behavior of the interphase region of an amphiphilic polymer conetworks wollen in polar and nonpolar solvent, Macromolecules, 2012, 45, 1037–1040 CrossRef.
- C. Zhou, L. H. Deng, F. Yao, L. Q. Xu, J. Zhou and G. D. Fu, A well-defined amphiphilic polymer conetwork from sequence control of the cross-linking in polymer chains, Ind. Eng. Chem. Res., 2014, 53, 19239–19248 CrossRef CAS.
- S. Nakagawa, X. Li and M. Shibayama, Insight into the microscopic structure of module- assembled thermoresponsive conetwork hydrogels, Macromolecules, 2018, 51, 6645–6652 CrossRef CAS.
- Ch. C. Zhou, X. Y. Zhou and X. K. Su, Noncytotoxic polycaprolactone polyethyleneglycol-ε-poly (L-lysine) triblock copolymey systhsized and self-assembled as an antibacterial drug carrier, RSC Adv., 2017, 7, 39718–39725 RSC.
- K. J. Mochizuki and D. B. Amotz, Hydration-shell transformation of thermosensitive aqueous polymers, J. Phys. Chem. Lett., 2017, 8, 1360–1364 CrossRef CAS PubMed.
- C. Krumm, S. Konieczny, G. J. Dropalla, M. Milbradt and J. C. Tiller, Amphiphilic polymer conetworks based on end group cross-linked poly(2-oxazoline) homo- and triblock copolymers, Macromolecules, 2013, 46, 3234–3245 CrossRef CAS.
- J. F. Lutz, K. Weichenhan, Ö. Akdemir and A. Hoth, About the phase transitions in aqueous solutions of thermoresponsive copolymers and hydrogels based on 2-(2-methoxyethoxy) ethyl methacrylate and oligo(ethylene glycol) methacrylate, Macromolecules, 2007, 40, 2503–2508 CrossRef CAS.
- Y. Yasuyuki, K. Kawahara, K. Inamoto, S. Mitsumune, S. Ichikawa, A. Kuzuya and Y. Ohya, Biodegradable Injectable Polymer Systems Exhibiting Temperature Responsive Irreversible Sol-to-Gel Transition by Covalent Bond Formation, ACS Biomater. Sci. Eng., 2017, 3, 56–67 CrossRef CAS.
- N. M. B. Smeets, E. Bakaic, M. Patenaude and T. Hoare, Injectable and tunable poly(ethylene glycol) an alogue hydrogels based on poly(oligoethylene glycol methacrylate), Chem. Commun., 2014, 50, 3306–3309 RSC.
- C. L. Ma, P. J. Pan, G. R. Shan, Y. Z. Bao, M. Fujita and M. Maeda, Core−shell structure, biodegradation, and drug release behavior of poly(lactic acid)/poly(ethyl- -ene glycol) block copolymer micelles tuned by macromolecular stereostructure, Langmuir, 2015, 31, 1527–1536 CrossRef CAS PubMed.
- J. R. Sarasua, E. P. Robert, M. Wisniewski, A. L. Borgne and N. Spassky, Crystallization and melting behavior of polylactides, Macromolecules, 1998, 31, 3895–3905 CrossRef CAS.
- J. Shao, S. Xiang, X. C. Bian, J. R. Sun, G. Li and X. S. Chen, Remarkable melting behavior of PLA stereocomplex in linear PLLA/PDLA blends, Ind. Eng. Chem. Res., 2015, 54, 2246–2253 CrossRef CAS.
- J. Bai, J. Y. Wang, W. T. Wang, H. G. Fang, Z. H. Xu, X. S. Chen and Z. G. Wang, Stereocomplexcry stallite-assisted shear-induced crystallization kinetics at a high temperature for asymmetric agglomerate biodegradable PLLA/PDLA blends, ACS Sustainable Chem. Eng., 2016, 4, 273–283 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: