Open Access Article
Open Access ArticleCorrelating the size and cation inversion factor in context of magnetic and optical behavior of CoFe2O4 nanoparticles†
Jitendra Pal Singh *a,
Jae Yeon Park
*a,
Jae Yeon Park b,
Varsha Singh
b,
Varsha Singh c,
So Hee Kim
c,
So Hee Kim c,
Weon Cheol Lim
c,
Weon Cheol Lim c,
Hemaunt Kumar
c,
Hemaunt Kumar d,
Y. H. Kim
d,
Y. H. Kim a,
Sangsul Lee
a,
Sangsul Lee *ae and
Keun Hwa Chae
*ae and
Keun Hwa Chae *c
*c
aPohang Accelerator Lab, Pohang University of Science and Technology, Pohang 37673, Republic of Korea. E-mail: jitendra2029@postech.ac.kr; sangsul@postech.ac.kr
bRadiation Equipment Research Division, Korea Atomic Energy Research Institute, Jeongup 56212, Republic of Korea
cAdvanced Analysis Center, Korea Institute of Science and Technology, Seoul 02792, Republic of Korea. E-mail: khchae@kist.re.kr
dDepartment of Applied Sciences, Rajkiya Engineering College, Bijnor-246725, India
eXavisoptics Ltd., Pohang 37673, Republic of Korea
First published on 3rd June 2020
Abstract
Herein, the size dependent behavior of cobalt ferrite nanoparticles was investigated using synchrotron radiation based techniques. Scanning electron micrographs revealed the enhancement of particle/crystallite size with increase of annealing temperature. Moreover, the shape of these particles also changed with increase of crystallite size. Saturation magnetization increased with increase of crystallite size. The higher saturation magnetization for larger crystallite size nanoparticles was attributed to a cation distribution similar to that of bulk CoFe2O4. The optical band-gap of these nanoparticles decreased from 1.9 eV to 1.7 eV with increase of crystallite size. The enhancement of the optical band-gap for smaller crystallites was due to phenomena of optical confinement occurring in the nanoparticles. Fe L Co L-edge near edge extended X-ray absorption fine structure (NEXAFS) measurements showed that Fe and Co ions remain in the 3+ and 2+ state in these nanoparticles. The results obtained from Fe & Co K-edge X-ray absorption near edge structure (XANES)-imaging experiments further revealed that this oxidation state was possessed by even the crystallites. Extended X-ray absorption fine structure (EXAFS) measurements revealed distribution of Fe and Co ions among tetrahedral (A) and octahedral (B) sites of the spinel structure which corroborates the results obtained from Rietveld refinement of X-ray diffraction patterns (XRD). X-ray magnetic circular di-chroism (XMCD) measurements revealed negative exchange interaction among the ions situated in tetrahedral (A) and octahedral (B) sites. Theoretical and experimental calculated magnetic moments revealed the dominancy of size effects rather than the cation redistribution in the spinel lattice of CoFe2O4 nanoparticles.
Introduction
The underlying processes involved in the physical and chemical properties of nanoparticles are investigated via depicting the local electronic/atomic and magnetic structure using numerous techniques like electron energy-loss spectroscopy (EELS)1,2 and neutron diffraction.3 In addition to this, techniques based on synchrotron radiation show it's prominence over these techniques.4 X-ray absorption spectroscopy (XAS),5 X-ray magnetic circular dichroism (XMCD)6 and X-ray absorption near edge structure (XANES)-imaging7 are the well-known and effective tools based on this radiation. XAS is very much effective to determine local electronic/atomic structure in the nanoparticles whereas XMCD is able to provoke about the magnetic interaction in these nanoparticles.5,6 The XANES-imaging technique determines local electronic structure of the materials with spatial resolution additive.7 Thus, these techniques are able to give complete account of the local structure of the nanoparticle under investigation.5–7 Since, size dependence of nanoparticle is common phenomena and bulk characteristics of these nanoparticles are investigated using numerous techniques, however, the appropriate reason roots at the atomic/molecular level.8,9 Thus, a systematic and rigorous investigation using advanced techniques is needed at this level.The nanoparticles of CoFe2O4 show immense opportunities for utilization in various fields of technological importance.10,11 The nanoparticles of this material show potential for permanent magnet12 spintronics,13 photocatalysis,14 battery industry,15,16 biomedical applications17 and many more depending upon it's magnetic,12,13,17 optical10,11,14 and structural behavior.15,16 These various behaviors are controlled by the size, size distribution and occupancies of metal ions in the unit cell.10–17 The unit cell of this material in the bulk form represents the face centered cubic (fcc) structure configured by O2− ions by forming tetrahedral (A) and octahedral (B)-sites to be filled by metal (Co2+ and Fe3+) ions. Due to strong tendency of favoring octahedral coordination, whole Co2+ ions occupy B-site, however, Fe3+ ions distribute among A- and B-sites in equal proportion.18 This makes this material inverse spinel, however, pre and post-synthesis treatment leads to distribution of these ions among A- and B-sites10–17 as follows
| (CoλFe1−λ)A[Co1−λFe1+λ]BO4 | (1) |
Numerous studies are available determining the metal ion distribution using Mössbauer spectroscopy24 and neutron diffraction,25 however, this investigation lacks using XAS even showing superiority over these techniques.26 This technique is very much effective to determine metal ion distribution in nanoparticles27,28 and thin film.29 Hence, synchrotron radiation based techniques like XAS, XMCD and XANES-imaging are carried out to get deeper insights of size dependence of this material by investigating local electronic/atomic and magnetic structure.
Experimental
Synthesis procedure
Solutions of cobalt nitrate hexahydrate and ferric nitrate nonahydrate in stoichiometric proportion were mixed into citric acid solution by keeping the cations to citric acid ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.28 The mixture was kept on magnetic stirrer at 85 °C to get viscous solution for 2 h. The viscous solution was dried for almost 12 h by heating between 100 °C on a hot plate to form precursor. Precursor thus obtained was further annealed at 300, 500, 700 and 900 °C to synthesize nanoparticles of different size.
2.28 The mixture was kept on magnetic stirrer at 85 °C to get viscous solution for 2 h. The viscous solution was dried for almost 12 h by heating between 100 °C on a hot plate to form precursor. Precursor thus obtained was further annealed at 300, 500, 700 and 900 °C to synthesize nanoparticles of different size.
Characterization
X-ray diffraction (XRD) patterns of synthesized nanoparticles were recorded at D/MAX2500 (RIGAKU, Japan) X-ray diffractometer using Cu Kα (λ = 1.5418 Å) radiation. Scanning electron microscopic (SEM) measurements were carried out using Hitachi (S-4200) field emission scanning electron microscope (FE-SEM). Magnetic measurements at room temperature were performed on vibrating sample magnetometer (VSM). Optical behavior of these nanoparticles was investigated using Carry 5000 UV-Vis spectrometer.Synchrotron radiation based characterization
Various synchrotron radiation based experiments were performed at Pohang Accelerator Laboratory (PAL), Pohang, South Korea. This laboratory is able to operate at 3.0 GeV energy with a maximum storage current of 360 mA.30Near edge X-ray absorption fine structure (NEXAFS) measurements for these nanoparticles were performed at 10D XAS KIST (Korea Institute of Science and Technology) beamline. NEXAFS spectra of these nanoparticles were collected in the total electron yield (TEY) mode and were normalized with respect to post-edge height after background subtraction. Extended X-ray absorption structure (EXAFS) spectra were measured at 1D KIST beamline while detuning incident beam to 50% of maximum intensity, which enable removal of higher harmonics. The detailed experimental procedure is depicted elsewhere.27–29
Room temperature XMCD experiments at L2,3 edges of Fe were performed at 2A beamline which utilizes circularly polarized light with a degree of circular polarization around 80% and an electromagnet of magnetic field strength μ0H (between −0.75 and 0.75 T) for measurements.31 The absorption data were collected in the TEY mode by applying voltage of 200 V. The photon beam and the magnetic field were perpendicular to the sample plane. The base pressure of the system was 10−9 Torr. The absorption was normalized to the incoming photon beam intensity by measuring simultaneously the photocurrent at Au grid.
XANES-imaging measurements were carried out at 7C beamline, which utilizes off-axis illumination of X-ray to capture image using a charge-coupled device (CCD). Zone plate with 150 μm diameter and 40 nm outermost width was used to get transmission X-ray images. This arrangement is able to give spatial resolution of 40 nm and field of view of around 35 μm.32,33
For these measurements, X-ray energy was varied from −20 to 80 eV from the main edge energy of Fe and Co K-edge with an interval of 1 eV. The actual values of energy variation are shown in Table S1.†
Simulation details
XRD patterns of these nanoparticles were refined using FullProf program based on Rietveld analysis.22 The space group Fd3m was assumed, with the 8a, l6d cation sites and 32e O sites all fully occupied.14 Values of goodness of factor (GOF) obtained for these refined patterns vary from 1.1 to 1.5.The program Athena was used to sum the data, identify the beginning of the absorption edge (E0), fit pre- and post-edge backgrounds, and hence to obtain the normalized absorbance, χ, as a function of the modulus of the photoelectron wave vector, k.34 The EXAFS data is Fourier transformed to R-space to investigate the atomic structure and relative bond-lengths with respective to absorbing atoms.35 The fitting was carried out using ARTEMIS in the k range 3–11.5 Å−1 (Co) and 3–9 Å−1 (Fe). The theoretical structure for CoFe2O4 was generated using the ATOM and FEFF from the parameters obtained from Rietveld refinement.34,35
The transmission images were processed with background correction and image registration to extract XANES spectrum at single pixel using the lab-made software based on LabVIEW program.36 From these images, XANES spectra are extracted for single-pixel and for the whole image.
Results
Crystalline phase, size and strain analysis
Fig. 1 shows fitted XRD patterns of synthesized nanoparticles at annealing temperature of 300, 500, 700 and 900 °C. It is clear from Fig. 1 that both the experimental and simulated data points are in close correlation with each other. This indicates that all the peaks in XRD pattern are associated with Fd3m space group of spinel structure. Thus, synthesized nanoparticles exhibit presence of cubic spinel phase [JCPDS no. # 01-1121]. No other peak corresponding to any impurity phase is being detected in these patterns. Refined and simulated parameters from Rietveld refinement are collated in Table 1.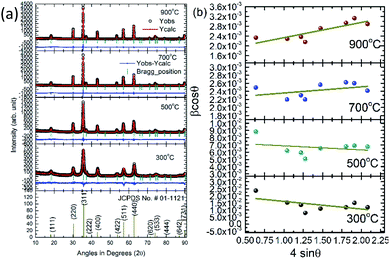 | ||
| Fig. 1 (a) XRD patterns along with JCPDS-01-1121 and (b) W–H plots of CoFe2O4 nanoparticles synthesized at 300, 500, 700 and 900 °C. | ||
| Parameters | CoFe2O4 nanoparticles | |||||
|---|---|---|---|---|---|---|
| 300 °C | 500 °C | 700 °C | 900 °C | |||
| Lattice parameter; a (Å) | 8.357 ± 0.005 | 8.382 ± 0.002 | 8.384 ± 0.008 | 8.386 ± 0.007 | ||
| Oxygen positional parameter; u (Å) | 0.2558 ± 0.0004 | 0.2562 ± 0.0003 | 0.2556 ± 0.0004 | 0.2551 ± 0.004 | ||
| Occupancy | A-site | Co2+ | 0.446 ± 0.003 | 0.376 ± 0.003 | 0.351 ± 0.004 | 0.294 ± 0.004 |
| Fe3+ | 0.556 ± 0.003 | 0.624 ± 0.003 | 0.649 ± 0.004 | 0.706 ± 0.004 | ||
| B-site | Co2+ | 0.556 ± 0.003 | 0.624 ± 0.003 | 0.649 ± 0.004 | 0.706 ± 0.004 | |
| Fe3+ | 1.446 ± 0.003 | 1.376 ± 0.003 | 1.351 ± 0.004 | 1.294 ± 0.004 | ||
| Bragg R-factor | 7.99 | 6.48 | 5.75 | 5.57 | ||
| Rf-factor | 5.52 | 3.43 | 3.47 | 3.10 | ||
| Goodness of fit (GOF)-index | 1.1 | 1.1 | 1.5 | 1.4 | ||
| Crystallite size; D (nm) | 9 ± 1 | 22 ± 2 | 49 ± 4 | 61 ± 6 | ||
| X-ray density; ρ (g cm3) | 5.31 ± 0.02 | 5.46 ± 0.02 | 5.61 ± 0.02 | 5.73 ± 0.02 | ||
| Crystallite size; DWH (nm) | 7 ± 1 | 19 ± 3 | 66 ± 6 | 84 ± 12 | ||
| Strain (×10−4), εi | −54 ± 32 | 5.6 ± 8.7 | 1.5 ± 1.3 | 6.2 ± 1.7 | ||
The values of lattice parameter are 8.357 ± 0.005, 8.382 ± 0.002, 8.384 ± 0.002 and 8.386 ± 0.007 Å for annealing temperature of 300, 500, 700 and 900 °C respectively. Thus, lattice parameter approaches towards the value of bulk CoFe2O4 with increase of annealing.37
Another, important observation from this refinement is the distribution of cations (Co2+ and Fe3+ ions) among A- and B-sites. The value of Fe3+ ion occupancy is 0.556 ± 0.003, 0.624 ± 0.003, 0.649 ± 0.004 and 0.706 ± 0.004 at B-site for annealing temperature of 300, 500, 700 and 900 °C. Thus, this value is approaching towards 1, which is the behavior of bulk CoFe2O4 and concurrent with the variation of lattice parameter with annealing as discussed earlier. This means higher annealing help to achieve the perfect inverse spinel structure for CoFe2O4.
Crystallite size (D) of these nanoparticles were estimated from most intense (311) peak using Scherrer's formula,26
D = 0.94λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (2) |
Thus, values of lattice parameters, cation occupancies and crystallite size envisage that higher annealing results in the bulk like characteristics of nanoparticles. This kind of behavior is also observed for other ferrite systems as well.
The X-ray density (ρx) of these nanoparticles is determined from following relation
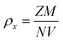 | (3) |
In most of the nanoparticle, strain also exhibits variation with change of nanoparticle size or processing parameters, hence, the strain is estimated by using Williamson–Hall (W–H) plot methods.38 The W–H plots based on the equation given below are shown in Fig. 1.
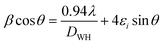 | (4) |
The estimated strain has negative value for the nanoparticles annealed at 300 and 500 °C. Negative value of strain is observed for numerous ferrite nanoparticle systems and associated with presence of compressive strain.39,40 Strain has positive value for the annealing temperature of 700 and 900 °C in agreement with previous studies on cobalt ferrite nanoparticles of similar size.41
Size and morphology
Fig. 2 shows the SEM micrographs of nanoparticles synthesized at 300, 500, 700 and 900 °C. SEM micrographs of nanoparticles synthesized at 300 and 500 °C exhibit bunch of several small particles. These nanoparticles contains particles with almost spherical shape. SEM micrograph of CoFe2O4 nanoparticles synthesized at 700 °C contains mixture of particles with hexagonal type shape and deviated spherical shape (Fig. 2). | ||
| Fig. 2 Scanning electron micrographs of CoFe2O4 nanoparticles having annealing temperature of 300, 500, 700 and 900 °C. | ||
When these nanoparticles are obtained at 900 °C, most of them attain hexagonal type shape. Almost spherical shape of nanoparticles is observed for ferrite nanoparticles and are reported by number of authors.42,43 These authors also observe formation of hexagonal type particle shape at higher sintering temperature. A schematic of shape modification is presented in Fig. 3a.
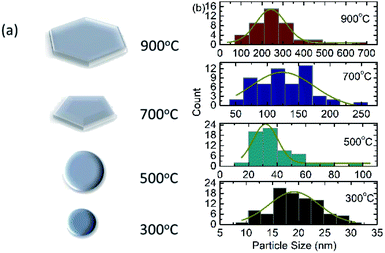 | ||
| Fig. 3 (a) Particle shape overview and (b) size distribution estimated from SEM for CoFe2O4 nanoparticles annealed at 300, 500, 700 and 900 °C. | ||
The information on particle size is obtained from these measurements. The size of almost 150 particles were measured using ImageJ software and size distribution curves of these nanoparticles are shown in Fig. 3b. The particle size estimated from these size distribution curves are 19 ± 9, 32 ± 17, 124 ± 101 and 242 ± 142 nm for annealing temperature of 300, 500, 700 and 900 °C respectively.44,45 Thus, variation of particle size with annealing temperature is concurrent with the crystallite size obtained from XRD pattern.
Magnetic behaviour
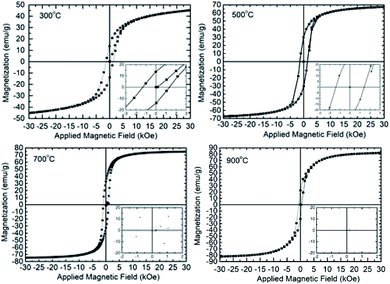
Fig. 4 shows the hysteresis curves of CoFe2O4 nanoparticles synthesized at 300, 500, 700 and 900 °C. These hysteresis curves show remanence and coercive behavior, which modifies with crystallite size.Thus, these curves exhibit ferrimagnetic kind behavior of synthesized nanoparticles. This behavior is general characteristics of ferrites nanoparticles and is observed by number of researchers.21,22,28,46
The saturation magnetization of these nanoparticles are 52 ± 2, 66 ± 2, 70 ± 2 and 81 ± 2 emu g−1 for annealing temperature of 300, 500, 700 and 900 °C (Fig. 5a). The value of saturation magnetization at 900 °C is close to the value of bulk CoFe2O4.37,42,43 Thus saturation magnetization (σs) increases with increase of crystallite size according to the following linear relation.
| σs = (0.5 ± 0.1) × D + (49 ± 4) | (5) |
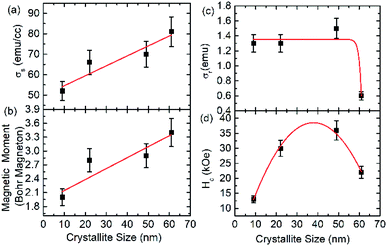 | ||
| Fig. 5 (a) Saturation magnetization, (b) magnetic moment (c) remanent magnetization and (d) coercivity as a function of crystallite size. | ||
The magnetic moment per formula unit for these nanoparticles is calculated from saturation magnetization (σs) and molecular weight (M) of the material using the relation given below28
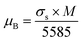 | (6) |
Behavior of magnetic moment with crystallite size is analogues to that of saturation magnetization (Fig. 5b).
The remnant magnetization (σr) of these nanoparticles is represented by exponential decay function as follow (Fig. 5c)
| σr = (1.3 ± 0.1) + (−1.2 × 10−25 ± 1.2 × 10−20) × e−(0.9±0.2)×D | (7) |
The behavior of coercivity (Hc) with crystallite size (D) is represented by a parabolic function (Fig. 5d).
| Hc = (2.3 ± 0.2) × D − (0.030 ± 0.002) × D2 − (5.5 ± 1.5) | (8) |
A detailed investigation carried out by Li et al. (2017) for Fe3O4 nanoparticles envisages decrease of both remanent magnetization and coercivity with increase of size even the saturation magnetization increases. This behavior was explained because of exceeding single domain size limit above certain particle size.47
Optical behaviour
UV-Vis spectra of cobalt ferrite nanoparticles in diffuse reflectance mode are shown in Fig. 6a. The optical band-gap of these nanoparticles are determined from following relation
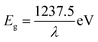 | (9) |
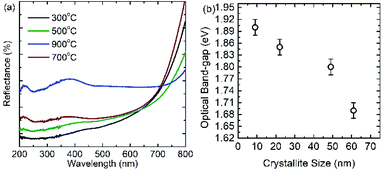 | ||
| Fig. 6 (a) Optical behavior of CoFe2O4 nanoparticles synthesized at 300, 500, 700 and 900 °C. (b) Optical band-gap of nanoparticles as a function of crystallite size. | ||
The optical-band gaps of these nanoparticles are 1.90 ± 0.02, 1.85 ± 0.02, 1.80 ± 0.02 and 1.69 ± 0.02 eV for nanoparticles annealed at 300, 500, 700 and 900 °C. Thus, optical band-gap decreases with increase of crystallite size and enhances with decrease of crystallite size (Fig. 6b). Similar enhancement of optical band-gap with decrease of crystallite size for zinc ferrite nanoparticle is also observed.49 The enhancement of optical band-gap for small sized nanoparticle is explained using quantum confinement.49,50 According to quantum confinement the optical band-gap of nanoparticle is written as
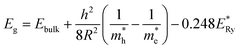 | (10) |
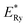 is the bulk excitation energy, h is Planck's constant, R is radius of nanocrystals. This clearly shows increase of optical band gap with decrease of crystallite size.
is the bulk excitation energy, h is Planck's constant, R is radius of nanocrystals. This clearly shows increase of optical band gap with decrease of crystallite size.
Synchrotron radiation based investigation
Oxidation state of metal ions. Fig. 7a shows Fe L-edge spectra of cobalt ferrite nanoparticles synthesized at 300, 500, 700 and 900 °C. Spectral features in the spectra of these nanoparticles are represented by A1, A2, and A3 centered at 708.3 ± 0.1, 719.9 ± 0.1 and 721.5 ± 0.1 eV, respectively. In addition to this, shoulders S1 and S2 centered at 706.5 ± 0.1 and 711.7 ± 0.1 eV also exist in the spectra of these nanoparticles. These spectral features are associated with presence of Fe3+ ions in tetrahedral and octahedral crystal field.26,27,51,52 Further, Fe K-edge spectra reflect spectral features A1′, A2′, A3′, A4′ and A5′ centered at 7132.1 ± 0.4, 7139.3 ± 0.4, 7146.7 ± 0.4, 7155.7 ± 0.4 and 7184.4 ± 0.4 for all nanoparticles synthesized at various annealing temperatures. In addition, a pre-edge spectral feature Ao′, centered at 7114.1 ± 0.4 eV is observed in the spectra of these nanoparticles. Presence of pre-edge in the Fe K-edge spectra of ferrites is common. The intensity of pre-edge spectral features modifies with annealing temperature (Fig. 7b: inset).
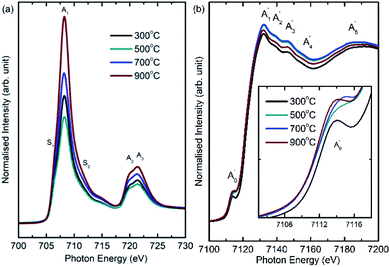 | ||
| Fig. 7 (a) Fe L-edge and (b) Fe K-edge spectra of CoFe2O4 nanoparticles synthesized at 300, 500, 700 and 900 °C. | ||
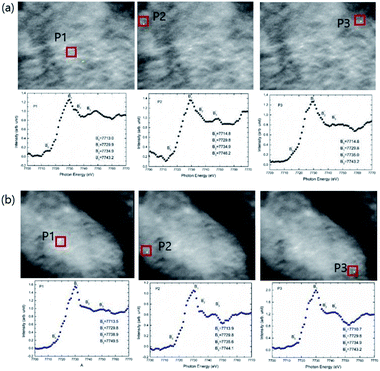
The main edge for these nanoparticles occurs at 7121.7 ± 0.4, 7121.1 ± 0.4, 7121.0 ± 0.4 and 7120.1 ± 0.4 eV for annealing temperature of 300, 500, 700 and 900 °C. Thus, main edge slightly shifts to lower value with increase of annealing temperature (Fig. 7b). The main energy exhibits a shift of around 1.6 ± 0.8 eV when annealing temperature increases to 900 °C from 300 °C. Hence, nanoparticles synthesized at 300 and 900 °C are investigated using XANES-imaging.
XANES spectra extracted from these images from bulk mode (whole image) exhibit similar behavior with annealing temperature (Fig. S2†). In addition, main edge energy shift of Fe K-edge estimated from these measurements is 0.6 ± 2 eV (Fig. 8b) which appears to be similar (considering error values) to that determined from the XANES spectra shown in Fig. 8b.
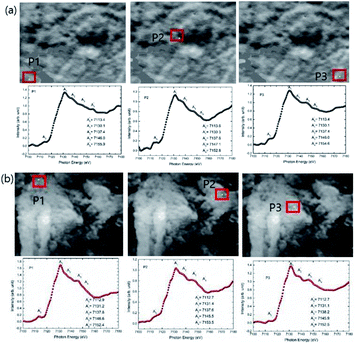 | ||
| Fig. 8 The XANES spectra extracted on the 2-dimensional XANES-imaging results at Fe K-edge for CoFe2O4 nanoparticles synthesized at (a) 300 and (b) 900 °C for selected pixels P1, P2 and P3. | ||
As mentioned earlier, the XANES imaging measurements are able to give spatially resolved chemical information, hence, XANES spectrum are also extracted from single pixels by taking different pixels P1, P2 and P3. Fe K-edge XANES spectra, extracted from P1, P2 and P3 pixels of TXM images for CoFe2O4 nanoparticles synthesized at 300 and 900 °C are shown in Fig. 8. These measurements reveal that Fe K-edge XANES spectra remain almost similar in different regions of materials for lateral resolution down to 35 nm (Fig. 8a and b).
Co L-edge spectra of these materials show spectral features B1 and B2 (Fig. 9a). These spectral features occur at 774.9 ± 0.1 and 778.0 ± 0.1 in the Co L-edge spectra of these nanoparticles. In addition, a shoulder around 776.1 ± 0.1 eV (shown by dotted arrow) is also observed in the spectra of all nanoparticles. Similar nature of Co L-edge spectra is reported for different kind of nanostructures of CoFe2O4.52 Presence of these spectral features in Co L-edge spectra is associated with 2+ oxidation state in both tetrahedral and octahedral environment.52 Co K-edge spectra show spectral features B1′, B2′ and B3′ centered at 7726.0 ± 0.4, 7733.7 ± 0.4 and 7741.8 ± 0.4 eV for all annealing temperatures (Fig. 9b). A minor pre-edge spectral feature, Bo′, centered at 7708.9 ± 0.4 occurs in the spectra of these nanoparticles. Main edge occurs at 7716.6 ± 0.4, 7716.3 ± 0.4, 7716.2 ± 0.4 and 7716.1 ± 0.4 eV for nanoparticles synthesized at 300, 500, 700 and 900 °C. The main edge shifts towards lower value with annealing temperature in case of Co K-edge revealing the same oxidation state at all temperature. This main edge energy shift is 0.5 ± 0.8 eV when annealing temperature increases from 300 and 900 °C. These results are further supported by XANES-imaging spectra at Co K-edges for CoFe2O4 nanoparticles obtained at 300 and 900 °C. XANES (Fig. S3†). Moreover, Co K-edge spectra for different pixels P1, P2 and P3 exhibit almost similar spectral features for each spectra of different nanoparticle system (Fig. 10a and b) revealing almost same local electronic structure of individual particles. These observations are concurrent with that obtained from Fe K-edge measurements.
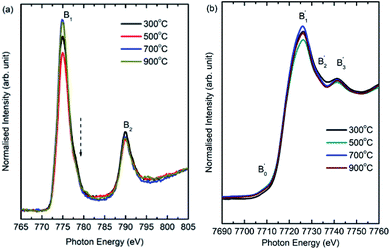 | ||
| Fig. 9 (a) Co L-edge and (b) Co K-edge spectra of CoFe2O4 nanoparticles synthesized at 300, 500, 700 and 900 °C. | ||
Covalence and metal oxygen hybridization
To reveal the nature of metal oxygen hybridization in these nanoparticles, O K-edge NEXAFS measurements were performed and shown in Fig. 11. Pre-edge spectral features C1 and C2 appear at 529.1 ± 0.1 and 530.4 ± 0.1 eV at all annealing temperatures (Fig. 11a). Post-edge spectral features C3, C4, C5 and C6 centered at 536.9 ± 0.1, 540.2 ± 0.1, 546.8 ± 0.1 and 560.7 ± 0.1 eV appear in the O K-edge spectra of all nanoparticles (Fig. 11a). Observed pre- and post-edge spectral features are characteristics of O K-edge spectrum of CoFe2O4 and reported in our previous studies as well as work from other groups.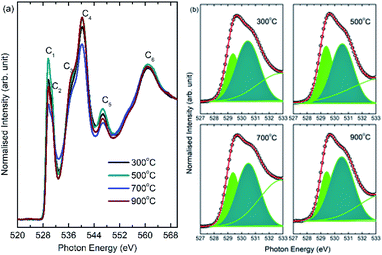 | ||
| Fig. 11 (a) O K-edge spectra of CoFe2O4 nanoparticles annealed at 300, 500, 700 and 900 °C. (b) De-convoluted pre-edge region of O K-edge spectra at corresponding annealing temperature. | ||
Since, it is well established that pre-edge region of O K-edge spectrum reflect the extent of hybridization in ferrite/transition metal based nanoparticles,8,53,54 hence, pre-edge region is critically analyzed. Spectral features C1 and C2 are associated with metal–oxygen hybridization in these nanoparticles and reflect the t2g and eg symmetry states. Thus, to determine the extent of hybridization in these nanoparticles, pre-edge region is de-convoluted into spectral features C1 and C2. The de-convoluted pre-edge regions for different annealing temperatures are shown in Fig. 11b. Ratio of t2g/eg was determined from these de-convoluted features to get quantitative information of metal–oxygen hybridization. t2g/eg ratio for these nanoparticles are 0.4, 0.6, 0.5 and 0.4 for annealing temperatures of 300, 500, 700 and 900 °C. Thus, these studies envisaged modulation of extent of hybridization with annealing temperature.54,55
Local atomic structure
To get insights of local atomic structure, EXAFS spectra at Co K and Fe K-edge are measured (Fig. S4†). These EXAFS spectra are simulated by considering the distribution of cations between A-site and B-site of spinel structure. Crystal information files (CIF) for these sites are generated based on positions of metal and oxygen ions in the unit cell of spinel structures using ATOMS (Table S2†).12Co K-edge EXAFS investigations
The simulated chik spectra (Fig. S5†) and Fourier transform at Co K-edge for various annealing temperature are shown in Fig. 12a. Simulated parameters are collated in Table 2. Bond-distance for Co–O and Co–Co shells among A- and B-site along with fractions of metal ions (n1 and n2) are shown for various annealing temperatures. Co–O shell bond distances are 1.96 and 2.06 Å for A-site and B-site respectively. Co–Co shell bond distances are 3.48 and 2.94 Å for A-site and B-site respectively. Fractions of Co2+ ions attached to A-site (n1) are 0.27 ± 0.05, 0.42 ± 0.06, 0.48 ± 0.03 and 0.34 ± 0.05 for annealing temperature of 300, 500, 700 and 900 °C. Fractions of these ions attached to B-site (n2) are 0.58 ± 0.02, 0.51 ± 0.01, 0.65 ± 0.02 and 0.65 ± 0.04 for annealing temperature of 300, 500, 700 and 900 °C.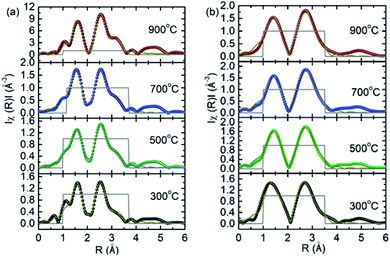 | ||
| Fig. 12 Simulated non-phase corrected Fourier transform of (a) Co and (b) Fe K-edge EXAFS spectra for CoFe2O4 nanoparticles synthesized at 300, 500, 700 and 900 °C. | ||
| S.N. | Atoms | N | R (Å) | σ2 (Å2) | Atoms | N | R (Å) | σ2 (Å2) |
|---|---|---|---|---|---|---|---|---|
| 300 °C; εo = 0.11 eV; R-factor: 0.002 | ||||||||
| A-site (n1 = 0.27 ± 0.05) | B-site (n2 = 0.58 ± 0.02) | |||||||
| 1 | O | 4 | 1.96 | 0.004 | O | 6 | 2.06 | 0.004 |
| 2 | Co | 12 | 3.48 | 0.023 | Co | 6 | 2.94 | 0.005 |
| 3 | Co | 6 | 3.48 | 0.006 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| 500 °C; εo = −0.039 eV; R-factor: 0.003 | ||||||||
| A-site (n1 = 0.42 ± 0.06) | B-site (n2 = 0.51 ± 0.02) | |||||||
| 1 | O | 4 | 1.96 | 0.004 | O | 6 | 2.07 | 0.004 |
| 2 | Co | 12 | 3.46 | 0.012 | Co | 6 | 2.94 | 0.005 |
| Co | 6 | 3.46 | 0.012 | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| 700 °C; εo = −0.78 eV; R-factor: 0.001 | ||||||||
| A-site (n1 = 0.48 ± 0.03) | B-site (n2 = 0.65 ± 0.02) | |||||||
| 1 | O | 4 | 1.96 | 0.001 | O | 6 | 2.07 | 0.003 |
| 2 | Co | 12 | 3.47 | 0.011 | Co | 6 | 2.93 | 0.005 |
| Co | 6 | 3.4 | 0.009 | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| 900 °C; εo = 0.14 eV; R-factor: 0.004 | ||||||||
| A-site (n1 = 0.34 ± 0.05) | B-site (n1 = 0.65 ± 0.04) | |||||||
| 1 | O | 4 | 1.96 | 0.005 | O | 6 | 2.07 | 0.004 |
| 2 | Co | 12 | 3.47 | 0.010 | Co | 6 | 2.94 | 0.005 |
| Co | 6 | 3.47 | 0.009 | |||||
Fe K-edge EXAFS investigations
The simulated chik spectra (Fig. S6†) and Fourier transform at Fe K-edge are shown in Fig. 12b. Simulated parameters are collated in Table 3. Bond-distance for Fe–O and Fe–Fe shells among A- and B-site along-with fractions of Fe3+ ions (n1 and n2) are shown for various annealing temperatures. Fe–O shell bond distances are 1.82 and 2.01.| S.N. | Atoms | N | R (Å) | σ2 (Å2) | Atoms | N | R (Å) | σ2 (Å2) |
|---|---|---|---|---|---|---|---|---|
| 300 °C; εo = −0.79 eV; R-factor: 0.01 | ||||||||
| A-site (n1 = 0.33 ± 0.08) | B-site (n2 = 0.89 ± 0.06) | |||||||
| 1 | O | 4 | 1.82 | −0.006 | O | 6 | 2.01 | 0.004 |
| 2 | Fe | 12 | 3.51 | 0.009 | Fe | 6 | 2.99 | 0.008 |
| Fe | 6 | 3.50803 | 0.015 | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| 500 °C; εo = −2.32 eV; R-factor: 0.006 | ||||||||
| A-site (n1 = 0.43 ± 0.08) | B-site (n2 = 0.83 ± 0.05) | |||||||
| 1 | O | 4 | 1.85 | 0.001 | O | 6 | 1.99 | 0.006 |
| 2 | Fe | 12 | 3.47 | 0.008 | Fe | 6 | 2.96 | 0.007 |
| Fe | 6 | 3.47 | 0.008 | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| 700 °C; εo = −1.64 eV; R-factor: 0.006 | ||||||||
| A-site (n1 = 0.40 ± 0.05) | B-site (n2 = 0.80 ± 0.05) | |||||||
| 1 | O | 4 | 1.84 | 0.003 | O | 6 | 1.98 | 0.006 |
| 2 | Fe | 12 | 3.48 | 0.006 | Fe | 6 | 2.96686 | 0.006 |
| Fe | 6 | 3.48287 | 0.006 | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| 900 °C; εo = 4.1 ± 1.6 eV; R-factor: 0.01 | ||||||||
| A-site (n1 = 0.5 ± 0.2) | B-site (n2 = 0.9 ± 0.1) | |||||||
| 1 | O | 4 | 1.82 | 0.003 | O | 6 | 1.98 | 0.004 |
| 2 | Fe | 12 | 3.47 | 0.009 | Fe | 6 | 2.97 | 0.008 |
| Fe | 6 | 3.47 | 0.009 | |||||
Å for A- and B-site respectively. Fe–Fe shell bond distances are 3.51 and 2.98 Å for A-site and B-site respectively. Fractions of Fe3+ ions attached to A-site (n1) are 0.33 ± 0.08, 0.43 ± 0.08, 0.40 ± 0.05 and 0.5 ± 0.2 for annealing temperature of 300, 500, 700 and 900 °C. Fractions of these ions attached to B-site (n2) are 0.89 ± 0.06, 0.83 ± 0.05, 0.80 ± 0.05 and 0.9 ± 0.1 for annealing temperature of 300, 500, 700 and 900 °C.
Local magnetic structure
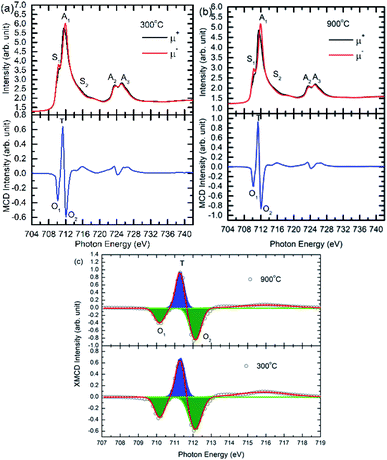
To understand the magnetic interaction in these nanoparticles, XMCD spectra for nanoparticles synthesized at 300 and 900 °C are shown in Fig. 13. XAS spectra under different helicity of X-ray exhibit spectral features S1, A1, S2, A3 and A4 for these annealing temperatures (Fig. 13a and b: Upper panels). Origin of these spectral features are already discussed. Fig. 13a and b (Lower panels) also depicts MCD signal for these nanoparticles. Feature assigned as T is associated with presence of Fe3+ ions among tetrahedral sites, however, spectral features O1 and O2 is due to presence of these ions among octahedral sites.Positive spectral features, T and negative spectral features O1, O2 in the MCD spectra of these nanoparticles are due to negative exchange interaction among Fe3+ ions situated at A- and B-sites.56,57 Area of these spectral features is determined by de-convoluting these features (Fig. 13c). This envisages that almost 34% of Fe3+ ions reside on A-site, however, this fraction is almost 66% at A-site for nanoparticle synthesized at 300 °C. In case of nanoparticles synthesized at 900 °C, these fractions are 31% and 69% for A- and B-sites respectively.
Discussion
Thus, EXAFS and XMCD investigations envisage redistribution of cations with annealing temperature/crystallite size. To determine exact occupancy of metal ions from EXAFS following relation was used.For Co2+ ions,
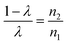 | (11) |
For Fe3+ ions,
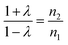 | (12) |
Values of λ are estimated from these relations that are used to determine occupancies of Co2+ and Fe3+ ions (Table 4). Occupancies determined from EXAFS are similar to that obtained from XRD (Tables 1 and 4).
| Parameters | CoFe2O4 nanoparticles | |||||
|---|---|---|---|---|---|---|
| 300 °C | 500 °C | 700 °C | 900 °C | |||
| Occupancy | A-site | Co2+ | 0.31 | 0.45 | 0.42 | 0.35 |
| Fe3+ | 0.54 | 0.60 | 0.67 | 0.71 | ||
| B-site | Co2+ | 0.49 | 0.55 | 0.58 | 0.65 | |
| Fe3+ | 1.46 | 1.40 | 1.33 | 1.29 | ||
| Magnetic moment | A-site | 5.2 | 5.3 | 5.6 | 5.6 | |
| B-site | 10.5 | 10.4 | 10.1 | 10.1 | ||
| Effective magnetic moment (μB) | 5.3 | 5.0 | 4.5 | 4.5 | ||
| Experimental magnetic moment (μB) | 2.2 | 2.8 | 2.9 | 3.4 | ||
The theoretical magnetic moment of ferrite is given as
| m = mB-site − mA-site | (13) |
Based on structural formula mention in eqn (1), the magnetic moment of ferrite can be derived directly from magnetic moments of it's constituent Co2+ and Fe3+ ions from the equation given below
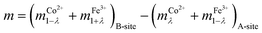 | (14) |
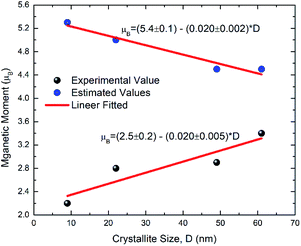
The magnetic moment of each nanoparticles was determined using eqn (14) by taking magnetic moment of Fe3+ and Co2+ ions as 5.92 and 3.87 μB.58 The magnetic moments estimated from these relation are 5.3, 5.0, 4.5 and 4.5 μB for nanoparticles synthesized at 300, 500, 700 and 900 °C (Table 4).
The experimentally observed value of magnetic moment for these nanoparticles are 2.2, 2.8, 2.9 and 3.4 μB (Table 4). Thus, difference among the theoretical and experimentally observed values of magnetic moment is large for lower crystallite size nanoparticles (Fig. 14). The other factors which affect the magnetization are spin canting59 and formation of spin-glass state at the surface.60,61 These factors diminishes the magnetization in ferrite nanoparticles.59–62 Hence, the spin canting and spin-glass state effects rather than particle size effects or cation redistribution influences the magnetic behavior of these nanoparticles.
Conclusions
In conclusion, pure cubic spinel phase of synthesized CoFe2O4 nanoparticles was observed. Both the crystallite/particle size increases with annealing temperature. The particle shape also modifies from spherical to hexagonal type with increase of annealing. All nanoparticles exhibit ferrimagnetic behavior with linear increase of saturation magnetization and magnetic moment with enhancement of crystallite size. Variation of other magnetic parameters like remanent magnetization and coercivity reflects the single domain behavior below 55 nm. Moreover, optical band-gap of these nanoparticle is influenced by optical confinement.The nanoparticles exhibit modification of Co, Fe (3d)–O (2p) hybridized states with increase of annealing temperature as envisaged from the O K-edge NEXAFS spectra. The presence of Fe and Co ions in 3+ and 2+ state is evidenced from the Fe and Co L-edge NEXAFS, XANES and spatially resolved XANES-imaging techniques. Distribution of Fe and Co ions estimated from EXAFS simulation corroborates the results obtained from Rietveld refinement of X-ray diffraction patterns. XMCD revealed the negative exchange interaction among the metal ions situated at A- and B-sites.
Author's contribution
JPS conceptualised the work, performed analysis and wrote the manuscript in consultation with SL and KHC. JPS, JYP and SL performed the XANES-imaging experiment and analysed the results. SHK, WCL performed the SEM, UV-Vis and XRD measurements. JPS, HK measured and analysed the hysteresis curves. VS and KHC performed the XAS experiments and simulated the obtained results. JPS, YHK and KHC carried out the XMCD measurements.Conflicts of interest
Authors declare no conflicts of interests.Acknowledgements
The research program of Commercializations Promotion Agency for R&D Outcomes (COMPA) funded by the Ministry of Science and ICT (1711080502), financially supports this research. KHC acknowledges the financial support received by Korea Institute of Science and Technology, Seoul, Republic of Korea through project no. 2V08170.Notes and references
- X. Cai, K. Chen, X. Gao, C. Xu, M. Sun, G. Liu, X. Guo, Y. Cai, B. Huang, J. Deng, J. Z. Liu, A. Tricoli, N. Wang, C. Dwyer and Y. Zhu, Chem. Mater., 2019, 31, 5769 CrossRef CAS.
- T. Bernges, J. Peilstöcker, M. Dutta, S. Ohno, S. P. Culver and K. Biswas, Inorg. Chem., 2019, 58, 9236 CrossRef CAS PubMed.
- C. V. Ovidiu Garlea, V. Yannello, H. Cao and A. F. Bangura, Inorganic Chemistry, 2019, 58(9), 5799 CrossRef PubMed.
- C. Richard and A. Catlow, Philos. Trans. R. Soc., A, 2015, 373, 20130162 CrossRef PubMed.
- X. Liu and T.-C. Weng, MRS Bull., 2016, 41, 466 CrossRef CAS.
- G. van der Laan and A. I. Figueroa, Coord. Chem. Rev., 2014, 95, 129 Search PubMed.
- A. Sakdinawat and D. Attwood, Nat. Photonics, 2010, 4, 840 CrossRef CAS.
- J. P. Singh, S. H. Kim, S. O. Won, W. C. Lim, I. Lee and K. Hwa Chae, CrystEngComm, 2016, 18, 2701 RSC.
- K. Kumari, R. Naji Aljawfi, Y. S. Katharria, S. Dwivedi, K. H. Chae, R. Kumar, A. Alshoaibi, P. A. Alvi, S. Dalela and S. Kumar, J. Electron Spectrosc. Relat. Phenom., 2019, 235, 29 CrossRef CAS.
- S. Jauhar, J. Kaur, A. Goyal and S. Singhal, RSC Adv., 2016, 6, 97694 RSC.
- A. Varma, A. S. Mukasya, A. S. Rogachev and K. V. Manukyan, Chem. Rev., 2016, 116, 14493–14586 CrossRef CAS PubMed.
- E. Lottini, A. López-Ortega, G. Bertoni, S. Turner, M. Meledina, G. Van Tendeloo, C. de Julián Fernández and C. Sangregorio, Chem. Mater., 2016, 28, 4214 CrossRef CAS.
- A. V. Ramosa, M.-J. Guittet, J.-B. Moussy, R. Mattana, C. Deranlot, F. Petroff and C. Gatel, Appl. Phys. Lett., 2007, 91, 122107 CrossRef.
- D. Moitra, M. Chandel, B. Kumar Ghosh, R. Kumar Jani, M. Kumar Patra, S. Raj Vadera and N. N. Ghosh, RSC Adv., 2016, 6, 76759 RSC.
- N. Xu, J. Qiao, Q. Nie, M. Wang, He Xu, Y. Wang and X. Zhou, Catal. Today, 2018, 315, 144 CrossRef.
- S. Permien, S. Indris, U. Schürmann, L. Kienle, S. Zander, S. Doyle and W. Bensch, Chem. Mater., 2016, 28, 434 CrossRef CAS.
- S. Y. Srinivasan, K. M. Paknikar, D. Bodas and V. Gajbhiye, Nanomedicine, 2018, 13, 1221 CrossRef CAS PubMed.
- B. D. Cullity, Introduction to Magnetic Mater, 1972 Search PubMed.
- H. Kumar, R. C. Srivastava, J. P. Singh, P. Negi, H. M. Agrawal, D. Das and K. Hwa Chae, J. Magn. Magn. Mater., 2016, 401, 16 CrossRef CAS.
- M. Artus, L. Ben Tahar, F. Herbst, L. Smiri, F. Villain, N. Yaacoub, J. Grenèche, S. Ammar and F. Fiévet, J. Phys.: Condens. Matter, 2011, 23, 506001 CrossRef PubMed.
- H. Kumar, J. P. Singh, R. Chandra Srivastava, P. Negi, H. M. Agrawal, K. Asokan, S. Ok Won and K. Hwa Chae, J. Alloys Compd., 2015, 645, 274 CrossRef CAS.
- D. Sharma and N. Khare, AIP Adv., 2016, 6, 085005 CrossRef.
- C. Singh, A. Goyal and S. Singhal, Nanoscale, 2014, 6, 7959 RSC.
- J. P. Singh, G. Dixit, R. C. Srivastava, H. M. Agrawal, V. R. Reddy and A. Gupta, J. Magn. Magn. Mater., 2012, 324, 2553 CrossRef CAS.
- M. Tsvetkov, M. Milanova, I. Ivanova, D. Neov, Z. Cherkezova-Zheleva, J. Zaharieva and M. Abrashev, J. Mol. Struct., 2019, 1179, 233 CrossRef CAS.
- J. P. Singh, S. Ok Won, W. Cheol Lim, I. Jae Lee and K. H. Chae, J. Mol. Struct., 2016, 1108, 444 CrossRef CAS.
- J. P. Singh, B. Kaur, A. Sharma, S. Hee Kim, S. Gautam, R. C. Srivastava, N. Goyal, W. Cheol Lim, H.-J. Lin, J. M. Chen, K. Asokan, D. Kanjilal, S. O. Won, I.-J. Lee and K. H. Chae, Phys. Chem. Chem. Phys., 2018, 20, 12084 RSC.
- H. Kumar, J. P. Singh, R. C. Srivastava, K. R. Patel and K. Hwa Chae, Superlattices Microstruct., 2017, 109, 296 CrossRef CAS.
- J. P. Singh, S. Lee and K. H. Chae, Vacuum, 2019, 168, 108848 CrossRef CAS.
- H. Hwi Lee, M. Kumar and H. Joon Shin, Physics and Advanced Technology, 2017, 26, 7 Search PubMed.
- H. J. Lee, G. Kim, D. H. Kim, J.-S. Kang, C. L. Zhang, S.-W. Cheong, J. H. Shim, S. Lee, H. Lee and J.-Y. Kim, J. Phys.: Condens. Matter, 2008, 20, 295203 CrossRef.
- S. Lee, I. H. Kwon, J.-Y. Kim, S. S. Yang, S. Kang and J. Lim, J. Synchrotron Radiat., 2017, 24, 1276 CrossRef CAS PubMed.
- J. Y. Park, J. P. Singh, J. Lim, K. H. Chae and S. Lee, Mater. Lett., 2020, 261, 126983 CrossRef CAS.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537 CrossRef CAS PubMed.
- A. L. Ankudinov, B. Ravel, J. J. Rehr and S. D. Conradson, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 7565 CrossRef CAS.
- J. Y. Park, J. P. Singh, J. Lim and S. Lee, J. Synchrotron Radiat., 2020, 27, 545 CrossRef CAS PubMed.
- T. Meron, Y. Rosenberg, Y. Lereah and G. Markovich, J. Magn. Magn. Mater., 2005, 292, 11 CrossRef CAS.
- J. P. Singh, R. C. Srivastava, H. M. Agrawal and P. Chand, Int. J. Nanosci., 2009, 8, 523 CrossRef CAS.
- G. A. Kaur, M. Shandilya, P. Rana, S. Thakur and P. Uniyal, Nano-Structures & Nano-Objects, 2020, 22, 100428 Search PubMed.
- D. M. Ghone, V. L. Mathe, K. K. Patankar and S. D. Kaushik, J. Alloys Compd., 2018, 739, 52 CrossRef CAS.
- A. S. Ponce, E. F. Chagas, R. J. Prado, C. H. M. Fernades, A. J. Terezo and E. B. Saitovitch, J. Magn. Magn. Mater., 2013, 344, 182 CrossRef CAS.
- A. López-Ortega, E. Lottini, C. de Julián Fernández and C. Sangregorio, Chem. Mater., 2015, 27, 4048 CrossRef.
- J. P. Singh, G. Dixit, R. C. Srivastava, H. M. Agrawal and R. Kumar, J. Alloys Compd., 2013, 551, 370 CrossRef CAS.
- R. S. Yadav, I. Kuřitka, J. Vilcakova, J. Havlica, J. Masilko, L. Kalina, J. Tkacz, J. Švec, V. Enev and M. Hajdúchová, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2017, 8, 045002 Search PubMed.
- Z. Ž. Lazarević, Č. Jovalekić, D. Sekulić, M. Slankamenac, M. Romceevic, A. Milutinović and N. Ž. Romčević, Sci. Sintering, 2012, 44, 331 CrossRef.
- R. Zhang, Q. Yuan, R. Ma, X. Liu, C. Gao, M. Liu, C.-L. Jia and H. Wang, RSC Adv., 2017, 7, 21926 RSC.
- Q. Li, C. W. Kartikowati, S. Horie, T. Ogi, T. Iwaki and K. Okuyama, Sci. Rep., 2017, 7, 9894 CrossRef.
- J. Llanos, C. Mujica and A. Buljan, J. Alloys Compd., 2001, 316, 146–152 CrossRef CAS.
- J. P. Singh, R. C. Srivastava and H. M. Agrawal, AIP Conf. Proc., 2010, 1276, 137 CrossRef.
- S. T. Tan, B. J. Chen, X. W. Sun, W. J. Fan, H. S. Kowk, X. H. Xhang and S. J. Chua, J. Appl. Physiol., 2005, 98, 013505 CrossRef.
- B. Gilbert, J. E. Katz, J. D. Denlinger, Y. Yin, R. Falcone and G. A. Waychunas, J. Phys. Chem. C, 2010, 114, 21994 CrossRef CAS.
- S. Nappini, E. Magnano, F. Bondino, P. Igor, A. Barla, E. Fantechi, F. Pineider, C. Sangregorio, L. Vaccari, L. Venturelli and P. Baglioni, J. Phys. Chem. C, 2015, 119, 25529 CrossRef CAS.
- J. P. Singh, J. Y. Park, K. H. Chae, D. Ahn and S. Lee, Nanomaterials, 2020, 10, 759 CrossRef PubMed.
- S. Shen, J. Zhou, C.-L. Dong, Y. Hu, E. Nestor Tseng, P. Guo, L. Guo and S. S. Mao, Sci. Rep., 2014, 4, 6627 CrossRef CAS.
- J. P. Singh, H. Kuang, S. Lee and K. H. Chae, Investigation of Metal–Oxygen Hybridization Process during the Growth of ZnFe2O4 Films on MgO (100) Substrates, (to be communicated).
- P. M. Zélis, G. A. Pasquevich, K. L. S. Rodríguez, F. H. Sánchez and C. E. Rodríguez Torres, J. Magn. Magn. Mater., 2016, 419, 98 CrossRef.
- C. E. R. Torres, G. A. Pasquevich, P. Mendoza Zélis, F. Golmar, S. P. Heluani, S. K. Nayak, W. A. Adeagbo, W. Hergert, M. Hoffmann, A. Ernst, P. Esquinazi and S. J. Stewart, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 104411 CrossRef.
- K. C. de Berg and K. J. Chapman, J. Chem. Educ., 2001, 78, 670 CrossRef CAS.
- T. J. Daou, J. M. Greneche, S. J. Lee, S. Lee, C. Lefevre, S. Bégin-Colin and G. Pourroy, J. Phys. Chem. C, 2010, 114, 8794 CrossRef CAS.
- F. Zeb, W. Sarwer, K. Nadeem, M. Kamran, M. Mumtaz, H. Krenn and I. Letofsky-Papst, J. Magn. Magn. Mater., 2016, 407, 241 CrossRef CAS.
- J. Mantilla, L. León Félix, M. A. R. Martinez, P. Souza, P. A. M. Rodrigues, L. C. Figueiredo, S. W. da Silva, J. A. H. Coaquira, F. F. H. Aragón and P. C. Morais, J. Magn. Magn. Mater., 2019, 476, 392 CrossRef CAS.
- J. P. Singh, R. C. Srivastava, H. M. Agrawal, R. Kumar, V. R. Reddy and A. Gupta, J. Magn. Magn. Mater., 2010, 322, 1701 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra01653e |
| This journal is © The Royal Society of Chemistry 2020 |