Open Access Article
Open Access ArticleAssembly of graphene oxide on cotton fiber through dyeing and their properties
Jie Zhoua,
Qiulan Luob,
Pu Gaob and
Hui Ma *a
*a
aChina-Australia Institute for Advanced Materials and Manufacturing, College of Material and Textile Engineering, Jiaxing University, Jiaxing, 314001, PR China. E-mail: mahone1136@163.com; Fax: +86-573-83640322; Tel: +86-573-83641175
bCollege of Nanhu, Jiaxing University, Jiaxing, Zhejiang 314001, PR China
First published on 24th March 2020
Abstract
Materials with electrical conductivity are preferred for electronics, medical, space and other applications. Flexible, stretchable and washable conductive fabrics have been preferred over metallic materials. However, the currently available conductive fabrics are mainly made using a dip-drying process which makes it difficult to obtain a regular assembly structure of graphene sheets on the fibers. In this research, we report the development of conductive cotton fabrics through simple dyeing, graphene oxide (GO) with two distinct sizes was used to dye the fabrics which were later reduced using hydrazine hydrate. The regularity of graphene sheets on the surface of the cotton fiber can be improved by level assembly, it is beneficial to the conductive stability of the later drawing, bending and friction process. The results show that, the fabrics coated with graphene had excellent fastness to washing, friction and bending. After 20 washings and exposure to 2000 rubbing and 1000 bending cycles, the fabrics had excellent conductivity retention of 86%, 55% and 99%, respectively. In addition, the introduction of graphene causes the dyed fabric to have good infrared absorption and excellent UV resistance. Using cotton fabrics and GO to impart conductivity and UV resistance would be an affordable, sustainable and novel approach to develop functionalized materials for various applications.
1. Introduction
Functionalized materials, particularly those made from natural polymers, provide a unique opportunity to develop devices, sensors and other aids for various applications. Among the various functionalizations, making polymers conductive is desired for biosensors, actuators, rechargeable batteries, separator films etc.1 Similarly, functionalization has also been done to improve anti-flammable, antibacterial and pH and temperature responsive materials.2 A recent trend has been to develop functionalized fabrics for energy storage, health care and protective applications.3,4 Various forms of materials including fibers, particles, nanowires and fabrics have been developed. Fabrics offer higher flexibility both in terms of design and applications and hence are widely functionalized for specific uses. In addition to fabrics, fibers have also been developed using conductive polymers such as polyaniline and polypyrrole.5Cotton is the most commonly available and used natural cellulose fiber. Fabrics have been made from cotton since time immemorial not only for clothing but also as bandages, gauze and for wide variety of applications. Cotton fabrics are not only inexpensive, biodegradable and available in different shapes, sizes and configurations, they are also easily modifiable and are hydrophilic. Many attempts have been made to functionalize cotton fabrics to meet specific needs. Treating cotton fabrics with silver nanowires provided considerable resistance against Gram positive and Gram negative bacterial and also able to efficiently block UV rays with a UV protection factor of 113 and increase hydrophobicity of the fabrics up to a contact angle of 156°.6 ZnO nanoparticles were dyed onto cotton fabrics (up to 10% on weight of the fabrics) resulting in excellent UV resistance up to 20 cycles after washing.7 Thermal and flame resistant properties of cotton fabrics were substantially improved after functionalizing with a novel nitrogen containing carboxyl functionalized organophosphorous.8
Graphene based materials, particularly graphene oxide has been extensively used to modify polymers including fabrics. Synthetic graphene oxide was deposited (up to 19.5%) onto silk fabrics by dry coating and reducing using L-ascorbic acid solution. Modified silk fabrics had improved fire resistance, smoke suppression and sheet resistance. The GO modified silk fabrics were assembled into a sensitive and flexible sensor for incorporating into a wearable device.9 GO was dyed onto acrylic yarns and immobilized and later converted into graphene by treating with sodium hydrosulfite solution. The modified fibers had electrical resistivity ranging from 102 to 1010 Ω cm−1.10 Thermally reduced graphene oxide nanosheets were coated onto flexible carbon fabric for developing supercapacitors. The GO containing carbon acted as excellent supercapacitors with capacitance values as high as 70 F g−1 at 5 mV s−1.11
Studies have reported the use of graphene and graphene oxide to modify the functions and stability of cotton fabrics as well. For instance, flexible conductive cotton fabrics were developed by depositing graphene oxide dispersion and later reducing the GO by hot pressing at 180 °C for 60 minutes. Modified fabrics had good performance even after 400 cycles with electrical resistance varying from −3500 kΩ to −10 kΩ.12 Instead of GO, graphene nanoribbons were wet coated onto cotton fabrics with uniform distribution and good interaction. In addition to increase in strength and modulus by 59 and 61%, respectively, the fabrics were also conductive and suitable for fabricating smart textiles.13 Using three different approaches (direct adsorption, radiation induced crosslinking and chemical crosslinking), GO was fixed onto cotton fabrics to improve their antibacterial performance. An antibacterial activity of up to 98% before washing and >90% even after 100 washing cycles was reported.14 Using a simple heat treatment, GO was reduced onto cotton fabrics to obtain good electrical conductivity, surface hydrophobicity and ultraviolet protection even after considerable bending and washing.15
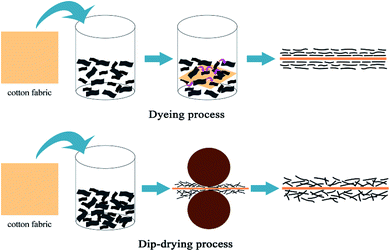
However, most conductive GO-based fabrics are fabricated by dip-drying process, and there are no reports on dyeing GO particles onto cotton fabrics to improve conductive stability and other properties, including infrared adsorption and UV resistance. In the dyeing process, the free enthalpy of graphene sheets in water is lower, the direction of graphene sheets in water is easier, and the arrangement of graphene flakes on the fiber surface is more uniform and regular compared to dip-drying process, the differences between the two methods are shown in Fig. 1. In this paper, cotton fabrics have been dyed with GO of two distinct sizes and the morphology, structure, conductive stability in the drawing, bending and friction process and other properties (infrared adsorption and UV resistance) of modified fabrics have been studied.
2. Experiment section
2.1. Materials
Graphite powder (particle size < 30 μm) was purchased from Sino pharm Chemical Reagent Co. Ltd., China. Commercial 100% cotton woven fabrics (plain weave, 560 × 276, 109 g m−2), other agents, chemicals including sulfuric acid (H2SO4, 98 wt%), hydrogen peroxide (H2O2, 30 wt%), NaOH, hydrazine hydrate (N2H4·H2O), sodium nitrate (NaNO3) and potassium permanganate (KMnO4), were supplied by the Sino pharm Chemical Reagent Co. Ltd., Shanghai, China. Deionized water was used for all the experiments in this study.2.2. Experimental
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
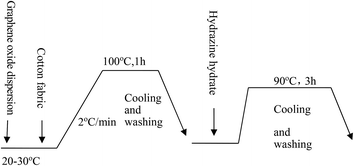
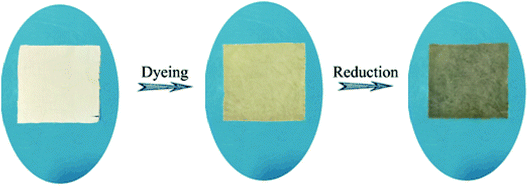
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. The dyeing time was maintained at 60 min. After dyeing, the fabrics were reduced by hydrazine hydrate at 90 °C for 3 h and then washed with cold water to dry. The dyeing process curve and the appearance of the fabric before and after dyeing is shown in Fig. 3 and 2, respectively.
30. The dyeing time was maintained at 60 min. After dyeing, the fabrics were reduced by hydrazine hydrate at 90 °C for 3 h and then washed with cold water to dry. The dyeing process curve and the appearance of the fabric before and after dyeing is shown in Fig. 3 and 2, respectively.
2.3. Characterization
The morphology of the prepared GO samples was analyzed by field emission scanning electron microscopy (FE-SEM). The prepared GO was tested for changes in the carboxyl group content using X-ray photoelectron spectroscopy (XPS). The electrical conductivity of the fabric was tested using a universal meter. The thermal conductivity of the fabric was tested using an infrared thermal imager (FLK-TIR32 9HZ, Fluke, USA). The UV resistance of the fabric was tested in an UV tester (UV-2000, Labsphere Inc. USA).3. Results and discussion
3.1. Morphology of GO
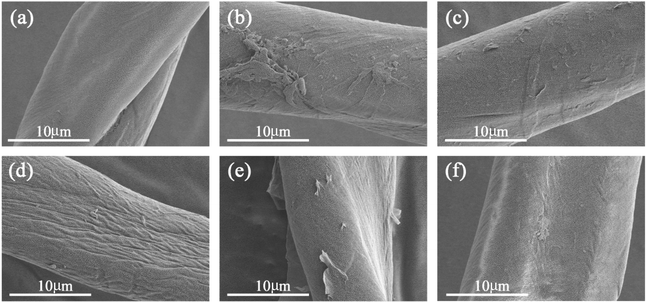
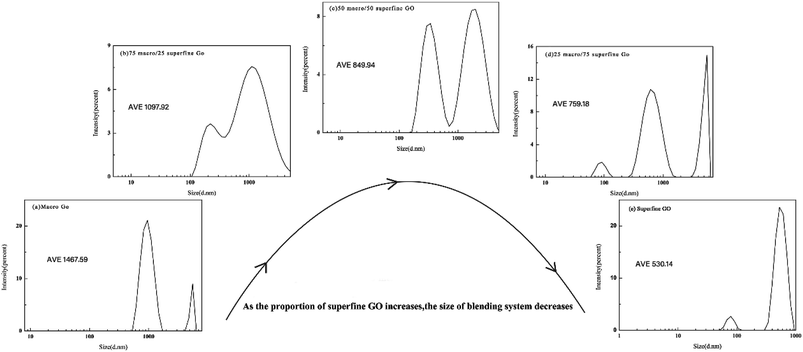
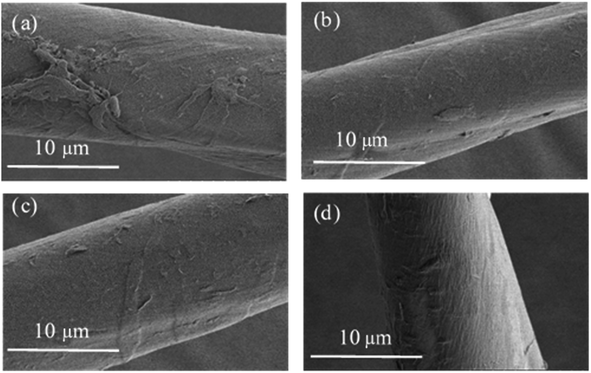
Considerable changes can be observed in the surface features of cotton fibers before and after treating with GO of different particle sizes as shown in Fig. 4. Untreated fibers have smooth and clear appearance (a). The surface of cotton fiber treated with pure macro GO becomes more rougher, and show considerable amounts of deposits on the surface (b). With increasing the ratio of superfine size GO in the blend makes the surface smoother gradually (as shown in (c) and (d)), especially for the cotton fiber dyed with a ratio of 50 macro/50 superfine GO as shown in Fig. 4(d), there is almost no stacking phenomenon and has an unique ridged shape morphology. However, as the proportion of the superfine layers increases further, there is a peeling phenomenon in Fig. 4(e), and also deposited unevenly on the surface of fiber by pure superfine GO in Fig. 4(f). With the increase of the proportion of superfine GO, there are strong π–π conjugation and van der Waals interaction between GO sheets enlarging the specific surface area, and therefore, it is prone to agglomeration. This formation has a direct relationship with subsequent performance.Combining macro GO particles with superfine ones increases their average size as seen from Fig. 5. Without any superfine particles, the size of GO is 1467 nm, and decreases to 759 nm when the proportion is 25 macro/75 superfine GO, the average particle size of pure superfine GO is about 530 nm.
3.2. Carboxyl content of GO surface
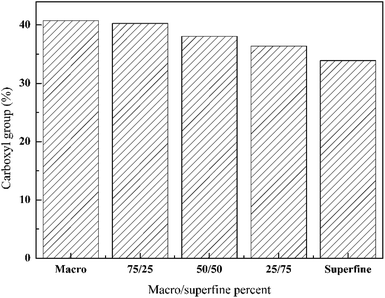
A progressive decrease in carboxyl content on the surface of the fibers occurs as the ratio of the smaller size GO increases. As seen from Fig. 6, highest carboxyl group content of 40% was obtained when the macro GO was used. The number of functional groups decrease because of the formation of layers and also due to oxidation17,18 which decreases the number of groups. Also, large sized particles not only have higher number of carboxyl group content but also have better sorption on the fibers due to their higher polarity. Although the smaller GO has lower number of carboxyl group content, they have a larger specific surface area and also tend to form aggregates. When the macro and superfine pieces of GO are mixed, the aggregation on the fiber surface can be reduced. An optimum ratio of small and large particles is necessary to achieve the desired properties.3.3. Electrical performance of cotton fabrics
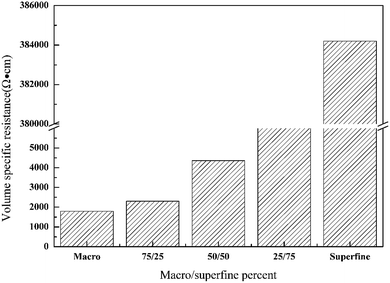
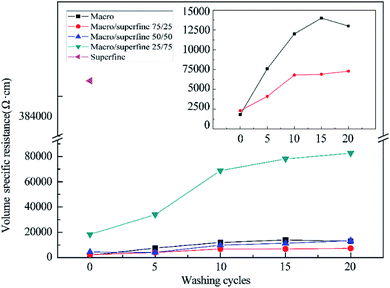
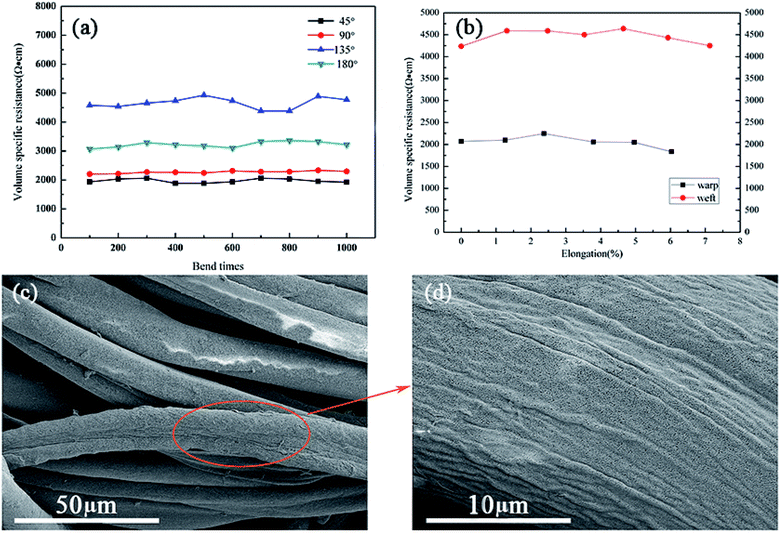
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Ω cm (622%) after 20 washing cycles for the fabrics containing larger GO. Reduction in conductivity (increase in resistance) was lower for the fabrics treated with blended GO. As seen from Fig. 8, the change in resistance was from 2300 to 7300 Ω cm (217%) when a blend of 75/25 of macro and superfine GO are used. The cotton fabric assembled with macro GO sheets has good conductivity, but the partial aggregations of reduced GO will fall off in the process of washing, resulting in the decrease of electrical conductivity. Nevertheless, by adding superfine GO, continuous and stable assemblies of macro and superfine graphene can be formed after reduction, which is beneficial to prevent the falling off of macro graphene sheets, thus improving the conductivity stability of the resulting materials.
000 Ω cm (622%) after 20 washing cycles for the fabrics containing larger GO. Reduction in conductivity (increase in resistance) was lower for the fabrics treated with blended GO. As seen from Fig. 8, the change in resistance was from 2300 to 7300 Ω cm (217%) when a blend of 75/25 of macro and superfine GO are used. The cotton fabric assembled with macro GO sheets has good conductivity, but the partial aggregations of reduced GO will fall off in the process of washing, resulting in the decrease of electrical conductivity. Nevertheless, by adding superfine GO, continuous and stable assemblies of macro and superfine graphene can be formed after reduction, which is beneficial to prevent the falling off of macro graphene sheets, thus improving the conductivity stability of the resulting materials.
Progressive removal of GO from the surface of the fibers with increase in washing cycles is evident from the SEM images shown in Fig. 9. Fabrics dyed with larger GO show considerable differences in morphology (Fig. 9(a and b)), whereas those dyed with 75/25 graphene particles have similar morphology before and after washing (Fig. 9(c and d)). This result can prove that the regularity of graphene assembly on the surface of cotton fiber is conducive to the stability of conductivity.
3.4. Applications of GO modified cotton fabrics
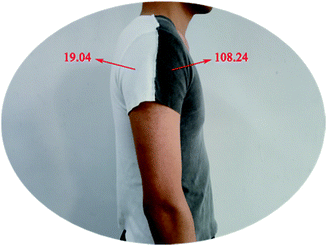
| Numerical value | Undyed | After dyed | Washing cycles | ||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | |||
| TUVA/% | 8.46 | 1.04 | 1.09 | 1.12 | 1.16 | 1.17 | 1.16 |
| TUVB/% | 4.25 | 0.88 | 0.92 | 0.97 | 0.98 | 0.98 | 0.99 |
| AVE UPF | 19.04 | 108.24 | 105.37 | 103.59 | 103.40 | 102.60 | 102.95 |
4. Conclusions
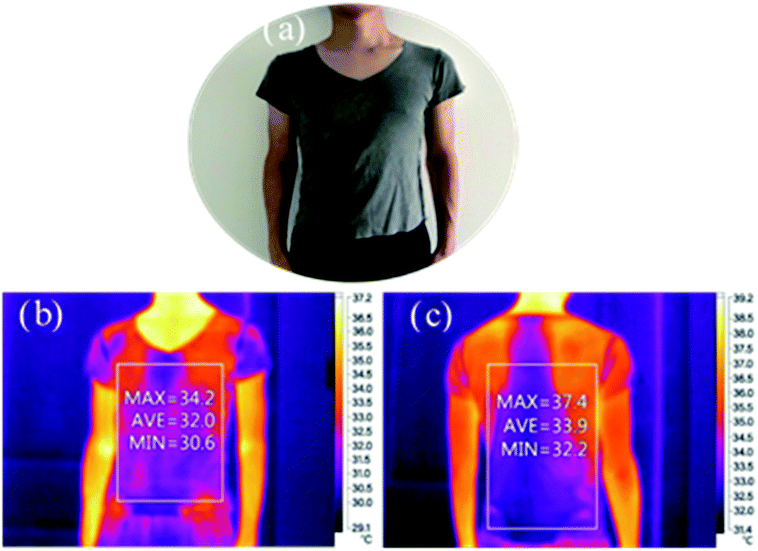
In our work, a simple approach of assembly of GO on cotton fiber through dyeing has been used to impart excellent conductive stability, infrared absorption and UV resistance. The extent of improvement in properties was dependent on the size of the GO used: the large size (macro) particles assembled on the fiber surface makes it have good electrical conductivity, whereas blended with superfine particles can make the surface smoother of fiber, and has better conductivity with a volume specific resistance of 2300 Ω cm. GO on the fabrics was resistant to washing and bending up to 1000 times. A high UPF of 102 was imparted after dyeing with GO even after 10 washing cycles. In addition, the dyed fabrics showed heat infrared absorption capability when worn on the body. GO dyed cotton fabrics show potential for use in electronics, space, medical and other applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (51703079), General Scientific Research Projects of Zhejiang Education Department (Y201738106), Key Laboratory of Yarn Materials Forming and Composite Processing Technology, Zhejiang Province, Jiaxing University (MTC-2020-12) and Key Program in College of Nanhu (N41472001-42).References
- T. Das, B. K. Sharma, A. K. Katiyar and J. H. Ahn, J. Semicond., 2018, 39, 011007 CrossRef.
- N. V. Bhat, D. T. Seshadri, M. M. Nate and A. V. Gore, J. Appl. Polym. Sci., 2006, 102, 4690–4695 CrossRef CAS.
- B. Ouadil, O. Cherkaoui, M. Safi and M. Zahouily, Appl. Surf. Sci., 2017, 414, 292–302 CrossRef CAS.
- D. P. Dubal, N. R. Chodankar, D. H. Kim and P. Gomezromero, Chem. Soc. Rev., 2018, 47, 2065–2129 RSC.
- Z. Stempien, T. Rybicki, E. Rybicki, M. Kozanecki and M. I. Szynkowska, Synth. Met., 2015, 202, 49–62 CrossRef CAS.
- M. R. Nateghi and M. Shateri-Khalilabad, Carbohydr. Polym., 2015, 117, 160–168 CrossRef CAS PubMed.
- L. E. Román, J. Huachani, C. Uribe, J. Solís, M. Gómez, S. Costa and S. Costa, Appl. Surf. Sci., 2019, 469, 204–212 CrossRef.
- G. Rosace, A. Castellano, V. Trovato, G. Iacono and G. Malucelli, Carbohydr. Polym., 2018, 196, 348–358 CrossRef CAS PubMed.
- Y. M. Ji, Y. Z. Li, G. Q. Chen and T. L. Xing, Mater. Des., 2017, 133, 528–535 CrossRef CAS.
- B. Fugetsu, E. Sano, H. W. Yu, K. Mori and T. Tanaka, Carbon, 2010, 12, 3340–3345 CrossRef.
- A. Ramadoss, B. Saravanakumar and S. J. Kim, Nano Energy, 2015, 15, 587–597 CrossRef CAS.
- J. S. Ren, C. X. Wang, X. Zhang, T. Carey, K. L. Chen, Y. J. Yin and F. Torrisi, Carbon, 2017, 111, 622–630 CrossRef CAS.
- L. Gan, S. M. Shang, C. W. M. Yuen and S. X. Jiang, Compos. Sci. Technol., 2015, 117, 208–214 CrossRef CAS.
- J. M. Zhao, B. Deng, M. Lv, J. Y. Li, Y. J. Zhang, H. Q. Jiang, C. Peng, J. Li, J. Y. Shi, Q. Huang and C. H. Fan, Adv. Healthcare Mater., 2013, 2, 1259–1266 CrossRef CAS.
- G. M. Cai, Z. L. Xu, M. Y. Yang, B. Tang and X. G. Wang, Appl. Surf. Sci., 2017, 393, 441–448 CrossRef CAS.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- M. Terrones, A. R. Botello-Méndez, J. Campos-Delgado, F. López-Urías, Y. I. Vega-Cantú, F. J. Rodríguez-Macías, A. L. Elías, E. Muñoz-Sandoval, A. G. Cano-Márquez, J.-C. Charlier and H. Terrones, Nano Today, 2010, 5, 351–372 CrossRef.
- Y. S. Jun, S. Sy, W. Ahn, H. Zarrin, L. Rasen, R. Tjandra, B. M. Amoli, B. X. Zhao, G. Chiu and A. P. Yu, Carbon, 2015, 95, 653–658 CrossRef CAS.
- H. Ma, W. Wu, J. D. Cao, B. B. Yue and H. X. Zhang, Carbon, 2017, 114, 731–739 CrossRef CAS.
- X. H. Wu, J. Lyu, G. Hong, X. C. Liu and X. T. Zhang, Langmuir, 2018, 34, 9004–9014 CrossRef CAS PubMed.
- K. J. Yuan, H. C. Wang, J. Liu, X. M. Fang and Z. G. Zhang, Sol. Energy Mater. Sol. Cells, 2015, 143, 29–37 CrossRef CAS.
- M. W. Tian, X. L. Hu, L. Q. Qu, S. F. Zhu, Y. N. Sun and G. T. Han, Carbon, 2016, 96, 1166–1174 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |