Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Microwave-assisted simple synthesis of 2-anilinopyrimidines by the reaction of 2-chloro-4,6-dimethylpyrimidine with aniline derivatives†
Cristina Campestre a,
György Keglevich
a,
György Keglevich b,
János Kótic,
Luca Scotti
b,
János Kótic,
Luca Scotti d,
Carla Gasbarri
d,
Carla Gasbarri a and
Guido Angelini
a and
Guido Angelini *a
*a
aDepartment of Pharmacy, University “G. d’Annunzio” of Chieti-Pescara, via dei Vestini, 66100 Chieti, Italy. E-mail: guido.angelini@unich.it; Tel: +39-0871-3554785
bDepartment of Organic Chemistry and Technology, Budapest University of Technology and Economics, 1521 Budapest, Hungary
cSpectroscopic Research Division, Gedeon Richter Plc., 1475 Budapest, Hungary
dDepartment of Medical, Oral and Biotechnological Sciences, University “G. d’Annunzio” of Chieti-Pescara, via dei Vestini, 66100 Chieti, Italy
First published on 25th March 2020
Abstract
A series of 2-anilinopyrimidines including novel derivatives has been obtained from 2-chloro-4,6-dimethylpyrimidine by aromatic nucleophilic substitution with differently substituted anilines under microwave conditions. The substituents had a significant impact on the course and efficiency of the reaction. The results reported herein demonstrate the efficacy of microwaves in the synthesis of the title heterocyclic compounds as compared to the results obtained with conventional heating. The 2-anilinopyrimidines described are of potential bioactivity.
Introduction
The biological activity of anilinopyrimidines as fungicides and pesticides is well-known and widely reported.1–7 Recently, a few 2-anilinopyrimidine derivatives have been evaluated as kinase inhibitors having antiproliferative activity against cancer cell lines.8–11 The role of this class of compounds in the generation of supramolecular networks for molecular recognition has also been demonstrated.12Different methods have been proposed to synthesize anilinopyrimidines: (a) the cyclization between guanidines (generally obtained from isothiourea salts and alkyl(aryl)amines in the presence of strong bases) and β-diketones, ethyl acetoacetate or ethyl cyanoacetate under refluxing for several hours (b) the transition metal-free cross-coupling reactions, (c) the aromatic nucleophilic substitution of halogen pyrimidines or substituted heterocycles having an alkylsulfonyl group with anilines.13–16 In particular, the substitution of the halogen atom in 2-aminopyrimidines by alkyl- or arylamines occurs under acidic conditions.17,18 The main disadvantage of these procedures is the requirement for drastic conditions and long reaction times.
In the last years a large number of heterocyclic compounds including anilinopyrimidines has been synthesized by microwave (MW) irradiation.19–21 The main advantage of the use of this technique is the decrease of the reaction time from several hours to a few minutes or seconds in comparison to the results obtained on conventional heating.22,23 Moreover, less by-products are formed in MW-assisted reactions.24 However, in most cases, it is not possible to predict if a given reaction will be improved under MWs or not.25 At the same time, if the energetics of the target reaction is known, it is possible to judge in advance about the appropriateness of the application of MWs. The ideal subject of MW-promoted reactions are those that have a relatively high enthalpy of activation, and are thermoneutral.26–28
In this article we describe the simple synthesis of a series of anilinopyrimidine derivatives including three new compounds by the reaction of 2-chloro-4,6-dimethylpyrimidine with substituted anilines under MW irradiation. This method represents a novel approach to the synthesis of the target compounds, and allows high yields in eco-friendly conditions. Recently, some green and recyclable reaction media have been proposed, such as water, PEG-200, and 2-methyltetrahydrofuran.29–31 The use of ethanol, one of the environmentally preferable solvents, confers the eco-friendly character to the synthesis of the investigated compounds.32–34
Results and discussion
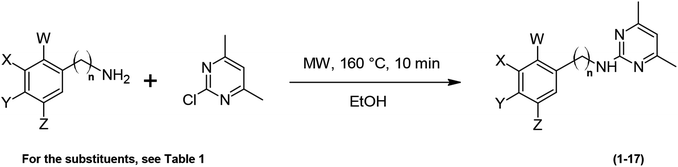
Recently, it has been demonstrated that acid catalysis promoted the reaction of chloro-N-heterocycles with anilines.19,35 The reaction between 2-chloro-4,6-dimethylpyrimidine and substituted anilines in ethanol at 160 °C under MW irradiation for 10 min, is an aromatic nucleophilic substitution taking place via the corresponding Meisenheimer complexes, and affording 2-anilinopyrimidines. An aminopyrimidine from benzylamine has also been synthesized as shown in Scheme 1.Yields of the 2-anilinopyrimidine derivatives (1–16) and benzylaminopyrimidine (17) are listed in Table 1. All the target compounds were prepared with high yields (71–99%) except compound (10). Pure products were obtained after column chromatography, and their structure was confirmed by 1H and 13C-NMR as well as mass spectral data.
| Compound | X | Y | Z | W | n | Yield/% | mp (lit)/°C |
|---|---|---|---|---|---|---|---|
| 1 | OCH3 | OCH3 | OCH3 | H | 0 | 90 | 167.5–169 |
| 2 | H | H | H | H | 0 | 91 | 96–98 (96–97)21 |
| 3 | H | OCH3 | H | H | 0 | 90 | 90–92 (88–89)21 |
| 4 | H | OH | H | H | 0 | 92 | 170–172 (170–172)36 |
| 5 | H | CH3 | H | H | 0 | 99 | 116–117 (107–108)36 |
| 6 | H | Br | H | H | 0 | 98 | 123–124 (123–124)36 |
| 7 | H | Cl | H | H | 0 | 82 | 118–120 (153)36 |
| 8 | H | F | H | H | 0 | 71 | 91–93 (91–93)36 |
| 9 | H | CF3 | H | H | 0 | 87 | 106.5–108 |
| 10 | H | NO2 | H | H | 0 | 39 | 221–223 (230)36 |
| 11 | H | C6H5 | H | H | 0 | 91 | 114–115.5 |
| 12 | H | N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C6H5 N–C6H5 |
H | H | 0 | 91 | 159.5–161 |
| 13 | CH2OH | H | H | H | 0 | 97 | 106–108 |
| 14 | F | H | H | H | 0 | 97 | 132–134 (132–134)36 |
| 15 | OH | H | H | H | 0 | 85 | 137.5–139 (157)36 |
| 16 | H | H | H | CH3 | 0 | 90 | 111–112 (92–93)36 |
| 17 | H | H | H | H | 1 | 95 | 109–111 (111–112)21 |
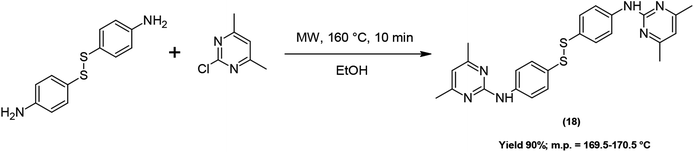
The bis-derivative (18) was obtained according to Scheme 2, under the same conditions as compounds (1–17), starting from 2-chloro-4,6-dimethylpyrimidine and 4,4′-dithiobis(benzenamine).
In order to verify the role of MWs in the investigated reactions, compound (1) was also synthesized on conventional heating at reflux for 72 h. After the work-up described in the general procedure, product (1) was isolated in a yield of 68% that is significantly lower than that obtained by MW irradiation (90%). It has been demonstrated that in the MW-assisted synthesis, the polarity and polarizability of the reagents, transition states and intermediates may influence the absorption of MWs and hence the heating.37
The presence of substituents, their electronic effects and their position in the aromatic ring may influence the properties of the compounds, and the rate of their reactions.38–43 In the investigated SNAr reactions, the electron-donating effect (+I) of the alkyl group increases the nucleophilic nature of the amino group of the aniline moiety, as it was demonstrated by the high yields obtained for compounds (5) and (16) due to the presence of the CH3 group in the para or ortho position, respectively (Table 1). A similar effect was observed in the phenyl substituted instance (11). In case of products (3) and (4), the high yields of 90 and 92%, respectively, are the consequence of the electron-releasing MeO and HO groups. These substituents can promote the reactivity of the aniline molecule because of the major +M effect versus the −I effect. On the other hand, regarding compound (10), in which the nitro group posseses an −M effect, nucleophilicity of the amino group is decreased, as it was demonstrated by the lower yield of 39%.
Compounds (1), (2) and (3) are well-known molecules: heterocycle (1) has been recently patented for its antimitotic activity as a topical formulation against psoriasis,44 while derivatives (2) and (3) are commercial fungicides pyrimethanil and andoprim, respectively.2,45,46 In this work, these species and their analogues have been synthesized in a one pot reaction instead of the guanidine route.14 To the best of our knowledge, compounds (11), (12) and (18) are reported for the first time.
Conclusions
MW irradiation allowed rapid and high yield “green” synthesis of a series of 2-anilinopyrimidines. In this work, 18 products were prepared, including new compounds (11), (12) and (18) and the benzylamine derivative (17). The electronic effect of the substituents had an impact on the outcome of the reaction by influencing the nucleophilic character of the amino group of the aniline molecule. As N-heterocycle-based molecules are important due to their applicability in medicinal chemistry,47 the compounds synthesized beyond the antimitotic (1) and fungicidal (2) and (3) may also posses bioactive properties.Experimental
Material and methods
All the reagents, were purchased from commercial suppliers and employed without further purification. Reactions were monitored by thin layer chromatography (TLC) on silica gel plates (60 F254) that were visualized under UV light (254 nm). The silica gel (Kieselgel 4°, 0.04–0.063 mm) used in the column chromatography was purchased from Merck. The MW-assisted reactions were performed using a Biotage Initiator Plus oven. Infrared absorption spectra were recorded on a Varian 1000 Fourier-transform infrared spectrometer. The 1H and 13C-NMR spectra were recorded on a Varian-Mercury 300 spectrometer, operating at a frequency of 300 MHz and 75 MHz, respectively. Melting points were determined on a Büchi B-540 apparatus and are uncorrected. Electrospray high-resolution MS measurements were performed on a Thermo Velos Pro Orbitrap Elite Hybrid Mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). The ionization method was ESI and operated in positive ion mode. The capillary temperature was set at 275 °C. Samples were infused into the ESI source MeOH solutions at a flow rate of 3 μL min−1. Resolving power of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (FWHM) at m/z 400. Data acquisition and analysis were accomplished with Xcalibur software version 3.0 (Thermo Fisher Scientific Inc.).
000 (FWHM) at m/z 400. Data acquisition and analysis were accomplished with Xcalibur software version 3.0 (Thermo Fisher Scientific Inc.).
General procedure for the synthesis of compounds (1–17)
The appropriate aniline (1.0 mmol), 2-chloro-4,6-dimethylpyrimidine (114 mg, 0.80 mmol), and ethanol (4 mL) were placed in a 5 mL reaction vial, sealed and irradiated at 160 °C for 10 min under magnetic stirring. The solid residue was taken up in 30 mL of CH2Cl2, and the solution washed with 0.25 M Na2CO3 (2 × 20 mL) and dried over Na2SO4. The solvent was evaporated, and the crude product so obtained purified by silica gel column chromatography. The yields were calculated combining the fractions having only one spot on TLC. According to the NMR spectra, purity of the compounds is ≥ 99%. The following products were thus prepared:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the eluent.
2) as the eluent.IR (KBr) νmax cm−1 3317, 2951, 1612, 1593, 1544; 1H-NMR (CDCl3): δ 2.37 (s, 6H, 2CH3), 3.80 (s, 3H, OCH3), 3.85 (s, 6H, 2 OCH3), 6.49 (s, 1H, Ar Pyr), 6.99 (s, 2H, Ar), 7.32 (s, 1H, NH); 13C-NMR (CDCl3): δ 23.9, 56.0, 61.1, 96.8, 111.6, 135.9, 153.3, 159.3, 167.6; ESI-HRMS [M + H]+ found = 290.14950, C15H20N3O3 requires = 290.14992.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the eluent.
2) as the eluent.IR (KBr) νmax cm−1 3260, 2967, 1614, 1589, 1566, 1548; 1H-NMR (CDCl3): δ 2.37 (s, 6H, 2CH3), 6.48 (s, 1H, Ar Pyr), 6.98–7.03 (m, 1H, Ar), 7.29–7.34 (m, 2H, Ar), 7.43 (s, 1H, NH), 7.66–7.69 (m, 2H, Ar); 13C-NMR (CDCl3): δ 23.9, 111.7, 118.9, 122.1, 128.9, 139.9, 159.7, 167.6; ESI-HRMS [M + H]+ found = 200.11781, C12H14N3 requires = 200.11822.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the eluent.
2) as the eluent.IR (KBr) νmax cm−1 3266, 2960, 1602, 1583, 1563, 1545; 1H-NMR (CDCl3): δ 2.33 (s, 6H, 2CH3), 3.78 (s, 3H, OCH3), 6.43 (s, 1H, Ar Pyr), 6.86 (d, J = 8.7, 2H, Ar), 7.34 (s, 1H, NH), 7.53 (d, J = 8.7, 2H, Ar); 13C-NMR (CDCl3): δ 23.9, 55.6, 111.2, 114.2, 121.1, 133.1, 155.2, 159.9, 167.6; ESI-HRMS [M + H]+ found = 230.12824, C13H16N3O requires = 230.12879.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the eluent.
4) as the eluent.IR (KBr) νmax cm−1 3217, 2958, 1598, 1585, 1561, 1546; 1H-NMR (CD3OD): δ 2.27 (s, 6H, 2CH3), 4.88 (s, 1H, NH) (s, 1H, OH), 6.47 (s, 1H, Ar Pyr), 6.73 (d, J = 8.9, 2H, Ar), 7.39 (d, J = 8.9, 2H, Ar), 13C-NMR (CD3OD): δ 26.3, 114.1, 118.7, 125.8, 135.9, 156.6, 164.3, 171.65; ESI-HRMS [M + H]+ found = 216.11271, C12H14N3O requires = 216.11314.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent.
1) as the eluent.IR (KBr) νmax cm−1 3263, 2964, 1609, 1585, 1565, 1541; 1H-NMR (CDCl3): δ 2.31 (s, 3H, CH3), 2.35 (s, 6H, 2CH3), 6.45 (s, 1H, Ar Pyr), 7.12 (d, J = 8.1, 2H, Ar), 7.43 (s, 1H, NH), 7.54 (d, J = 8.1, 2H, Ar); 13C-NMR (CDCl3): δ 20.8, 23.9, 111.3, 119.2, 129.4, 131.6, 137.3, 159.8, 167.6; ESI-HRMS [M + H]+ found = 214.13349, C13H16N3 requires = 214.13387.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as the eluent.
5) as the eluent.IR (KBr) νmax cm−1 3408, 2962, 1595, 1564, 1519; 1H-NMR (CDCl3): δ 2.33 (s, 6H, 2CH3), 6.47 (s, 1H, Ar Pyr), 7.36 (d, J = 8.7, 2H, Ar), 7.54 (d, J = 8.7, 2H, Ar), 7.67 (s, 1H, NH); 13C-NMR (CDCl3): δ 23.9, 111.9, 114.1, 120.4, 131.6, 139.1, 159.4, 167.6; ESI-HRMS [M + H]+ found = 278.02861, C12H13BrN3 requires = 278.02874.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the eluent.
2) as the eluent.IR (KBr) νmax cm−1 3317, 2949, 1612, 1593, 1544,; 1H-NMR (acetone d6): δ 2.31 (s, 6H, 2CH3), 6.59 (s, 1H, Ar Pyr), 7.29 (d, J = 8.8, 2H, Ar), 7.95 (d, J = 8.8, 2H, Ar), 8.59 (s, 1H, NH); 13C-NMR (acetone d6): δ 23.9, 112.2, 120.9, 126.1, 129.1, 140.9, 160.7, 168.2; ESI-HRMS [M + H]+ found = 234.07884, C12H13ClN3 requires = 234.07925.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the eluent.
2) as the eluent.IR (KBr) νmax cm−1 3267, 2964, 1614, 1595, 1566, 1550; 1H-NMR (CDCl3): δ 2.34 (s, 6H, 2CH3), 6.47 (s, 1H, Ar Pyr), 6.95–7.01 (m, 2H, Ar), 7.48 (s, 1H, NH), 7.56–7.60 (m, 2H, Ar); 13C-NMR (CDCl3): δ 23.8, 111.6, 115.3 (d, J = 22), 120.6 (d, J = 6.7), 135.8, 158.3 (d, J = 242), 159.4, 167.6; ESI-HRMS [M + H]+ found = 218.10849, C12H13FN3 requires = 218.10880.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent.
1) as the eluent.IR (KBr) νmax cm-1 3435, 2989, 1618, 1600, 1598, 1535; 1H-NMR (CDCl3): δ 2.39 (s, 6H, 2CH3), 6.55 (s, 1H, Ar Pyr), 7.53 (d, J = 8.4, 2H, Ar), 7.73 (s, 1H, NH), 7.78 (d, J = 8.4, 2H, Ar); 13C-NMR (CDCl3): δ 23.9, 112.5, 118.1, 122.5, 126.1, 143.1, 159.2, 167.8; ESI-HRMS [M + H]+ found = 268.10489, C13H13F3N3 requires = 268.10561.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the eluent.
2) as the eluent.IR (KBr) νmax cm−1 3383, 2985, 1600, 1564, 1541, 1506; 1H-NMR (CDCl3): δ 2.49 (s, 6H, 2CH3), 6.68 (s, 1H, Ar Pyr), 7.86 (d, J = 8.7, 2H, Ar), 8.21 (d, J = 8.7, 2H, Ar), 8.70 (s, 1H, NH); 13C-NMR (CDCl3): δ 23.7, 113.3, 118.5, 125.4, 142.4, 144.9, 156.9, 168.1; ESI-HRMS [M + H]+ found = 245.10289, C12H13N4O2 requires = 245.10330.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8), to CH2Cl2 as the eluent.
8), to CH2Cl2 as the eluent.IR (KBr) νmax cm−1 3242, 2962, 1602, 1573, 1561, 1534; 1H-NMR (CDCl3): δ 2.41 (s, 6H, 2CH3), 6.50 (s, 1H, Ar Pyr), 7.31–7.36 (m, 1H, Ar), 7.43–7.48 (m, 2H, Ar), 7.58–7.64 (m, 4H, Ar, s, 1H, NH), 7.78–7.82 (m, 2H, Ar), 13C-NMR (CDCl3): δ 23.9, 111.7, 119.2, 126.7, 127.5, 128.8, 134.8, 139.4, 140.9, 159.6, 167.6; ESI-HRMS [M + H]+ found = 276.14892, C18H18N3 requires = 276.14952.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent.
1) as the eluent.IR (KBr) νmax cm−1 3278, 2960, 1604, 1585, 1562, 1535; 1H-NMR (CDCl3): δ 2.39 (s, 6H, 2CH3), 6.52 (s, 1H, Ar Pyr), 7.39–7.52 (m, 3H, Ar), 7.83–7.96 (m, 7H, Ar, NH); 13C-NMR (CDCl3): δ 23.9, 112.5, 118.5, 122.6, 124.2, 129.1, 130.3, 142.9, 147.5, 152.9, 159.1, 167.7; ESI-HRMS [M + H]+ found = 304.15492, C18H18N5 requires = 304.15567.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent.
1) as the eluent.IR (KBr) νmax cm−1 3271, 2939, 1618, 1593, 1556, 1548; 1H-NMR (CDCl3): δ 2.32 (s, 6H, 2CH3), 3.73 (bs, 1H, OH), 4.62 (s, 2H, CH2), 6.43 (s, 1H, Ar Pyr), 6.93–6.95 (m, 1H, Ar), 7.19–7.25 (m, 1H, Ar), 7.37 (s, 1H, NH), 7.49 (bs, 1H, Ar), 7.58–7.61 (m, 1H, Ar); 13C-NMR (CDCl3): δ 23.8, 64.8, 111.6, 117.5, 118.1, 120.7, 128.9, 139.9, 141.9, 159.4, 167.6; ESI-HRMS [M + H]+ found = 230.12849, C13H16N3O requires = 230.12879.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as the eluent.
5) as the eluent.IR (KBr) νmax cm−1 3419, 2922, 1602, 1587, 1566, 1539; 1H-NMR (CDCl3): δ 2.37 (s, 6H, 2CH3), 6.51 (s, 1H, Ar Pyr), 6.63–6.71 (m, 1H, Ar), 7.12–7.24 (m, 2H, Ar), 7.62 (s, 1H, NH), 7.78–7.83 (m, 1H, Ar); 13C-NMR (CDCl3): δ 23.9, 105.9 (d, J = 27.0), 108.5 (d, J = 22), 112.2, 114.1 (d, J = 2.5), 129.8 (d, J = 9.9), 141.7 (d, J = 5.8), 159.4, 163.3 (d, J = 242), 167.7; ESI-HRMS [M + H]+ found = 218.10851, C12H13FN3 requires = 218.10880.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to CH2Cl2–isopropanol (8
1) to CH2Cl2–isopropanol (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the eluent.
2) as the eluent.IR (KBr) νmax cm−1 3365, 2995, 1620, 1610, 1589, 1568, 1548; 1H-NMR (acetone d6): δ 2.30 (s, 6H, 2CH3), 6.45–6.48 (m, 1H, Ar), 6.54 (s, 1H, Ar Pyr), 7.05–7.11 (m, 1H, Ar), 7.26–7.30 (m, 1H, Ar), 7.66–7.68 (m, 1H, Ar), 8.37 (s, 1H, NH); 13C-NMR (acetone d6): δ 23.8, 106.6, 109.1, 110.8, 111.7, 129.9, 142.9, 158.5, 160.8, 168.1; ESI-HRMS [M + H]+ found = 216.11285, C12H14N3O requires = 216.11314.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the eluent.
2) as the eluent.IR (KBr) νmax cm−1 3218, 2964, 1600, 1568, 1555; 1H-NMR (CDCl3): δ 2.32 (s, 3H, CH3), 2.36 (s, 6H, 2CH3), 6.48 (s, 1H, Ar Pyr), 6.87 (s, 1H, 1N), 6.96–7.01 (m, 1H, Ar), 7.17–7.25 (m, 2H, Ar), 8.17–8.19 (m, 1H, Ar); 13C-NMR (CDCl3): δ 18.3, 24.0, 111.7, 120.9, 122.9, 126.6, 127.7, 130.5, 137.9, 160.1, 167.7; ESI-HRMS [M + H]+ found = 214.13352, C13H16N3 requires = 214.13387.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the eluent.
2) as the eluent.IR (KBr) νmax cm−1 3254, 2964, 1600, 1583, 1570, 1559; 1H-NMR (CDCl3): δ 2.26 (s, 3H, CH3), 4.66 (d, J = 5.7, 2H), 5.85 (s, 1H), 6.29 (s, 1H, Ar Pyr), 7.22–7.36 (m, 5H, Ar); 13C-NMR (CDCl3): δ 24.0, 45.3, 109.9, 127.0, 127.5, 128.5, 139.7, 162.2, 167.5; ESI-HRMS [M + H]+ found = 214.13349, C13H16N3 requires = 214.13387.
Procedure for the synthesis of compound (18)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the eluent.
2) as the eluent.IR (KBr) νmax cm−1 3259, 2962, 1610, 1580, 1568, 1563, 1553; 1H-NMR (CDCl3): δ 2.35 (s, 6H, CH3), 6.48 (s, 1H, Ar Pyr), 7.42 (d, J = 8.6, 2H, Ar), 7.61 (d, J = 8.6, 2H, Ar), 7.76 (s, 1H, 1N); 13C-NMR (CDCl3): δ 23.9, 112.0, 119.2, 129.6, 131.2, 140.1, 159.3, 167.6; ESI-HRMS [M + H]+ found = 461.15649, C24H25N6S2 requires = 461.15766.
Conflicts of interest
There are no conflict to declare.Acknowledgements
This work was supported by Italian Ministry of Education MIUR (60% Grant 2018) and University “G. d'Annunzio” of Chieti-Pescara.Notes and references
- B. Forster and T. Staub, Crop Prot., 1996, 15(6), 529–537 CrossRef CAS.
- M. A. Waly, E. T. Bader-Eldien, M. E. Aboudobarah and E. S. T. Aboumosalam, Med. Chem. Res., 2013, 22(11), 5267–5273 CrossRef CAS.
- G. Sandmann, Pestic. Biochem. Physiol., 2001, 70, 86–91 CrossRef CAS.
- M. P. McQuilken and J. Thomson, Pest Manage. Sci., 2008, 64, 748–754 CrossRef CAS PubMed.
- M. Dömötörová, A. Hercegová and E. Matisová, Czech J. Food Sci., 2006, 24(2), 84–92 CrossRef.
- F. A. Esteve-Turrillas, J. V. Mercader, C. Agulló, A. Abad-Somovilla and A. Abad-Fuentes, Analyst, 2017, 142, 3975–3985 RSC.
- J. G. Cumming, C. L. McKenzie, S. G. Bowden, D. Campbell, D. J. Masters, J. Breed and P. J. Jewsbury, Bioorg. Med. Chem. Lett., 2004, 14, 5389–5394 CrossRef CAS PubMed.
- A. A. Romu, Z. Lei, B. Zhou, Z. S. Chen and V. Korlipara, Bioorg. Med. Chem. Lett., 2017, 27, 4832–4837 CrossRef CAS PubMed.
- J. Jo, S. H. Kim, H. Kim, M. Jeong, J. H. Kwak, Y. T. Han, J. Y. Jeong, Y. S. Jung and H. Yun, Bioorg. Med. Chem. Lett., 2019, 29, 62–65 CrossRef CAS PubMed.
- R. Determann, J. Dreher, K. Baumann, L. Preu, P. G. Jones, F. Totzke, C. Schächtele, M. H. Kubbutat and C. Kunick, Eur. J. Med. Chem., 2012, 53, 254–263 CrossRef CAS PubMed.
- Y. Lv, M. Li, S. Cao, L. Tong, T. Peng, L. Wei, H. Xie, J. Ding and W. Duan, Med. Chem. Commun., 2015, 6, 1375–1380 RSC.
- S. Goswami, S. Jana, N. K. Das, H. K. Fun and S. Chantrapromma, J. Mol. Struct., 2008, 876, 313–321 CrossRef CAS.
- Y. Ma, L. Margarida, J. Brookes, G. M. Makara and S. C. Berk, J. Comb. Chem., 2004, 6, 426–430 CrossRef CAS PubMed.
- C. Liu, Z. Cui, X. Yan, Z. Qi, M. Ji and X. Li, Molecules, 2016, 21, 828 CrossRef PubMed.
- K. Walsh, H. F. Sneddon and C. J. Moody, ChemSusChem, 2013, 6, 1455–1460 CrossRef CAS PubMed.
- L. A. Ho, C. L. Raston and K. A. Stubbs, Eur. J. Org. Chem., 2016, 5957–5963 CrossRef CAS.
- F. Marchetti, C. Cano, N. J. Curtin, B. T. Golding, R. J. Griffin, K. Haggerty, D. R. Newell, R. J. Parsons, S. L. Payne, L. Z. Wang and I. R. Hardcastle, Org. Biomol. Chem., 2010, 8, 2397–2407 RSC.
- M. G. Bursavich, S. Lombardi and A. M. Gilbert, Org. Lett., 2005, 7(19), 4113–4116 CrossRef CAS PubMed.
- B. Carbain, C. R. Coxon, H. Lebraud, K. J. Elliott, C. J. Matheson, E. Meschini, A. R. Roberts, D. M. Turner, C. Wong, C. Cano, R. J. Griffin, I. R. Hardcastle and B. T. Golding, Chem.–Eur. J., 2014, 20, 2311–2317 CrossRef CAS PubMed.
- C. O. Kappe, Chem. Rec., 2019, 19, 15–39 CrossRef CAS PubMed.
- S. Goswami, A. Hazra and S. Jana, Bull. Chem. Soc. Jpn., 2009, 82, 1175–1181 CrossRef CAS.
- G. Keglevich, Milestones in Microwave Chemistry – Springer Briefs in Molecular Science, ed. G. Keglevich, Springer, Switzerland, 2016 Search PubMed.
- R. Gedye, F. Smith, K. Westaway, H. Ali, L. Baldisera, L. Laberge and J. Rousell, Tetrahedron Lett., 1986, 27, 279–282 CrossRef CAS.
- M. Driowya, A. Saber, H. Marzag, L. Demange, R. Benhida and K. Bougrin, Molecules, 2016, 21(4), 492 CrossRef PubMed.
- A. Díaz-Ortiz, P. Prieto and A. de la Hoz, Chem. Rec., 2019, 19, 85–97 CrossRef PubMed.
- G. Keglevich, N. Z. Kiss, Z. Mucsi and T. Körtvélyesi, Org. Biomol. Chem., 2012, 10, 2011–2018 RSC.
- G. Keglevich, N. Z. Kiss, A. Grün, E. Bálint and T. Kovács, Synthesis, 2017, 49, 3069–3083 CrossRef CAS.
- G. Keglevich and Z. Mucsi, Microwave Chemistry, ed. G. Cravotto and D. Carnaroglio, De Gruyter, Berlin, 2017, ch. 4, pp. 53–64 Search PubMed.
- S. Peng, D. Hu, J. L. Hu, Y. W. Lin, S. S. Tang, H. S. Tang, J. Y. He, Z. Cao and W. M. He, Adv. Synth. Catal., 2019, 361, 5721–5726 CrossRef CAS.
- L. Y. Xie, L. L. Jiang, J. X. Tan, Y. Wang, X. Q. Xu, B. Zhang, Z. Cao and W. M. He, ACS Sustainable Chem. Eng., 2019, 7, 14153–14160 CrossRef CAS.
- W. H. Bao, Z. Wang, X. Tang, Y. F. Zhang, J. X. Tan, Q. Zhu, Z. Cao, Y. W. Lin and W. M. He, Chin. Chem. Lett., 2019, 30, 2259–2262 CrossRef CAS.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC.
- D. Daoqing, C. Wenjing, C. Demao, L. Lixia, L. Guanghui, W. Zuli, D. Qi and L. Shu, Chin. J. Org. Chem., 2019, 39, 3190–3198 CrossRef.
- K. Singh, K. Singh, B. Wan, S. Franzblau, K. Chibale and J. Balzarini, Eur. J. Med. Chem., 2011, 46, 2290–2294 CrossRef CAS PubMed.
- R. Ormazabal-Toledo, J. G. Santos, P. Ríos, E. A. Castro, P. R. Campodónico and R. Contreras, J. Phys. Chem. B, 2013, 117, 5908–5915 CrossRef CAS PubMed.
- F. Franke, M. Klepel, G. Krause, H. Lehmann and B. Braemer, German patent, patent number DD151404A1, 1981.
- C. Gabriel, S. Gabriel, E. H. Grant, B. S. Halstead and D. M. P. Mingos, Chem. Soc. Rev., 1998, 27, 213–224 RSC.
- G. Angelini, N. Canilho, M. Emo, M. Kingsley and C. Gasbarri, J. Org. Chem., 2015, 80, 7430–7434 CrossRef CAS PubMed.
- D. Duraczyńska, E. M. Serwicka, A. Drelinkiewicz, R. P. Socha, M. Zimowska, L. Lityńska-Dobrzyńska and A. Bukowska, Mol. Catal., 2019, 470, 145–151 CrossRef.
- B. Sánchez, C. Calderón, R. A. Tapia, R. Contreras and P. R. Campodónico, Front. Chem., 2018, 6, 509 CrossRef PubMed.
- C. Gasbarri and G. Angelini, J. Mol. Liq., 2017, 229, 185–188 CrossRef CAS.
- J. Gao, B. J. Jankiewicz, H. Sheng, L. Kirkpatrick, X. Ma, J. J. Nash and H. I. Kenttämaa, Eur. J. Org. Chem., 2018, 6582–6589 CrossRef CAS PubMed.
- C. Gasbarri and G. Angelini, RSC Adv., 2014, 4, 17840–17845 RSC.
- D. Biagioni, A. Calcaterra, C. De Angelis and L. Scappaticci, patent number WO2016001419, 2016.
- G. Saladin, C. Magné and C. Clément, Pest Manage. Sci., 2003, 59, 1083–1092 CrossRef CAS PubMed.
- S. A. Deepak, G. Chaluvaraju, P. Basavaraju, K. N. Amuthesh, H. Shekar Shetty and G. Oros, Int. J. Pest Manage., 2005, 51(1), 7–16 CrossRef CAS.
- M. Chauhan and R. Kumar, Med. Chem. Res., 2015, 24, 2259–2282 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C-NMR spectra, and HRMS for the compounds (11), (12) and (18); Table S1: microwave conditions for the synthesis of compound (1). See DOI: 10.1039/d0ra00833h |
| This journal is © The Royal Society of Chemistry 2020 |