Open Access Article
Open Access ArticleAn overview of the chemical constituents from the genus Delphinium reported in the last four decades†
Tianpeng Yin ab,
Le Cai*b and
Zhongtao Ding
ab,
Le Cai*b and
Zhongtao Ding *b
*b
aZhuhai Key Laboratory of Fundamental and Applied Research in Traditional Chinese Medicine, Department of Bioengineering, Zhuhai Campus of Zunyi Medical University, Zhuhai 519041, China
bFunctional Molecules Analysis and Biotransformation Key Laboratory of Universities in Yunnan Province, Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan University, Kunming 650091, Kunming 650091, China. E-mail: ztding@ynu.edu.cn; caile@ynu.edu.cn
First published on 3rd April 2020
Abstract
Species of the genus Delphinium have been extensively used for different purposes by various civilizations worldwide since antiquity. Phytochemical investigations on Delphinium plants in the last four decades (1980–2019) have afforded a total of 453 new compounds, most of which are diterpenoid alkaloids. These constituents are of great research significance due to their novel structures and broad bioactivities. This review addresses, for the first time, the chemical constituents of Delphinium plants and the biological properties of these compounds to facilitate future research.
1. Introduction
The genus Delphinium (Larkspur), an important member of the family Ranunculaceae, comprises approximately 365 species, which are distributed mainly in northern temperate regions, including in Asia, Europe, and North America.1 There are also a few species growing in North Africa, such as D. cossonianum and D. staphisagria in Morocco,2,3 D. macrocentrum in Kenya,4 and D. leroyi in Ethiopia.5 Notably, among the 365 Delphinium species, 232 (200 endemic) have been found in China.6 Delphinium plants prefer cool and moist conditions and mainly grow in alpine-cold regions, such as the Hengduan Mountains region in Southwest China, which is the most important centre of diversity and speciation of this genus, as at least 167 Delphinium species have been found in this region.Delphinium plants have been extensively used for different purposes by various civilizations worldwide since antiquity. Delphinium plants feature various coloured flowers ranging from white, yellow, and red to blue, and they have been cultivated as horticultural plants in Europe since the 17th century. Currently, Delphinium plants are one of the most famous and popular horticultural plants around the world, and thousands of ornamental varieties of Delphinium have been cultivated and applied widely in bonsai, gardens, and greenbelts. Delphinium flowers are also an important source of natural dyes; for example, yellow dye for silk has been extracted from D. zalil flowers for a long period of time in Iran and India.7 In addition, Delphinium plants are traditionally used as herbal pesticides against lice and scorpions since the time of Dioscorides (in the 1st century A.D.), approximately two thousand years ago.8 During the battle at Waterloo, the British army also used the powders of D. staphisagra and D. peregrinum to prevent and kill lice.9 In China, there are five Delphinium species, namely, D. grandiflorum, D. albocoeruleum var. przewalskii, D. chefoense, D. korshinskyanum, and D. likiangense, that have been used to kill the larvae of mosquito, lice, and flies.6 Most importantly, for centuries, plants of this genus, mainly their tubers and roots, have been extensively used as herbal medicines—in Turkey to treat epilepsy, tetanus, rabies, and emesis; in Iran to treat disorders of the spleen, jaundice, a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nd dropsy; and in Nepal to treat fever and wounds.7,8 In China, Delphinium plants have a long history as folk medicines for the treatment of many kinds of diseases, such as traumatic injury, rheumatism, enteritis, influenza, oedema, asthma, ringworm, scabies and other skin diseases, as well as stomach ache, migraine, tooth ache, neuralgia, and other kinds of pain. At least 18 species of Delphinium have been used medically in Chinese traditional medicine (TCM) because of their unique and proven therapeutic effects.6
nd dropsy; and in Nepal to treat fever and wounds.7,8 In China, Delphinium plants have a long history as folk medicines for the treatment of many kinds of diseases, such as traumatic injury, rheumatism, enteritis, influenza, oedema, asthma, ringworm, scabies and other skin diseases, as well as stomach ache, migraine, tooth ache, neuralgia, and other kinds of pain. At least 18 species of Delphinium have been used medically in Chinese traditional medicine (TCM) because of their unique and proven therapeutic effects.6
Since the end of the 18th century, the chemical constituents in Delphinium plants have been investigated. Several earlier studies have attempted to isolate anthocyanin pigments from Delphinium flowers, and the first anthocyanin (delphinin) was identified from D. consolida in 1915 by Willstätter et al.10 At almost the same time, research on Delphinium alkaloids, mainly the diterpenoid alkaloid (DA) components, was also conducted.11 The DAs in Delphinium plants have attracted the attention of scientists for a long time, and most studies on these plants have been devoted to the DA components. In addition, the non-alkaloidal constituents of Delphinium plants have also been studied. To date, thousands of components with diverse chemical structures, including alkaloids, flavonoids and other phenolic compounds, fatty acids, terpenoids and steroids, have been isolated from Delphinium plants. These constituents offer novel structures and broad and impressive biological activities, including antioxidant, antiparasitic, antiphlogistic, antineoplastic, and immunoregulatory effects.
Several review articles and monographs regarding the distribution and physiological and NMR spectroscopic data of naturally occurring DAs, which have mainly been isolated from Delphinium and its sibling genera Aconitum and Consolida, have been published.12–15 However, to date, there has been no individual and comprehensive review of the chemical constituents of the Delphinium genus. Therefore, this review was prepared to summarize the structural features and biological activities of the chemical constituents from Delphinium species for the first time. The aim of this review is to provide a complete overview of the chemical constituents of the Delphinium genus reported in the last four decades (from 1980 to 2019), which will facilitate further research and exploitation of this genus.
2. Alkaloids
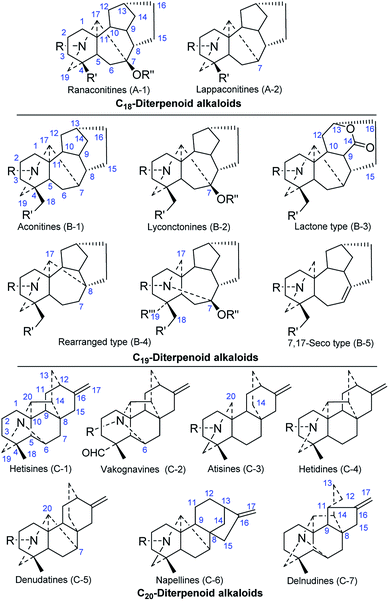
In addition to the genera Aconitum and Consolida, Delphinium is a genus in the Ranunculaceae family that is well known for its characteristic DA components.16,17 DAs are clearly the major constituents of Delphinium plants, and most of the published articles are devoted to DA components. In the past forty years, a large number of biologically active and structurally complex DAs have been isolated from various species of Delphinium. Table 1S† lists the names, plant sources, types, and the references of the new DAs isolated from Delphinium plants in the last four decades. Structurally, DAs are usually classified as C18-, C19-, or C20-DAs, which can be further divided into several to dozens of subtypes. Fig. 1 shows the fourteen subtypes of DAs that have been found in Delphinium plants in the last four decades. Herein, the new DAs as well as other alkaloids isolated from Delphinium plants are summarized by category.2.1 C18-Diterpenoid alkaloids
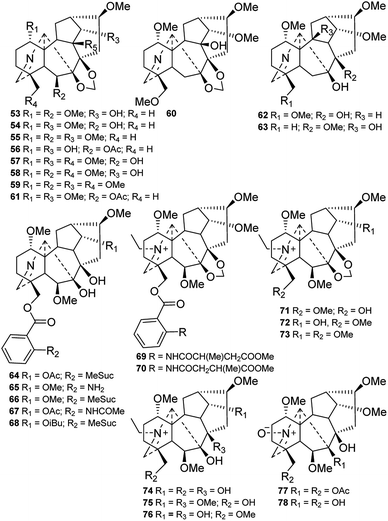
The C18-DAs, also called “bisnorditerpenoid alkaloids”, are a small sub-group of DAs.18 Compared with C19-DAs, C18-DAs are distinguished by the absence of C-18, and their C-4 moiety is a methine or an oxygenated quaternary carbon. C18-DAs can be classified into two subtypes based on whether an oxygen-containing functionality is attached at C-7, namely, lappaconitine-type compounds (A-2), which do not possess an oxygen-containing functionality at C-7, and ranaconitine-type compounds (A-1), which do have an oxygen-containing functionality at this position.To the best of our knowledge, only 23 new C18-DAs from Delphinium plant have been reported in the last four decades, and they were obtained from 7 different species (Fig. 2). Most of these compounds are ranaconitine-type C18-DAs, with the exception of giraldine I (21) from D. giraldii,19 delphicrispuline (22) from D. crispulum,20 and naviconine (23) from D. naviculare var. lasiocarpum,21 which are lappaconitine-type compounds. Sixteen ranaconitine-type C18-DAs possessing a 7,8-methylenedioxy group were reported, and these compounds are anthriscifolcones A and B (1 and 2) and anthriscifoltines A–G (3–9) from D. anthriscifolium var. majus,22–24 and anthriscifolcines A–G (10–16) from D. anthriscifolium var. savatieri.25,26 Most of them contain a 10-OH substituent, and the exceptions are alkaloids 10–11 and 16. Three of the ranaconitine-type C18-DAs, namely, delboxine (18) from D. bonvalotii,27 14-demethyltuguaconitine (19) from D. stapeliosum,28 and tiantaishansine (20) from D. tiantaishanense,29 contain a 3,4-epoxide unit. Giraldine I (21) was characterized by the lack of an oxygenated substituent at C-16.19 Alkaloids delphicrispuline (22) and naviconine (23) possess an anthranoyl group at C-18,20 and 23 features an N–CHO formamide group instead of an N-ethyl group.21
2.2 C19-Diterpenoid alkaloids
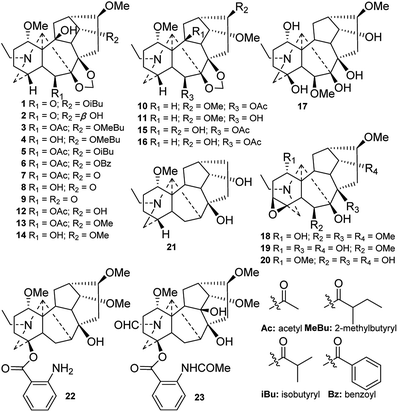
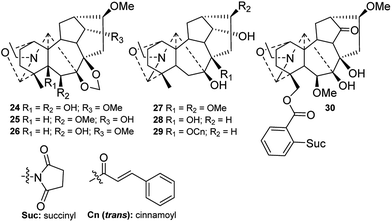
The majority of naturally occurring DAs are C19-DAs, and they are usually regarded as the representative type of DAs. In-depth investigations of C19-DAs in chemical and pharmacological fields have been carried, and more information is available on these compounds than on C18- or C20-DAs. According to their molecular skeletons, C19-DAs can be divided into six types, namely, lycaconitines (B-1), aconitines (B-2), lactones (B-3), 7,17-seco derivatives (B-4), rearranged compounds (B-5), and pyro derivatives. In the last four decades, a total of 299 new C19-DAs belonging to these five types have been isolated from Delphinium plants.In the last four decades, only seven lycaconitine-type DAs (24–30) with an N–C(19)–O–C(1) mixed acetal unit were found (Fig. 3). Among them, graciline (28) and 8-O-cinnamoylgraciline (29) are characterized by the absence of an oxygenated group at C-16,30 and alkaloid 29 features a cinnamoyl unit at C-8.2 In addition, laxicymine (24) from D. laxicymosum var. pilostachyum has a rare 5-OH group,31 and grandifloricine (30) from D. grandiflorum contains a ketone carbonyl at C-14 along with an N-(succinimido)anthranoyl group at C-18.32
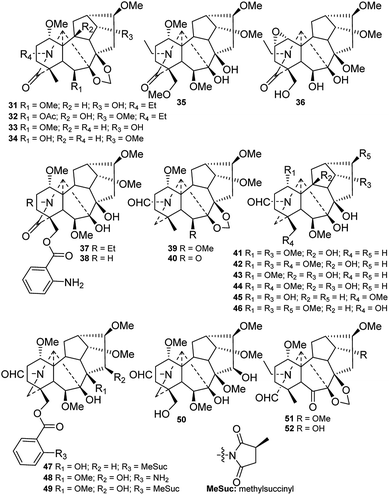
Twenty new amide lycaconitines were reported in the studied period (Fig. 4). Alkaloids 31–38 contain an N–C(19)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O lactam group, which might be formed by the carbonylation of 19-OH.33–37 Budelphine (36) from D. buschianum possesses a rare 1,2-epoxy group.36 There are 12 alkaloids (39–50) with an N–CHO formamide group formed from a C-21 aldehyde.4,38–45 Among these compounds, alkaloids 41–45 are DAs that have no oxygen-containing group at C-16.4,39–41 N-Formyl-4,19-secopacinine (51)45 and N-formyl-4,19-secoyunnadelphinine (52)38 from D. elatum contain another kind of formamide, which is formed from C-19 aldehydes.
O lactam group, which might be formed by the carbonylation of 19-OH.33–37 Budelphine (36) from D. buschianum possesses a rare 1,2-epoxy group.36 There are 12 alkaloids (39–50) with an N–CHO formamide group formed from a C-21 aldehyde.4,38–45 Among these compounds, alkaloids 41–45 are DAs that have no oxygen-containing group at C-16.4,39–41 N-Formyl-4,19-secopacinine (51)45 and N-formyl-4,19-secoyunnadelphinine (52)38 from D. elatum contain another kind of formamide, which is formed from C-19 aldehydes.
Sixteen lycaconitine-type DAs with an imine group at C-19 were isolated from Delphinium plants (Fig. 5). Nine of these DAs (53–61) contain a 7,8-methylenedioxy group,29,45–51 and of these, caerunine (60) from D. caeruleum possesses a 9-OH group.50 Another five imine DAs (64–68) contain a 7,8-diol group along with an anthranoyl group at C-18.7,37,52–54 In addition, orthocentrine (63) from D. orthocentrum possesses an 8-OMe moiety along with a 10-OH group.55 Eight quaternary ammonium bases, including pseudorenines A and B (69 and 70), and pseudophnines A–D (71, 73–74, and 76) from D. pseudoaemulans,48 and sharwuphinine B (72) from D. shawurense,56 naviculine (75) from D. naviculare var. lasiocarpum21 were reported, although these might be artefacts of the extraction and isolation procedure. Sharwuphinine A (76) from D. shawurense57 and chrysotrichumine A (77) from D. chrysotrichum58 are both alkaloids with a nitrone group between C-17 and C-19.
A total of 177 new amine lycaconitine-type C19-DAs have been reported, and they can be subdivided into three groups according to their oxygenated substituents at C-7 and C-18, namely, the 7,8-methylenedioxy group, the 7-OH group, and the 7-OH/18-anthranoyl group.
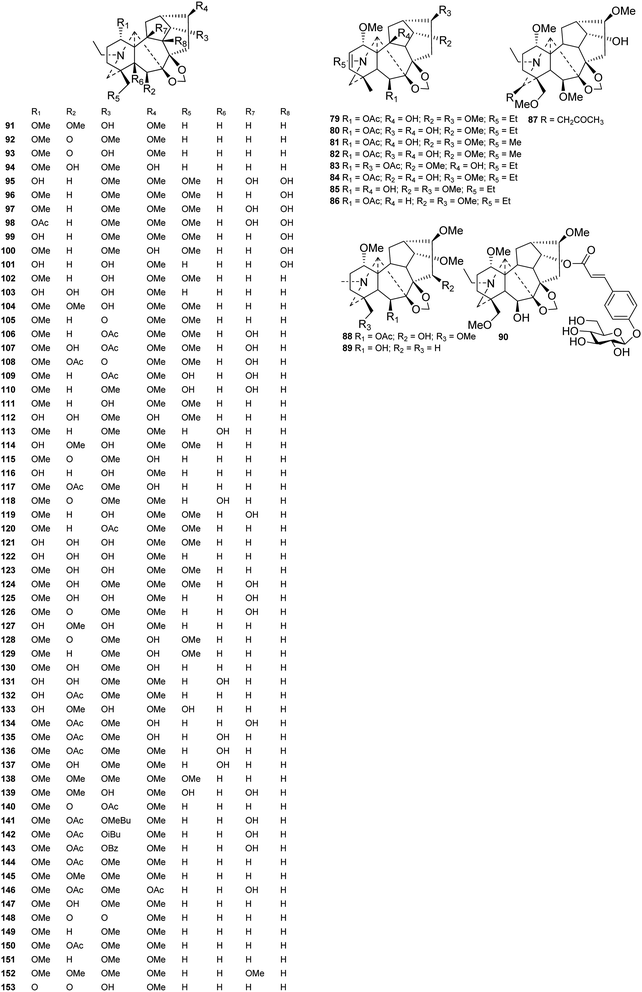
Seventy-six new lycaconitine-type alkaloids with a 7,8-methylenedioxy unit have been reported in the last four decades (Fig. 6). Among these alkaloids, eight (79–86) contain a Δ2,3 group, including siwanines A–D (79–82) from D. siwanense var. leptogen,59 siwanine E and F (82 and 83) from D. siwanense,60 deacetylswinanine A (85) from D. orthocentrum,55 and tatsiensine (86) from D. tatsienense.61 Notably, iliensine A (90) from D. iliense features a 4-O-β-D-glucose-cinnamate ester, making it the first example of a natural DA containing a glucose moiety.62 Pseudouridine B (87) from D. pseudoaemulans possesses a rare acetonyl group at C-19.48 The remaining alkaloids contain only common oxygenated groups, such as OH, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, OMe, and OAc groups, while OMeBu (2-methylbutyryl) and OiBu (isobutyryl) groups are less common. In most cases, these oxygenated groups are located at C-1, C-6, C-14, and C-18. There are also a small number of alkaloids with oxygenated groups at C-5, C-9, and C-10. Most of them possess a 16-OMe group, and some have a 16-OH substituent; delretine (146) from D. retropilosum has a rare 16-OAc.63
O, OMe, and OAc groups, while OMeBu (2-methylbutyryl) and OiBu (isobutyryl) groups are less common. In most cases, these oxygenated groups are located at C-1, C-6, C-14, and C-18. There are also a small number of alkaloids with oxygenated groups at C-5, C-9, and C-10. Most of them possess a 16-OMe group, and some have a 16-OH substituent; delretine (146) from D. retropilosum has a rare 16-OAc.63
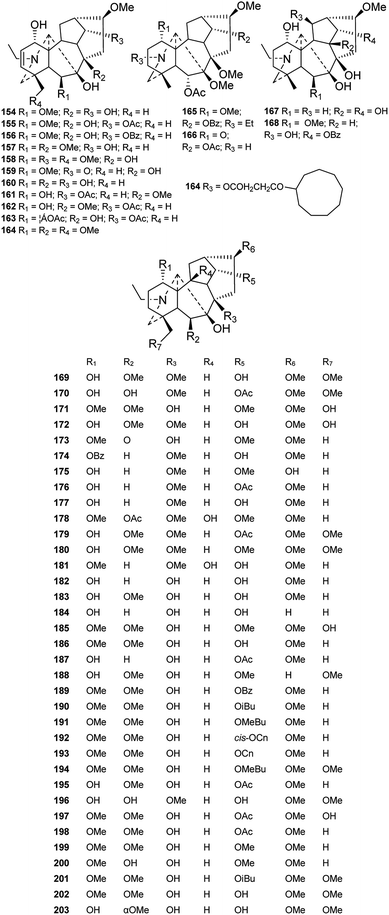
Fifty new amine lycaconitines possessing a 7-OH group were reported in the past forty years (Fig. 7). Eleven alkaloids (154–164) containing a Δ2,3 group were reported,26,64–68 and among these, majusine D (164) from D. majus possesses a novel 3-(cyclononyloxy)propanoate ester group at C-14.69 Alkaloids 192 and 193, a pair of isomers from D. cardiopetalum, possess cis- and trans-cinnamoyl groups at C-14, respectively.70 Gracinine (168) from D. gracile has a hydroxyl group at C-10, which is an infrequently substituted position.71 Pergilone (166) and delphiperegrine (165) from D. peregrinum uniquely feature a methoxy group at C-7.72
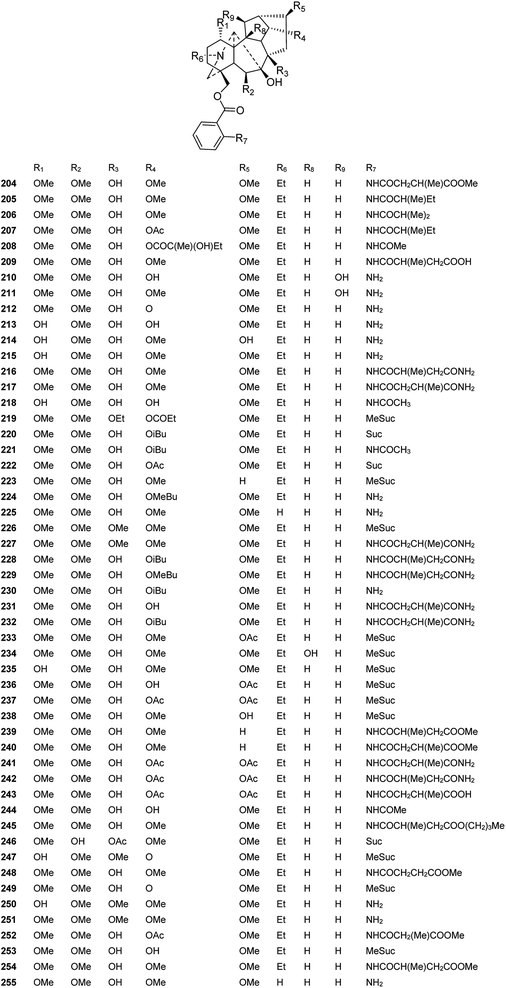
Fifty-two new DAs belonging to the 7-OH/18-anthranoyl group were reported (Fig. 8). These alkaloids are substituted with anthranilic acid derivatives at C-18. Amidogens are usually substituted by succinyl or methyl-succinyl groups or other amide side chains, which might be formed by the breakage of succinyl or methyl-succinyl groups. Ajanine (208) from D. ajacis possesses a 2-hydroxyl-2-methylbutyroyle ester chain at C-14,73 and alpinine (219) from D. alpinum possesses a propionyl group at C-14.74
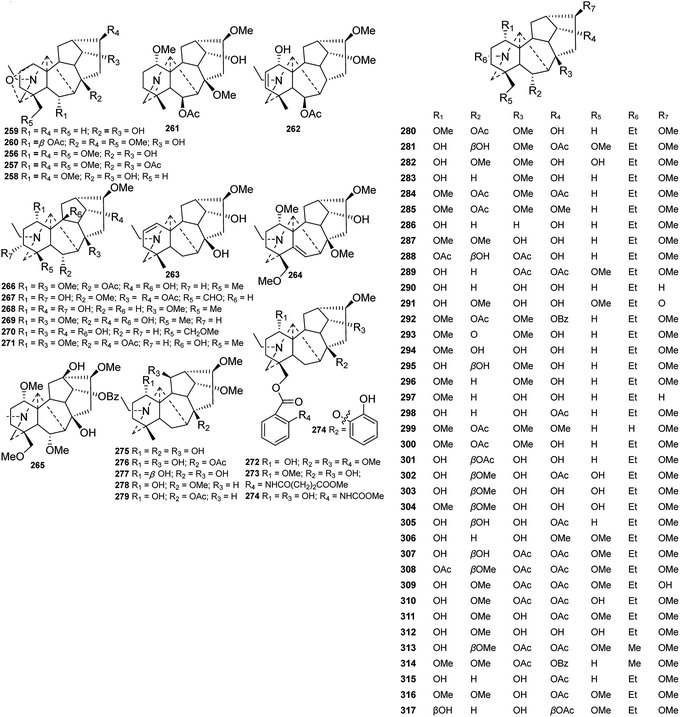
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(19) imine.82 Alkaloids 261 and 262 contain a β-oriented OAc group at C-6.75,82 The other alkaloids mainly vary in the quantity, position and orientation of common oxygenated substituents, including OH, OMe and OAc. Most of the oxygenated substituents are located at C-1, C-6, C-8, C-16, and C-19. Alkaloids 269–270 and 266 have a hydroxyl group at C-10,75,84,85 which is a rare substitution pattern. Generally, aconitine-type DAs have a 16-OMe moiety, but cardiopetaline (290) from D. cardiopetalum and souline (297) from D. souliei are exceptions to this statement, as they lack this group at C-16.81,86 Moreover, delstaphisine (309) from D. staphisagria has a 16-OH group,87 and staphisadrinine (291) from D. staphisagria features a ketone carbonyl at C-16.83
C(19) imine.82 Alkaloids 261 and 262 contain a β-oriented OAc group at C-6.75,82 The other alkaloids mainly vary in the quantity, position and orientation of common oxygenated substituents, including OH, OMe and OAc. Most of the oxygenated substituents are located at C-1, C-6, C-8, C-16, and C-19. Alkaloids 269–270 and 266 have a hydroxyl group at C-10,75,84,85 which is a rare substitution pattern. Generally, aconitine-type DAs have a 16-OMe moiety, but cardiopetaline (290) from D. cardiopetalum and souline (297) from D. souliei are exceptions to this statement, as they lack this group at C-16.81,86 Moreover, delstaphisine (309) from D. staphisagria has a 16-OH group,87 and staphisadrinine (291) from D. staphisagria features a ketone carbonyl at C-16.83
2.3 C20-Diterpenoid alkaloids
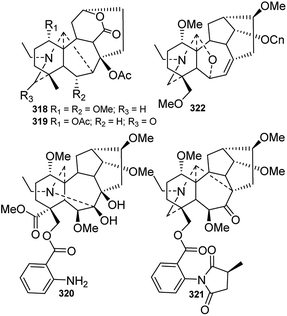
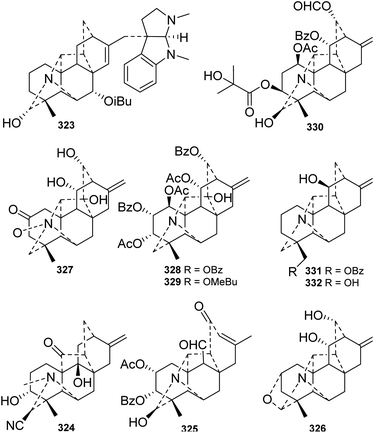
Although C20-type DAs account for a relatively small proportion of DAs in terms of quantity, they are much more structurally diverse than C19-type DAs. The skeletons of C20-DAs are fairly complex, and more than 20 subtypes have been defined.93 As listed in Table 1S,† approximately 89 new alkaloids belonging to seven of the subtypes of C20-DAs were isolated from Delphinium plants in the last four decades.Hetisine-type C20-DAs (C-1), which are characterized by a heptacyclic system with an N–C(6) bond, constitute the majority of the new C20-DAs from Delphinium plants. A total of 56 new hetisines were obtained from the Delphinium species (Fig. 11 and 12). These alkaloids vary mainly in the variety, quantity, position and orientation of their oxygenated substituents, including hydroxyl, acetyl, benzoyl, isobutyryl, 2-methylbutyryl, ketone and carbonyl groups. Anthriscifolmine J (330) from D. anthriscifolium var. savatieri features a unique 2-hydroxy-2-methylpropanoyloxy group at C-3 along with a formyloxy group at C-13,94 and grandiflodine A (324) from D. grandiflorum has a rare cyano group at C-18.90 14-Hydroxyhetisinone N-oxide (327) from D. gracile is a rare hetisine-type N-oxide,95 and delatisine (326) from D. elatum possesses an N–C(19)–O–C(2) mixed acetal unit.96 In addition, several structurally novel hetisine-type C20-DAs were reported. Anthriscifolsine A (325) from D. anthriscifolium var. majus features a seco C ring generated through unprecedented C(11)–C(12) bond cleavage in the hetisine skeleton.97 The N–C(17) bond in grandiflodine A (324) can be cleaved, forming an additional ketone carbonyl at C-17.90 Leptanine (323), which was isolated from D. leptocarpum, is a dimeric alkaloid consisting of a hetisine-type C20-DA part and an indolinonepyrrole fragment. According to X-ray crystal structure analysis, the indolinonepyrrole fragment was bound to the hetisine-type C20-DA part through an α-directed (relative to the indoline core) C(17)–C(3′) covalent bond.98
Vakognavine-type C20-DAs (C-2) have an N–C(19) seco hetisine skeleton in addition to a formyl group at C-4. During the past forty years, 17 new vakognavines were isolated from 6 Delphinium species (Fig. 13). Generally, vakognavine-type C20-DAs seldom have a C(15)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(16) bond, but anthriscifolmines G (393) and H (392) from D. anthriscifolium var. savatieri are exceptions to this statement.99 In addition, anthriscifolmines E–H (390–393) feature a rare formyloxy group at C-11 along with a unique 2-hydroxy-2-methylpropanoyloxy group or a 2-methylpropanoyloxy group at C-13.99
C(16) bond, but anthriscifolmines G (393) and H (392) from D. anthriscifolium var. savatieri are exceptions to this statement.99 In addition, anthriscifolmines E–H (390–393) feature a rare formyloxy group at C-11 along with a unique 2-hydroxy-2-methylpropanoyloxy group or a 2-methylpropanoyloxy group at C-13.99
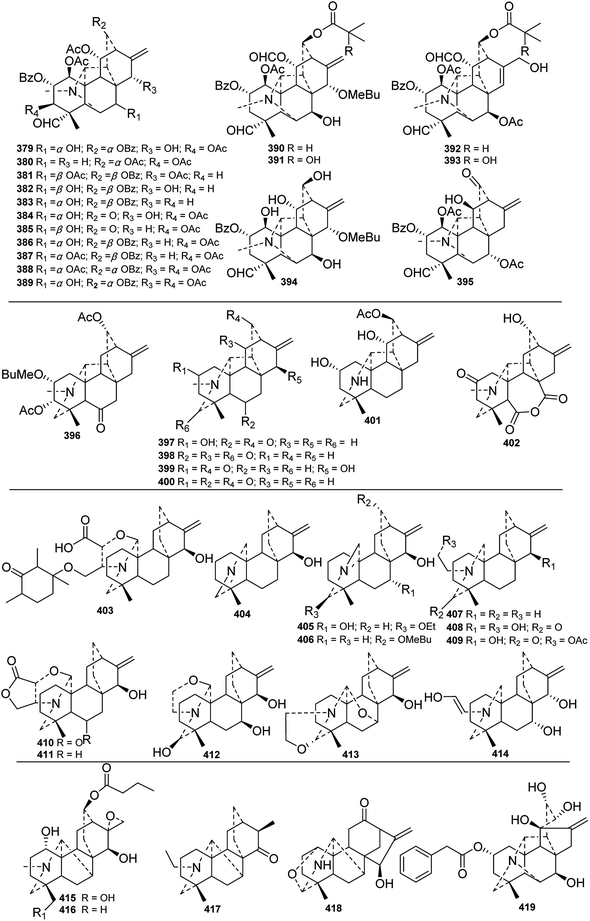 | ||
| Fig. 13 The vakognavine-type, hetidine-type, atisine-type, denudatine-type C20-DAs from Delphinium plants. | ||
Atisine-type C20-DAs (C-4) have always been regarded as the biosynthetic precursors of the other C20-DAs, possess a relatively simple pentacyclic framework. Twelve atisines from seven Delphinium species were reported (Fig. 13). Structurally, isoazitine (404) and 13-(2-methylbutyryl)azitine (406) each possess an azomethine between N and C-19 or C-17.3,100 Uncinatine (414) from D. uncinatum bears an uncommon N–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOH group.101 Delphatisine A (412)102 and delphatisine D (413)58 possess oxazolidine rings formed by a carbinolamine ether linkage between C-20 and C-17 or C-19, respectively. Delphatisines B (411) and C (410) feature a γ-lactone-fused oxazolidine ring.103 The γ-lactone ring in honatisine (403) was open, forming an extra carboxylic acid group at C-22. In addition, a unique 1′,3′,5′-trimethyl-4′-oxocyclohexyloxy unit was substituted at C-24.104
CHOH group.101 Delphatisine A (412)102 and delphatisine D (413)58 possess oxazolidine rings formed by a carbinolamine ether linkage between C-20 and C-17 or C-19, respectively. Delphatisines B (411) and C (410) feature a γ-lactone-fused oxazolidine ring.103 The γ-lactone ring in honatisine (403) was open, forming an extra carboxylic acid group at C-22. In addition, a unique 1′,3′,5′-trimethyl-4′-oxocyclohexyloxy unit was substituted at C-24.104
Other subtypes of C20-DAs were also reported. Six new hetidine-type C20-DAs (C-3), including anthriscifolmine I (396) from D. anthriscifolium var. savatieri,94 carduchoron (398) and delcarduchol (399) from D. carduchorum,105 macrocentrine (401) from D. macrocentrum,4 cardionidine (402) from D. cardiopetalum,106 and 2-dehydrodeacetylheterophylloidine (400) from D. pentagynum,52 were acquired. Among these alkaloids, cardionidine (402) features an anhydride function in its B ring.106 Three denudatine-type DAs (C-5), including anthriscifolmines A (416) and B (415) from D. anthriscifolium var. savatieri, which possess a 16,17-epoxy group and a butyryl group at C-13,107 and cordizine (417) from D. corymbosum, which possesses a CH3-17β angular methyl group, were reported.108 Moreover, a napelline-type C20-DA (C-6), norsongoramine (418), with an N–C(19)–O–C(1) mixed acetal unit was obtained from D. tamarae,109 and a delnudine-type C20-DA (C-7), trichodelphinine F (419), possessing a rare phenylacetyl group at C-2, was isolated from D. trichophorum.110
2.4 Other alkaloids
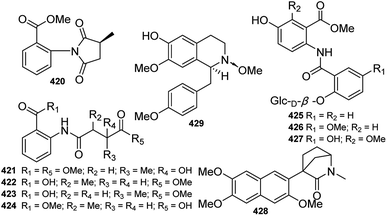
In addition to DAs, other types of alkaloids have also been isolated from Delphinium plants (Fig. 14). Several amide alkaloids from Delphinium plants have been reported. Five new acyl anilines, delamides A–E (420–424), which possessed an O-ester aniline bearing an amide side chain, were isolated from D. brunonianum by Zou et al.111 Diaz et al. reported the isolation of three new anthranilic acid derivatives (425–427), also called dianthramides, from tissue cell cultures of D. staphisagria.112 In addition, a novel lactam (428) possessing a 2-azabicyclo[2.2.1]heptane unit bearing a trimethoxy naphthalene ring, was isolated from D. caeruleum.113Tetrahydroisoquinoline alkaloids are widely distributed in the Ranunculaceae family. While little attention has been paid to the isoquinoline alkaloids in Delphinium plants, a new benzyltetrahydrobenzylisoquinoline alkaloid, O-methylroefractine N-oxide (429), was found in D. fangshanense.114
3. Flavonoids
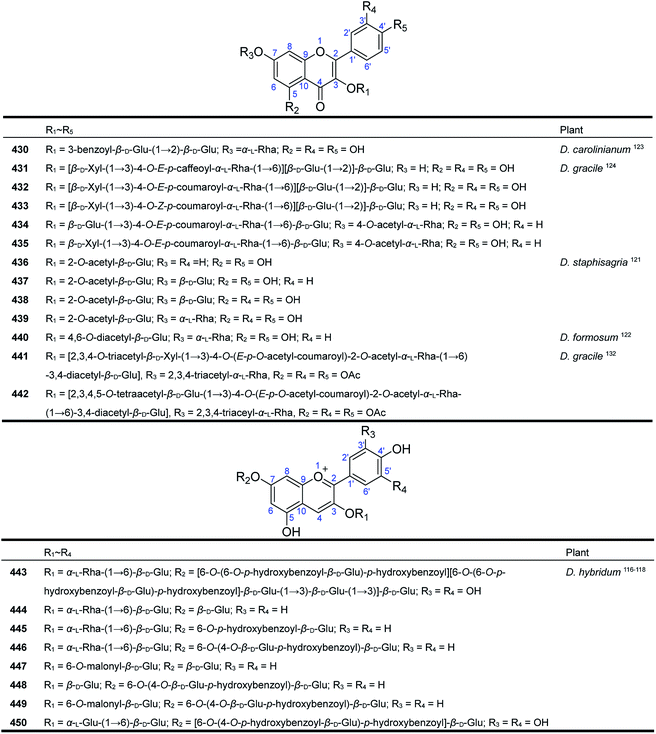
Delphinium, the flowers of which have petals of various colours, i.e., white, red, violet and blue, are widely cultivated as one of the most famous horticultural plants in the world. The anthocyanidin pigments in the Delphinium flowers have attracted considerable attention for a long time. As early as 1915, Willstitter isolated the first anthocyanidin pigment, delphinin, from the reddish-purple petals of D. consolida.10,115 During the last four decades, eight new anthocyanidins were reported from different cultivated varieties of D. hybridum (Fig. 14). Two new delphinin glycosides, violdelphin (450)116 and cyanodelphin (443),117 were isolated from the violet petals of D. hybridum cv “Blue Night” and the blue petals of D. hybridum cv “Blue Springs”, respectively. Structurally, violdelphin (450) contains two p-hydroxybenzoic acid units and four hexose substituents in addition to the delphinin core, and cyanodelphin (443) contains four p-hydroxybenzoic acid units and seven glucose units in its structure. In addition, six new acylated pelargonidin 3,7-glycosides (444–449) were isolated from the red petals of D. hybridum cv “Princess Caroline”.118 These pelargonidin glycosides possess various acylated glucoses and rhamnoses at C-3 and C-7. Characteristically, glycosides 447 and 449 are acylated at the 3-glucose residue with malonic acid.In addition to anthocyanidins, Delphinium plants are also rich in flavonol glycosides. In 1973, Arazashvili et al. first reported the identification of two known flavonol glycosides from the leaves of D. flexiosum and D. elisabethae.119 Dozens of flavonols and their glycosides were isolated from Delphinium plants during the next forty years, including some common and widespread constituents, such as rutin, quercetin, kaempferol, and luteolin as well as their glycosides.120 Eleven new compounds have been reported from four Delphinium species, with the aglycones being kaempferol (434–437 and 440) and quercetin derivatives (430–433 and 438–439) (Fig. 15). The novelty of these flavonol glycosides is mainly determined by the type and position of the acyl groups on the carbohydrate chains. Structurally, flavonol glycosides 436–439 from D. staphisagria possess a 2-O-acetyl glucosyl group at C-7,121 while flavonol glycoside 440 from D. formosum has a 4,6-O-diacetyl glucosyl group.122 Compound 430, a benzoylated quercetin glycoside, was isolated from D. carolinianum,123 and compounds 431–435 are a series of tetraglycosides acylated by caffeic acid and cumaric acid.124
4. Phenolics
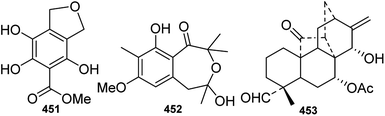
A certain number of phenolic compounds, such as benzoic and phenylacetic acid derivatives, have been identified from Delphinium plants.125–127 However, most of these phenolic compounds are common, structurally simple and widely distributed in the plant kingdom; new structures are rarely discovered. Only two new phenolic compounds, namely, 2,5,6-trihydroxypiperonylic acid methyl ester (451) from D. venulosum128 and oxformasine (452) from D. formosum,129 were reported during the studied period (Fig. 16). Oxformasine (452) represents the first benzoxepine derivative from Delphinium species.5. Terpenoids
In contrast to the wide variety of DAs present in Delphinium plants, terpenoids are rare. To date, only one new non-alkaloidal diterpenoid, campylopin (453) from D. campylocentrum, has been reported130 (Fig. 15). Campylopin (453) is the first naturally occurring hetidane-type diterpenoid, and it has great significance for the biosynthesis of diterpenoid alkaloids, as it implies a new biosynthetic pathway from atisane or hetidane-type C20-diterpenes to hetidine-type C20-diterpenoid alkaloids.1316. Bioactivities
In the past forty years, compounds isolated from Delphinium plants, mainly DAs and flavonols, have been screened for their multiple biological activities, including antineoplastic, antimicrobial, anti-inflammatory, and insecticidal and antifeedant activities, as well as cholinesterase inhibition effects. Some of the tested compounds showed considerable activities. Herein, the bioactivities of the compounds from the Delphinium plants are summarized.6.1 Anticancer activity
A certain number of natural DAs have been reported to possess antiproliferative activities against various human cancer cell lines, indicating their great potential as new drugs for treating the corresponding cancers.133 New DAs along with known DAs from Delphinium plants have also been reported to have in vitro anticancer activities (Table 1). The atisine-type DA delphatisine C (410) from D. chrysotrichum showed significant cytotoxic activity against A549 cells (IC50, 2.36 μM),103 and its analogue honatisine (403) from D. honanense also displayed impressive cytotoxic activity against MCF-7 cells with an IC50 value of 3.16 μM, making it more effective than the positive control etoposide (IC50, 7.53 μM).104 The cytotoxic activities of five hetisine-type C20-DAs, trichodelphinines A–E (350–354), and one delnudine-type C20-DA, trichodelphinine F (419), against A549 cells were tested.110 The most active compounds (351, 354 and 419) had low IC50 values (18.64, 12.03 and 16.55 μM, respectively), and the other compounds showed moderate cytotoxicities against A549 cells. In addition, known lycaconitine-type C19-DAs, including delpheline, delbrunine, siwanine E, delcorinine, uraphine, nordhagenine A, and delbrunine from D. chrysotrichum and D. honanense, also showed certain anticancer activities against A549 and MCF-7 cells with IC50 values ranging from 9.62 to 35.32 μM.104| Plants | Alkaloids | Type | IC50 (μM) | |
|---|---|---|---|---|
| MCF-7 | A549 | |||
| D. chrysotrichum | Delphatisine C (410) | C-4 | >50 | 2.36 |
| Delpheline | B-1 | 17.32 | >50 | |
| Delbrunine | B-1 | 16.50 | 10.63 | |
| Etoposide | — | 7.56 | 1.8 | |
| D. honanense | Honatisine (403) | C-4 | 3.16 | >50 |
| Siwanine E | B-1 | 35.32 | >50 | |
| Delcorinine | B-1 | 18.60 | 31.63 | |
| Uraphine | B-1 | 33.21 | 9.86 | |
| Nordhagenine A | B-1 | 17.38 | 9.62 | |
| Etoposide | — | 7.53 | 1.82 | |
| D. trichophorum | Trichodelphinine A (350) | C-1 | — | 27.62 |
| Trichodelphinine B (351) | C-1 | — | 18.64 | |
| Trichodelphinine C (352) | C-1 | — | 48.08 | |
| Trichodelphinine D (353) | C-1 | — | 52.79 | |
| Trichodelphinine E (354) | C-1 | — | 12.03 | |
| Trichodelphinine F (419) | C-1 | — | 16.55 | |
| Doxorubicin | — | — | 0.60 | |
Although no detailed structure–activity relationship (SAR) study has yet been reported, it seems that C20-DAs have shown more potential to be developed as antitumor drugs on account of their higher efficiency and lower toxicity.13 Especially, the hetisine-type C20-DAs, which have exhibited selective antiproliferative activity on human lung cancer cell A549, deserve further studies to identify more potent antitumor DAs. On the other hand, Delphinium plants have rarely been utilized for the treatment of cancer in TCM. The research presented above suggests that Delphinium plants with abundant DAs have great potential as herbal drugs for treating cancer, but more research is required to confirm this.
6.2 ChE inhibition effects
The discovery of natural ChE inhibitors is an active research area in natural medicinal chemistry due to the involvement of cholinesterases in Alzheimer's disease and related dementias.134 In the early 1990s, methyllycaconitine, one of the principal active constituents of Delphinium species, was found to be an effective ligand for neuronal nicotinic acetylcholine receptor (nAChR) subtypes, which attracted the attention of scientists to the screening of natural cholinesterase inhibitors from Delphinium species. Several Delphinium alkaloids have been reported to exhibit considerable ChE inhibitory effects (Table 2). The aconitine-type C19-DAs 1β-hydroxy, 14β-acetylcondelphine (317), jadwarine-A (270), jadwarine-B (262), and dihydropentagynine (203) from D. denudatum have been found to possess inhibitory effects of AChE and BChE with EC50 values ranging from 9.2 to 34.7 μM.75 Ahmad et al. reported that an isotalatazidine hydrate crystal isolated from D. denudatum showed competitive inhibition of both AChE and BChE with IC50 values of 12.13 μM and 21.41 μM, respectively.135 In addition, the amide alkaloid delamide A (420) from D. brunonianum also showed highly selective AChE inhibitory activity (EC50, 9.7 μM) and was shown to be a mixed-type reversible inhibitor of AChE by kinetic analysis.111| Plants | Compounds | Type | EC50 (μM) | |
|---|---|---|---|---|
| AChE | BChE | |||
| D. denudatum | 1β-hydroxy, 14β-acetyl condelphine (317) | B-2 | 19.8 | 31.5 |
| Jadwarine-A (270) | B-2 | 9.2 | 19.6 | |
| Jadwarine-B (262) | B-2 | 16.8 | 34.7 | |
| Isotalatizidine hydrate | B-2 | 12.1 | 21.4 | |
| Dihydropentagynine (203) | B-1 | 11.2 | 22.2 | |
| Allanzanthane A | — | 8.2 | 18 | |
| Galanthamine | — | 10.1 | 20.6 | |
| D. brunonianum | Delamide A (420) | Amide | 9.7 | >50 |
| Rivastigmine | — | 4.7 | >10 | |
6.3 Insecticidal and antiparasitic activities
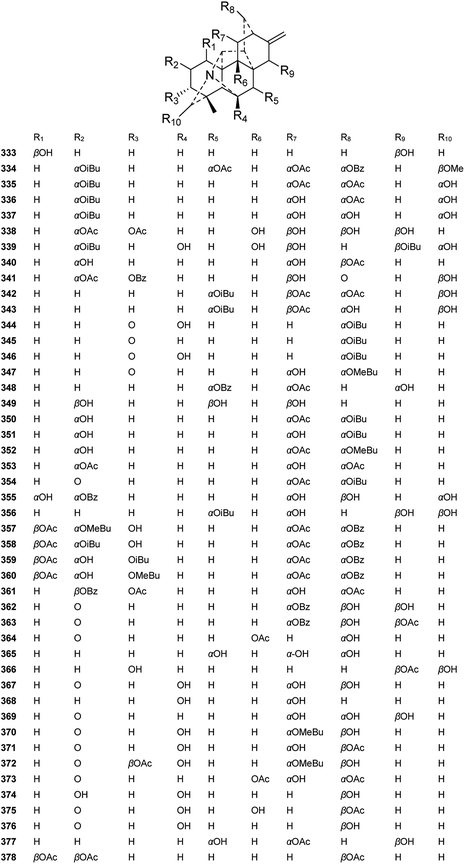
Delphinium plants have been used as natural insecticides since the time of Dioscorides. Previous studies have indicated that DAs might have evolved in nature to protect Delphinium and Aconitum plants against pests. Hence, searching for valuable natural insecticides from plants that are rich in DAs, which have been shown to be potent and selective ligands of the insect nicotinic receptor, is quite effective.136,137 A series of DAs from Delphinium plants have been shown to possess insecticidal and antifeedant activities. Ulubelen et al. tested the repellency of 8 new alkaloids along with 12 known alkaloids belonging to three subtypes of DAs from Turkish Delphinium species against Tribolium casteneum (Table 3).138 Most of the tested new alkaloids (280, 285, 299, 331, 368–369, and 378) had repellency class III values (40.1–60%) for a short period, and venuluson (369) gave the highest level of repellency (59.37%), suggesting it is a promising candidate for insecticide development.| Plants | Alkaloids | Type | Repellency (%) | Class |
|---|---|---|---|---|
| D. venulosum | Venulol (368) | C-1 | 31.25 | II |
| Venuluson (369) | C-1 | 56.25 | III | |
| Venudelphine (378) | C-1 | 40.62 | III | |
| Hetisine | C-1 | 59.12 | III | |
| Hetisinone | C-1 | 37.50 | II | |
| D. gueneri | 14-Methyl peregrine (285) | B-2 | 46.87 | III |
| N-Deethyl-14-O-methylperegrine (299) | B-2 | 40.62 | III | |
| Peregrine (280) | B-2 | 53.12 | III | |
| Peregrine alcohol | B-2 | 37.50 | II | |
| Talatisamine | B-2 | 34.37 | II | |
| 14-Acetyneoline | B-2 | 53.12 | III | |
| D. albiflorum | Lycoctonine | B-1 | 46.87 | III |
| D. davisii | 18-Benzoyldavisinol (331) | C-1 | 46.87 | III |
| Karakoline | B-2 | 37.50 | II | |
| D. uncinatum | 14-Acetylvirescenine | B-1 | 43.75 | III |
| Condelphine | B-2 | 40.62 | III | |
| D. formosum | 14-Demethylajacine (244) | B-1 | 40.62 | III |
| Delsemine B | B-1 | 37.50 | II | |
| Delsoline | B-1 | 37.50 | II | |
| D. crispulum | Browniine | B-1 | 46.87 | III |
| D. montanum | Gigactonine | B-1 | 43.75 | III |
Several investigations on the antifeedant activities of Delphinium alkaloids have been performed. The crude alkaloids of D. cyphoplectrum have slight antifeedant and insect repellent activities against the larvae of Spodoptera littoralis.139 González-Coloma et al. tested the insect antifeedant activities of 21 DAs isolated from Delphinium species on Spodoptera littoralis and Leptinotarsa decemlineata. The antifeedant effects of the test compounds were structure- and species-dependent (EC50 values ranging between 0.42–22.5 and 0.1–17.77 μg cm−2 for L. decemlineata and S. littoralis, respectively). The most active antifeedants to L. decemlineata and S. littoralis were found be to the cardiopetaline (259, EC50, 0.42 μg cm−2) and 19-oxodihydroatisine (408, EC50, 0.1 μg cm−2), respectively.140,141 Shawurensine (209) from D. naviculare var. lasiocarpum also showed considerably potent antifeedant activity with EC50 values of 0.42 and 0.81 mg cm−2 against the larvae of Spodoptera exigua in a choice test and no choice test, respectively.142 Generally, the antifeedant activities of C20-DAs are lower than those of C19-DAs, which might be attributed to the species- and structure-related differences in the taste receptor binding to these two classes of DAs,143 and this result suggest that further investigations on the antifeedant effects of these compounds should be concentrated on C19-DAs.
Among the new flavonol glycosides that have been isolated from Delphinium plants, a series of compounds (431–437, 439 and 441–442) have demonstrated high antiparasitic activities.132,144,145 In some cases, the antitrypanosomatid activities of these flavonol glycosides against Trypanosoma cruzi were more potent than that of the reference drug benznidazole. For example, compound 436 showed higher trypanocidal activity (IC50 = 6.5 μM) than benznidazole (IC50 = 15.8 μM) against the epimastigote form of T. cruzi, and compound 432 exhibited higher trypanocidal activity (IC50, 21.2 μM) than benznidazole (IC50, 23.3 μM) against the amastigote form of T. cruzi. These compounds also showed impressive leishmanicidal activities against both the extra- and intracellular forms of three Leishmania species (Leishmania infantum, L. braziliensis and L. donovani), and among these compounds, 439 presented the highest antileishmanial activity. Notably, all of these tested flavonol glycosides showed low toxicity to the corresponding host cells, resulting in higher selectivity indices than the reference drugs, which highlights their potential in the treatment of leishmaniasis and Chagas disease (Table 4).
| Plants | Alkaloids | Type | EC50 (μg cm−2) | |
|---|---|---|---|---|
| L. decemlineata | S. littoralis | |||
| D. cardiopetalum | Hetisinone | C-1 | 13.1 | >50 |
| Cardiopetamine (362) | C-1 | 22.5 | 5.5 | |
| 15-Acetyl-cardiopetamine (363) | C-1 | 12.9 | >100 | |
| Cardiodine (329) | C-1 | 2.2 | 4.4 | |
| D. gracile | Atisinium chloride | C-3 | 3.4 | 2.4 |
| D. stenocarpa | Ajaconine | C-3 | 5.1 | 8.2 |
| D. staphisagria | 19-Oxodihydroatisine (408) | C-3 | >50 | 0.1 |
| Azitine | C-3 | >50 | 1.1 | |
| Isoazitine (404) | C-3 | 6.9 | 4.1 | |
| D. cardiopetalum | Karakoline | B-2 | 0.44 | >50 |
| Cardiopetaline (259) | B-2 | 0.42 | ≈50 | |
| Cardiopetalidine (184) | B-1 | >50 | >50 | |
| 14-Benzoylgadesine | B-1 | >50 | 13.61 | |
| D. montanum | 8-O-Ethylaconine | B-2 | >50 | 8.29 |
| Neoline | B-2 | ≈50 | ≈50 | |
| Gigactonine | B-1 | 13.02 | 9.31 | |
| Delcosine | B-1 | 1.11 | 3.53 | |
| Methylicaconitine | B-1 | 2.78 | 17.77 | |
| D. pentagynum | Gadenine | B-1 | 11.93 | >50 |
6.4 Antifungal and antiviral activity
Delphinium species have been used for the treatment of itches and other skin eruptions in folk medicine, which indicates that the plants may possess constituents with antifungal activities. The new lactone-type C19-DA 8-acetylheterophyllisine (319) from D. denudatum showed antifungal activity against a number of human pathogenic fungi, including Allescheria boydii, Aspergillus niger, Epidermophyton floccosum, and Pleurotus ostreatus, with MIC values of 100, 200, 250, and 150 μg mL−1, respectively.88Delphinium-derived DAs also showed antiviral activity. The new lycaconitine-type C19-DAs ajacisines C–E (212–214), along with the known alkaloid isodelectine, which were isolated from D. ajacis, exhibited moderate to weak in vitro antiviral effects against respiratory syncytial virus (RSV) with IC50 values of 75.2, 35.1, 10.1, and 50.2 μM, respectively,146 while the positive control (ribavirin) showed an IC50 value of 3.1 μM. The rearranged-type C19-DA grandiflodine B (21), isolated from D. grandiflorum, also displayed a weak inhibitory effect on the growth of RSV with an IC50 value of 75.3 μM.90
7. Conclusions
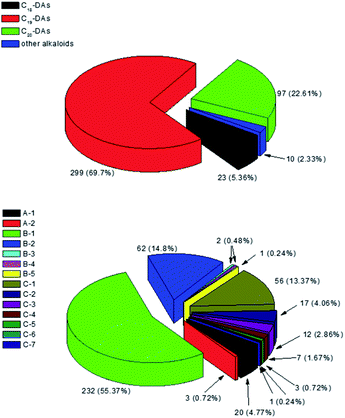
To the best of our knowledge, investigations on the chemical constituents of Delphinium in the last four decades have reported a total of 453 new compounds, including 429 alkaloids, 21 flavonoids, two phenolic compounds, and one diterpenoid. Among the 429 new alkaloids, 419 are DAs, including 23 C18-DAs, 299 C19-DAs, and 97 C20-DAs, which cover fourteen subtypes of DAs (Fig. 17). In view of the chemical diversity described, the lycaconitine sub-type of C19-DAs (B-1), with 230 new members, are the most abundant DAs in the Delphinium plants, as they accounted for the largest proportion of new compounds (55.37%), followed by aconitine-type C19-DAs (B-2) with 64 new members (14.8%) and hetisine-type C20-DAs (C-1) with 56 new members (13.37%). The other subtypes only account for only a small portion of compounds (less than 20%). Obviously, DAs, especially lycaconitine-type C19-DAs, are characteristic components of the genus Delphinium, which is distinguished from the genus Aconitum by the large number of aonitine-type C19-DAs. Among these new compounds, several possess unprecedented structures, and their various biological activities, including anticancer activity, cholinesterase inhibition effects, insecticidal and antiparasitic activities, and antifungal and antiviral activities, have been reported. These findings underscore the large chemical and biological diversity among the chemical constituents of Delphinium plants, which could not only serve as a vast resource for drug discovery but also help elucidate the therapeutic effects of Delphinium-derived herbal drugs.Although phytochemical and biological studies on the chemical constituents of Delphinium species have attracted considerable interest, some deficiencies remain. First, there are approximately 365 Delphinium species around the world, but the chemical constituents of only 87 species and 10 varietal have been studied in the last four decades. Among these species, D. elatum, D. staphisagria, D. anthriscifolium var. savatieri, D. nuttallianum, D. anthriscifolium var. majus, and D. cardiopetalum contributed relatively more new compounds than the other species. The biological constituents of other Delphinium species remain untapped. Hence, an extensive investigation of other species, especially species that are used medicinally, remains necessary. Second, all of the biological activities of the isolated compounds were investigated by using in vitro tests, namely, chemical and cellular models, and there is little research confirming the biological activities of Delphinium compounds using in vivo animal models or on their pharmacological mechanisms. It is necessary to evaluate the biological activities of Delphinium-derived constituents using both in vitro and in vivo models, which will facilitate further research and exploitation of this genus.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by a grant from the National Natural Science Foundation of China (No. 31860095), a grant from Guizhou Science and Technology Foundation of China (No. QKHJC[2018]1193), a program for Changjiang Scholars and Innovative Research Team in University (IRT_17R94), and a project of Yunling Scholars.References
- W. C. Wang, Guihaia, 2019, 39, 1425–1469 Search PubMed.
- G. de la Fuente, J. A. Gavin, R. D. Acosta and F. Sanchez-Ferrando, Phytochemistry, 1993, 34, 553–558 CrossRef CAS.
- J. G. Díaz, J. G. Ruiz and G. de la Fuente, J. Nat. Prod., 2000, 63, 1136–1139 CrossRef PubMed.
- M. H. Benn, I. Francis and R. M. Manavu, Phytochemistry, 1989, 28, 919–922 CrossRef CAS.
- Y. Bai and M. Benn, Phytochemistry, 1992, 31, 3243–3245 CrossRef CAS.
- L. Q. Li and K. Yuichi, Flora of China, 2001, vol. 6, pp. 149–222 Search PubMed.
- S. Fang and M. Benn, Phytochemistry, 1992, 31, 3247–3250 CrossRef.
- U. Kolak, M. Ozturk, F. Ozgokce and A. Ulubelen, Phytochemistry, 2006, 67, 2170–2175 CrossRef CAS PubMed.
- L. P. Yan, D. L. Chen and F. P. Wang, Chin. J. Org. Chem., 2007, 27, 976–980 CAS.
- R. Willstätter and W. Mieg, Ann. Chem., 1915, 408, 61 CrossRef.
- F. B. Ahrens, Ber. Dtsch. Chem. Ges., 1899, 32, 1581–1584 CrossRef CAS.
- T. P. Yin, Z. H. Luo, L. Cai and Z. T. Ding, Chin. J. Magn. Reson., 2018, 36, 113–126 Search PubMed.
- F. P. Wang, Q. H. Chen and X. Y. Liu, Nat. Prod. Rep., 2009, 27, 529–570 RSC.
- F. P. Wang and Q. H. Chen, The C19-diterpenoid alkaloids, 2010, vol. 69, pp. 1–577 Search PubMed.
- M. S. Yunusov, Nat. Prod. Rep., 1993, 10, 471–486 RSC.
- T. P. Yin, X. F. Hu, R. F. Mei, Y. Shu, D. Gan, L. Cai and Z. T. Ding, Phytochem. Lett., 2018, 25, 152–155 CrossRef CAS.
- T. P. Yin, Y. Shu, H. Zhou, L. Cai and Z. T. Ding, Fitoterapia, 2019, 135, 1–4 CrossRef CAS PubMed.
- F. P. Wang, Q. H. Chen and X. T. Liang, The C18-diterpenoid alkaloids, 2009, vol. 67, pp. 1–78 Search PubMed.
- X. L. Zhou, Q. H. Chen and F. P. Wang, Chem. Pharm. Bull., 2004, 52, 456–458 CrossRef CAS PubMed.
- A. Ulubelen, A. H. Meriçli, F. Meriçli, U. S. Kolak, R. Ilarslan and W. Voelter, Phytochemistry, 1999, 50, 513–516 CrossRef CAS.
- W. J. Xue, B. Zhao, J. Y. Zhao, S. Sh Sagdullaev and H. Akber Aisa, Phytochem. Lett., 2019, 33, 12–16 CrossRef CAS.
- S. Wang, X. L. Zhou, X. M. Gong, X. Y. Fan and M. S. Lan, J. Asian Nat. Prod. Res., 2016, 18, 141–146 CrossRef CAS PubMed.
- L. Shan, J. Zhang, L. Chen, J. Wang, S. Huang and X. Zhou, Nat. Prod. Commun., 2015, 10, 2067–2068 CrossRef PubMed.
- L. H. Shan, J. F. Zhang, F. Gao, S. Huang and X. L. Zhou, J. Asian Nat. Prod. Res., 2018, 20, 423–430 CrossRef CAS PubMed.
- L. Song, X. X. Liang, D. L. Chen, X. X. Jian and F. P. Wang, Chem. Pharm. Bull., 2007, 55, 918–921 CrossRef CAS.
- L. Song, X. Y. Liu, Q. H. Chen and F. P. Wang, Chem. Pharm. Bull., 2009, 57, 158–161 CrossRef CAS PubMed.
- Q. P. Jiang and W. L. Sung, Heterocycles, 1985, 23, 11–15 CrossRef CAS.
- P. M. Shrestha and A. Katz, J. Nat. Prod., 2000, 63, 2–5 CrossRef CAS PubMed.
- J. Li, D. L. Chen, X. X. Jian and F. P. Wang, Molecules, 2007, 12, 353–360 CrossRef CAS PubMed.
- A. G. Gonzalez, G. de la Fuente, M. Reina and I. Timon, Heterocycles, 1984, 22, 667–669 CrossRef CAS.
- P. Tang, D. L. Chen, Q. H. Chen, X. X. Jian and F. P. Wang, Chin. Chem. Lett., 2007, 18, 700–703 CrossRef CAS.
- C. J. Li and D. H. Chen, Acta Bot. Sin., 1992, 31, 466–469 Search PubMed.
- K. Wada, E. Asakawa, Y. Tosho, A. Nakata, Y. Hasegawa, K. Kaneda, M. Goto, H. Yamashita and K. H. Lee, Phytochem. Lett., 2016, 17, 190–193 CrossRef CAS.
- T. M. Gabbasov, E. M. Tsyrlina, L. V. Spirikhin and M. S. Yunusov, Chem. Nat. Compd., 2010, 46, 158–159 CrossRef CAS.
- X. Liang, S. A. Ross, Y. R. Sohni, H. M. Sayed, H. K. Desai, B. S. Joshi and S. W. Pelletier, J. Nat. Prod., 1991, 54, 1283–1287 CrossRef CAS.
- L. Bitiş, S. Suezgec, U. Sözer, H. Oezcelik, J. Zapp, A. K. Kiemer and A. H. Mericli, Helv. Chim. Acta, 2007, 90, 2217–2221 CrossRef.
- K. Wada, T. Yamamoto, H. Bando and N. Kawahara, Phytochemistry, 1992, 31, 2135–2138 CrossRef CAS.
- H. Yamashita, M. Katoh, A. Kokubun, A. Uchimura, S. Mikami, A. Takeuchi, K. Kaneda, Y. Suzuki, M. Mizukami, M. Goto, K. H. Lee and K. Wada, Phytochem. Lett., 2018, 24, 6–9 CrossRef CAS PubMed.
- A. S. Narzullaev, V. M. Matveev, S. S. Sabirov and M. Y. Yunusov, Chem. Nat. Compd., 1986, 22, 745–746 CrossRef.
- N. Batbayar, S. Enkhzaya, J. Tunsag, D. Batsuren, D. S. Rycroft, S. Sproll and F. Bracher, Phytochemistry, 2003, 62, 543–550 CrossRef CAS PubMed.
- S. W. Pelletier, N. V. Mody and R. C. Desai, Heterocycles, 1981, 16, 747–750 CrossRef CAS.
- X. X. Liang, D. L. Chen and F. P. Wang, Chin. Chem. Lett., 2006, 17, 1473–1476 CAS.
- H. Pu, Q. Xu, F. Wang and C. T. Che, Planta Med., 1996, 62, 462–464 CrossRef CAS PubMed.
- H. Y. Pu, F. P. Wang and C. T. Che, Phytochemistry, 1996, 43, 287–290 CrossRef CAS.
- K. Wada, R. Chiba, R. Kanazawa, K. Matsuoka, M. Suzuki, M. Ikuta, M. Goto, H. Yamashita and K. H. Lee, Phytochem. Lett., 2015, 12, 79–83 CrossRef CAS.
- B. S. Joshi, E. S. A. El-Kashoury, H. K. Desai, E. M. Holt, J. D. Olsen and S. W. Pelletier, Tetrahedron Lett., 1988, 29, 2397–2400 CrossRef CAS.
- B. Zhao, S. Usmanove and H. A. Aisa, Phytochem. Lett., 2014, 10, 189–192 CrossRef CAS.
- W. J. Xue, B. Zhao, Z. Ruzi, J. Y. Zhao and H. A. Aisa, Phytochemistry, 2018, 156, 234–240 CrossRef CAS.
- J. F. Zhang, L. H. Shan, F. Gao, S. Huang and X. L. Zhou, Chem. Biodiversity, 2017, 14, e1600297 CrossRef PubMed.
- Y. Wang, S. N. Chen, Y. Pan, J. Zhang and Y. Chen, Phytochemistry, 1996, 42, 569–571 CrossRef CAS.
- Y. Wang, Y. J. Pan, S. N. Chen and Y. Z. Chen, Chin. Chem. Lett., 1996, 7, 139–140 CAS.
- J. G. Diaz, J. G. Ruiz and W. Herz, Phytochemistry, 2004, 65, 2123–2127 CrossRef CAS PubMed.
- J. Z. Jin and M. C. Zhong, Chin. Tradit. Herb. Drugs, 1986, 17, 1–3 Search PubMed.
- H. K. Desai, B. T. Cartwright and S. W. Pelletier, J. Nat. Prod., 1994, 57, 677–682 CrossRef CAS.
- L. Ding, J. Wang, S. Peng and N. Chen, Acta Bot. Sin., 2000, 42, 523–525 CAS.
- B. Zhao, S. K. Usmanova, A. Yili, A. Kawuli, R. Abdulla and H. A. Aisa, Chem. Nat. Compd., 2015, 51, 519–522 CrossRef CAS.
- C. Li, Y. Hirasawa, H. Arai, H. Akber Aisa and H. Morita, Heterocycles, 2010, 80, 607–612 CrossRef CAS.
- Y. He, D. Zhang and L. M. West, Fitoterapia, 2019, 139, 104407 CrossRef CAS.
- S. Zhang and Q. Ou, Phytochemistry, 1998, 48, 191–196 CrossRef CAS.
- Z. Suoming, Z. Guiling and G. Lin, Phytochemistry, 1997, 45, 1713–1716 CrossRef.
- S. W. Pelleetier, J. A. Glinski, S. S. Joshi and C. Szu-ying, Heterocycles, 1983, 20, 1347–1354 CrossRef.
- J. F. Zhang, R. Y. Dai, L. H. Shan, L. Chen, L. Xu, M. Y. Wu, C. J. Wang, S. Huang and X. L. Zhou, Phytochem. Lett., 2016, 17, 299–303 CrossRef CAS.
- S. A. Saidkhodzhaeva and I. A. Bessonova, Chem. Nat. Compd., 1996, 32, 720–722 CrossRef.
- X. L. Zhou, Q. H. Chen, D. L. Chen and F. P. Wang, Chin. J. Chem., 2003, 21, 871–874 CrossRef CAS.
- X. L. Zhou, Q. H. Chen and F. P. Wang, Heterocycles, 2004, 63, 123–128 CrossRef CAS.
- L. S. Ding and W. X. Chen, Acta Pharmacol. Sin., 1990, 25, 438–440 CAS.
- J. Y. Sun and T. C. Li, J. Chem. Res., 2009, 2009, 306–307 CrossRef.
- F. Z. Chen, D. L. Chen, Q. H. Chen and F. P. Wang, J. Nat. Prod., 2009, 72, 18–23 CrossRef CAS PubMed.
- Q. Zhao, X. J. Gou, W. Liu, G. He, L. Liang and F. Z. Chen, Nat. Prod. Commun., 2015, 10, 2063–2064 Search PubMed.
- M. Reina, A. Madinaveitia and G. De La Fuente, Phytochemistry, 1997, 45, 1707–1711 CrossRef CAS.
- A. G. Gonzalez, R. Diaz Acosta, J. A. Gavin and G. De la Fuente, Heterocycles, 1986, 24, 2753–2756 CrossRef CAS.
- A. Ulubelen, A. H. Mericli, F. Mericli and R. Ilarslan, Phytochemistry, 1992, 31, 1019–1022 CrossRef CAS.
- J. Lu, H. K. Desai, S. A. Ross, H. M. Sayed and S. W. Pelletier, J. Nat. Prod., 1993, 56, 2098–2103 CrossRef CAS.
- E. D. Khairitdinova, E. M. Tsyrlina, L. V. Spirikhin, N. I. Fedorov and M. S. Yunusov, Chem. Nat. Compd., 2005, 41, 575–577 CrossRef CAS.
- H. Ahmad, S. Ahmad, M. Ali, A. Latif, S. A. A. Shah, H. Naz, N. Ur Rahman, F. Shaheen, A. Wadood, H. U. Khan and M. Ahmad, Bioorg. Chem., 2018, 78, 427–435 CrossRef CAS PubMed.
- Z. S. Boronova and M. N. Sultankhodzhaev, Chem. Nat. Compd., 2000, 36, 390–392 CrossRef CAS.
- Y. J. Pan, R. Wang, S. N. Chen and Y. Z. Chen, Chem. Res. Chin. Univ., 1992, 13, 1418–1419 CAS.
- S. W. Pelletier and M. M. Badawi, J. Nat. Prod., 1987, 50, 381–385 CrossRef CAS.
- S. A. Ross, H. K. Desai and S. W. Pelletier, Heterocycles, 1987, 26, 2895–2904 CrossRef CAS.
- A. G. Gonzalez, G. De la Fuente and R. Diaz, Phytochemistry, 1982, 21, 1781–1782 CrossRef CAS.
- A. G. Gonzalez, G. De la Fuente, M. Reina, V. Zabel and W. H. Watson, Tetrahedron Lett., 1980, 21, 1155–1158 CrossRef CAS.
- G. de la Fuente and L. Ruiz-Mesía, Phytochemistry, 1995, 39, 1459–1465 CrossRef CAS.
- X. Liang, H. K. Desai and S. W. Pelletier, J. Nat. Prod., 1990, 53, 1307–1311 CrossRef CAS.
- A. Ulubelen, A. H. Mericli and F. Mericli, Nat. Prod. Lett., 1994, 5, 135–140 CrossRef CAS.
- G. de la Fuente, A. H. Meriçli, L. Ruiz-Mesía, A. Ulubelen, F. Meriçli and R. Ilarslan, Phytochemistry, 1995, 39, 1467–1473 CrossRef CAS.
- K. Zhang, L. He, X. Pan and Y. Chen, Planta Med., 1998, 64, 580–581 CrossRef CAS.
- S. W. Pelletier and M. M. Badawi, Heterocycles, 1985, 23, 2873–2883 CrossRef CAS.
- A. Rahman, A. Nasreen, F. Akhtar, M. S. Shekhani, J. Clardy, M. Parvez and M. I. Choudhary, J. Nat. Prod., 1997, 60, 472–474 CrossRef.
- X. Pan, L. He, B. G. Li and Y. Z. Chen, Chin. Chem. Lett., 1998, 9, 57–59 CAS.
- N. H. Chen, Y. B. Zhang, W. Li, P. Li, L. F. Chen, Y. L. Li, G. Q. Li and G. C. Wang, RSC Adv., 2017, 7, 24129–24132 RSC.
- F. Z. Chen, Q. H. Chen and F. P. Wang, Helv. Chim. Acta, 2011, 94, 254–260 CrossRef CAS.
- D. L. Chen, L. Y. Lin, Q. H. Chen, X. X. Jian and F. P. Wang, J. Asian Nat. Prod. Res., 2003, 5, 209–213 CrossRef CAS PubMed.
- F. P. Wang, C20-diterpenoid alkaloids, 2002 Search PubMed.
- X. Y. Liu, L. Song, Q. H. Chen and F. P. Wang, Nat. Prod. Commun., 2010, 5, 1005–1008 CAS.
- M. Reina, R. Mancha, A. Gonzalez-Coloma, M. Bailen, M. L. Rodriguez and R. A. Martinez-Diaz, Nat. Prod. Res., 2007, 21, 1048–1055 CrossRef CAS PubMed.
- S. A. Ross, B. S. Joshi, H. K. Desai, S. W. Pelletier, M. G. Newton, X. Zhang and J. K. Snyder, Tetrahedron, 1991, 47, 9585–9598 CrossRef CAS.
- L. H. Shan, J. F. Zhang, F. Gao, S. Huang and X. L. Zhou, Sci. Rep., 2017, 7, 6063 CrossRef PubMed.
- U. K. Kurbanov, B. Tashkhodzhaev, K. K. Turgunov and N. I. Mukarramov, Chem. Nat. Compd., 2019, 55, 197–199 CrossRef CAS.
- X. Y. Liu, Q. H. Chen and F. P. Wang, Helv. Chim. Acta, 2009, 92, 745–752 CrossRef CAS.
- P. M. Shrestha and A. Katz, J. Nat. Prod., 2004, 67, 1574–1576 CrossRef CAS PubMed.
- A. Ulubelen, M. Arfan, U. Sönmez, A. H. Meriçli and F. Meriçli, Phytochemistry, 1998, 47, 1141–1144 CrossRef CAS.
- Y. Q. He, X. M. Wei, Y. L. Han and L. M. Gao, Chin. Chem. Lett., 2007, 18, 545–547 CrossRef CAS.
- Y. Q. He, Z. Y. Ma, X. M. Wei, B. Z. Du, Z. X. Jing, B. H. Yao and L. M. Gao, Fitoterapia, 2010, 81, 929–931 CrossRef CAS.
- Y. Q. He, Z. Y. Ma, X. M. Wei, D. J. Liu, B. Z. Du, B. H. Yao and L. M. Gao, Chem. Biodiversity, 2011, 8, 2104–2109 CrossRef CAS.
- A. H. Meriçli, F. Meriçli, E. Doğru, H. Özçelik and A. Ulubelen, Phytochemistry, 1999, 51, 337–340 CrossRef.
- M. Reina, A. Madinaveitia, G. de la Fuente, M. L. Rodriguez and I. Brito, Tetrahedron Lett., 1992, 33, 1661–1662 CrossRef CAS.
- X. Y. Liu, Q. H. Chen and F. P. Wang, Chin. Chem. Lett., 2009, 20, 698–701 CrossRef CAS.
- B. T. Salimov, Chem. Nat. Compd., 2004, 40, 579–581 CrossRef CAS.
- L. V. Beshitaishvili, M. N. Sultankhodzhaev, K. S. Mudzhiri and M. S. Yunusov, Chem. Nat. Compd., 1981, 17, 156–157 CrossRef.
- C. Z. Lin, Z. X. Zhao, S. M. Xie, J. H. Mao, C. C. Zhu, X. H. Li, B. Zeren-dawa, K. Suolang-qimei, D. Zhu, T. Q. Xiong and A. Z. Wu, Phytochemistry, 2014, 97, 88–95 CrossRef CAS PubMed.
- Y. S. Zou, Z. Dawa, C. Z. Lin, Q. Y. Zhang, Y. F. Yao, Y. Yuan, C. C. Zhu and Z. Y. Wang, Fitoterapia, 2019, 136, 104186 CrossRef CAS PubMed.
- J. G. Diaz, J. L. Marapara, F. Valdes, J. G. Sazatornil and W. Herz, Phytochemistry, 2005, 66, 733–739 CrossRef CAS PubMed.
- Y. Pan, C. Sun and Y. Chen, J. Zhejiang Univ., Sci., 2000, 1, 186–187 CrossRef CAS.
- S. Zhang, G. Zhao and G. Lin, Phytochemistry, 1999, 51, 333–336 CrossRef CAS.
- A. G. Perkin and J. A. Pilgrim, J. Chem. Soc., 1898, 73, 267–275 RSC.
- T. Kondo, K. Oki, K. Yoshida and T. Goto, Chem. Lett., 1990, 19, 137–138 CrossRef.
- T. Kondo, K. Suzuki, K. Yoshida, K. Oki, M. Ueda, M. Isobe and T. Goto, Tetrahedron Lett., 1991, 32, 6375–6378 CrossRef CAS.
- N. Saito, K. Toki, A. Suga and T. Honda, Phytochemistry, 1998, 49, 881–886 CrossRef CAS.
- A. I. Arazashvili, I. I. Moniava and E. P. Kemertelidze, Chem. Nat. Compd., 1973, 9, 556–557 CrossRef CAS.
- H. Yoshimitsu, M. Nishida, F. Hashimoto, M. Tanaka, Y. Sakata, M. Okawa and T. Nohara, J. Nat. Med., 2007, 61, 334–338 CrossRef CAS.
- J. G. Díaz, A. J. Carmona, P. P. de Paz and W. Herz, Phytochem. Lett., 2008, 1, 125–129 CrossRef.
- S. Özden, N. Dürüst, K. Toki, N. Saito and T. Honda, Phytochemistry, 1998, 49, 241–245 CrossRef.
- M. J. Warnock, Y. L. Liu and T. J. Mabry, Phytochemistry, 1983, 22, 1834–1835 CrossRef CAS.
- J. G. Diaz and W. Herz, Phytochemistry, 2010, 71, 463–468 CrossRef CAS PubMed.
- F. R. Kolar, S. R. Ghatge, V. V. Kedage and G. B. Dixit, Turk. J. Biochem., 2014, 39, 277–284 CrossRef.
- S. J. Liu, Z. X. Liao, Z. S. Tang, C. L. Cui, H. B. Liu, Y. N. Liang and Y. Zhang, J. Chin. Med. Mater., 2016, 39, 318–321 CAS.
- Z. D. Nan, X. A. Li, Y. X. Chen, H. Z. Ren, J. Xu and L. J. Zhou, J. Chin. Med. Mater., 2017, 40, 2077–2080 Search PubMed.
- A. H. Mericli, F. Mericli, A. Ulubelen and R. Ilarslan, Phytochemistry, 1991, 30, 4195–4196 CrossRef CAS.
- F. Mericli, A. H. Mericli, H. Becker and A. Ulubelen, Phytochemistry, 1996, 42, 1257–1258 CrossRef CAS.
- F. P. Wang and L. P. Yan, Tetrahedron, 2007, 63, 1417–1420 CrossRef CAS.
- P. Tang, Q. H. Chen and F. P. Wang, Tetrahedron Lett., 2009, 50, 460–462 CrossRef CAS.
- C. Marín, J. G. Díaz, D. Irure Maiques, I. Ramírez-Macías, M. J. Rosales, R. Guitierrez-Sánchez, R. Cañas and M. Sánchez-Moreno, Phytochem. Lett., 2017, 19, 196–209 CrossRef.
- X. X. Liang, Y. Y. Gao and S. X. Luan, RSC Adv., 2018, 8, 23937–23946 RSC.
- H. Ahmad, S. Ahmad, S. A. A. Shah, A. Latif, M. Ali, F. A. Khan, M. N. Tahir, F. Shaheen, A. Wadood and M. Ahmad, Bioorg. Med. Chem., 2017, 25, 3368–3376 CrossRef CAS PubMed.
- H. Ahmad, S. Ahmad, E. Khan, A. Shahzad, M. Ali, M. N. Tahir, F. Shaheen and M. Ahmad, Pharm. Biol., 2017, 55, 680–686 CrossRef CAS PubMed.
- L. Chen, L. Shan, J. Zhang, W. Xu, M. Wu, S. Huang and X. L. Zhou, Nat. Prod. Commun., 2015, 10, 2063–2065 Search PubMed.
- J. F. Zhang, L. Chen, S. Huang, L. H. Shan, F. Gao and X. L. Zhou, J. Nat. Prod., 2017, 80, 3136–3142 CrossRef CAS PubMed.
- A. Ulubelen, A. H. Meriçli, F. Meriçli, N. Kilinçer, A. G. Ferizli, M. Emekci and S. W. Pelletier, Phytother. Res., 2001, 15, 170–171 CrossRef CAS PubMed.
- A. H. Mericli, F. Mericli, G. V. Seyhan, H. Özçelik, N. Kılınçer, A. G. Ferizli and A. Ulubelen, Heterocycles, 1999, 8, 1843–1848 Search PubMed.
- A. González-Coloma, M. Reina, A. Guadaño, R. Martínez-Díaz, J. G. Díaz, J. García-Rodriguez and M. Grandez, Chem. Biodiversity, 2004, 1, 1327–1335 CrossRef PubMed.
- A. González-Coloma, A. Guadano, C. Gutiérrez, R. Cabrera, E. De La Pena, G. De La Fuente and M. Reina, J. Agric. Food Chem., 1998, 46, 286–290 CrossRef PubMed.
- L. Shan, L. Chen, F. Gao and X. Zhou, Nat. Prod. Res., 2018, 33, 3254–3259 CrossRef.
- M. Reina and A. González-Coloma, Phytochem. Rev., 2007, 6, 81–95 CrossRef CAS.
- C. Marin, I. Ramirez-Macias, A. Lopez-Cespedes, F. Olmo, N. Villegas, J. G. Diaz, M. J. Rosales, R. Gutierrez-Sanchez and M. Sanchez-Moreno, J. Nat. Prod., 2011, 74, 744–750 CrossRef CAS.
- I. Ramirez-Macias, C. Marin, J. G. Diaz, M. J. Rosales, R. Gutierrez-Sanchez and M. Sanchez-Moreno, Sci. World J., 2012, 2012, 203646 Search PubMed.
- L. Yang, Y. B. Zhang, L. Zhuang, T. Li, N. H. Chen, Z. N. Wu, P. Li, Y. L. Li and G. C. Wang, Planta Med., 2017, 83, 111–116 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra00813c |
| This journal is © The Royal Society of Chemistry 2020 |