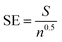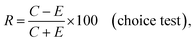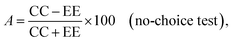Open Access Article
Open Access ArticleThird-generation ionic liquids with N-alkylated 1,4-diazabicyclo[2.2.2]octane cations and pelargonate anions†
Anna Turguła a,
Konrad Stęsika,
Katarzyna Materna
a,
Konrad Stęsika,
Katarzyna Materna a,
Tomasz Klejdyszb,
Tadeusz Praczykb and
Juliusz Pernak
a,
Tomasz Klejdyszb,
Tadeusz Praczykb and
Juliusz Pernak *a
*a
aFaculty of Chemical Technology, Poznan University of Technology, ul. Berdychowo 4, Poznan 60-965, Poland. E-mail: juliusz.pernak@put.poznan.pl
bInstitute of Plant Protection – National Research Institute, ul. Władysława Węgorka 20, Poznan 60-318, Poland
First published on 27th February 2020
Abstract
Ionic liquids that belong to the third-generation designs due to their intended biological activity are compounds with high potential applications as plant-protection products. The present study describes the synthesis and characterization of novel ionic liquids with cations based on the alkyl derivatives of 1,4-diazabicyclo[2.2.2]octane (DABCO) and an anion derived from naturally occurring pelargonic acid. The developed synthesis method allowed obtaining products with a high yield (≥96%), and the liquids were characterized as high-viscosity liquids at room temperature. This allowed classifying the products as ionic liquids (ILs). The structures of the obtained ILs were confirmed on the basis of their NMR and IR spectra as well as by elemental analysis. All the products exhibited surface activity and were capable of partially wetting a hydrophobic surface. The tested ionic liquids exhibited higher herbicidal activity against winter oilseed rape (Brassica napus L.) and common lambsquarters (Chenopodium album L.) at a lower dose compared to a commercial preparation in greenhouse studies. The studied ionic liquids also exhibited different effects as antifeedants on various insect species. The best results were obtained against beetles belonging to the granary weevil species (Sitophilus granarius L.). The relation between the surface-tension-reduction efficiency pC20 and biological activity was investigated. The herbicidal activity was also correlated with the value of the contact angles for the studied pelargonates. All the obtained results indicate that the designed and synthesized ionic liquids possess double biological functions: herbicidal activity and deterrent activity.
1. Introduction
Ionic liquids (ILs) are a group of compounds with different properties. Continuous development in the field in terms of their synthesis methods and applications has been observed for almost two decades.1,2 The classification of ILs into three generations has proved to be effective and important in terms of their application.3 Third-generation ILs are characterized by their biological activity, such as bactericidal,4,5 bacteriostatic,6 fungicidal,7 herbicidal8,9 or deterrent.10,11 Their biological activity is usually associated with the anion, while the cation is designed to increase their application potential.The first herbicidal ionic liquids (HILs), which incorporated MCPA and 2,4-D, were reported in 2011 and their high herbicidal activity as well as the ability to regulate the toxicity of the herbicide by selecting an appropriate cation was highlighted.8 To date, HILs with the following herbicides in the anion structure have been described in the literature: 2,4-DP,12 MCPB,13 MCPP,14 dicamba,15 fomesafen,16 clopyralid,17 bentazone,18 glyphosate,19,20 metsulfuron methyl,21 nicosulfuron,9 nonanoic acid22 and picloram.23 The low volatility, which is characteristic for HILs, allows limiting the negative health effects during the application of the product.
Food deterrents, also known as antifeedants, are substances or mixtures that limit the feeding of pests by affecting the taste receptors of both the larval and adult forms of the pests. Obtaining natural deterrent agents is highly expensive, leading to the search for synthetic replacements. Sweet ILs that possess deterrent properties include anions such as saccharinate,10 cyclamate24 or acesulfame.25 Deterrent ILs with a pelargonate anion have also been reported.26
1,4-Diazabicyclo[2.2.2]octane (common name DABCO) is a bicyclic compound that belongs to the group of tertiary diamines. It is widely used as a catalyst for many reactions, both in pristine and modified forms.27–29 Long-chain alkyl DABCO derivatives, which are quaternary halides, qualify as surfactants due to their amphiphilic properties. The carbon chain of such compounds is the hydrophobic fragment, whereas the structure of the quaternized DABCO amine is the hydrophilic “head”.30 Quaternary DABCO derivatives exhibit bactericidal activity. Polycations containing DABCO moieties in their structure have exhibited activity against Gram-negative bacteria.31 Compounds containing 1,4-diazabicyclo[2.2.2]octane in their structure have proved useful in genetic engineering, especially during transfection of the gene, as they can inhibitor the mRNA sequence encoding the luciferase protein.32 Recently, HILs containing a monoalkyl derivative of DABCO as the cation and a herbicidal anion were shown to possess double biological activity. The anion derived from 4-chloro-2-methylphenoxyacid (MCPA) provided plant protection, while the cation introduced surfactant and bactericidal properties.33
Pelargonic acid is a naturally occurring compound in geranium flowers, where it is present in the form of essential oils. It exhibits non-selective herbicidal activity; however, the currently used commercial preparations are economically inefficient due to the necessity to apply high doses of around 12–16 L per hectare. Additionally, the deterrent activity of pelargonic acid has been confirmed against the common forest pest belonging to the large pine weevil species (Hylobius abietis)34 and the storage pest belonging to the hide beetle species (Dermestes maculatus).35
The third generation of ILs may incorporate a potentially wide range of numerous commercial and widely used pesticides, and have been well researched in terms of their properties, including toxicity in relation to non-target organisms. A thorough review of pesticides in the EU resulted in the withdrawal of many effective measures that had been used for a long period. It remains an open question as to whether the replacement of the active substance of a pesticide in the form of an ionic liquid may lead to a change in its properties and eliminate the reasons why the substance was withdrawn from the market.
This study describes novel ILs with an alkyl derivative of 1,4-diazabicyclo[2.2.2]octane as the cation and an anion based on pelargonic acid. The obtained ILs combined two functions: herbicidal activity and deterrent activity.
2. Experimental
2.1. Materials
1,4-Diazabicyclo[2.2.2]octane (CAS 280-57-9, purity ≥99%), 1-bromobutane (CAS 109-65-9, purity 99%), 1-bromohexane (CAS 111-25-1, purity 98%), 1-bromooctane (CAS 111-83-1, purity 99%), 1-bromodecane (CAS 112-29-8, purity 98%), 1-bromododecane (CAS 143-15-7, purity 97%), 1-bromotetradecane (CAS 112-71-0, purity 97%), 1-bromohexadecane (CAS 112-82-3, purity 97%), 1-bromooctadecane (CAS 112-89-0, purity ≥97%), potassium hydroxide (CAS 1310-58-3), nonanoic acid (pelargonic acid, CAS 112-05-0) and all solvents were purchased from Sigma Aldrich and used without further purification.2.2. Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C curves) using a linear regression analysis method. Surface excess concentrations at the saturated interface (Γmax), the minimum surface occupied by a molecule at the interface (Amin) and the adsorption efficiency, pC20, were calculated. Calculations of the surface excess concentrations Γmax and the minimum surface area occupied by a molecule at the interface Amin were presented in our earlier report.13 pC20 is defined as the negative logarithm of the surfactant concentration in the bulk phase required to reduce the surface tension of the water by 20 mN m−1, which represents the efficiency of surface adsorption on an air–water interface.
C curves) using a linear regression analysis method. Surface excess concentrations at the saturated interface (Γmax), the minimum surface occupied by a molecule at the interface (Amin) and the adsorption efficiency, pC20, were calculated. Calculations of the surface excess concentrations Γmax and the minimum surface area occupied by a molecule at the interface Amin were presented in our earlier report.13 pC20 is defined as the negative logarithm of the surfactant concentration in the bulk phase required to reduce the surface tension of the water by 20 mN m−1, which represents the efficiency of surface adsorption on an air–water interface.The prepared ILs were dissolved in a mixture of water and methanol (the concentration of methanol was equal to 5%).
The prepared ILs were dissolved in a mixture of water and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at the amount corresponding to the doses of 5440 g and 8160 g of pelargonic acid per 1 ha. These doses accounted for ½ and ¾ of the recommended dose, respectively. The commercial product was a formulation containing 680 g of pelargonic acid in 1 L Beloukha 680 EC (Belchim Crop Protection, Londerzeel, Belgium). It was dissolved in water at doses of 5440 and 8160 g per ha of active substance. The applications were conducted at the 6 leaf growth stage using a moving sprayer (APORO, Poznan, Poland) with a TeeJet® VP 110/02 (TeeJet Technologies, Wheaton, IL, USA) flat-fan nozzle capable of delivering 200 L per ha of spray solution at 0.2 MPa operating pressure. The efficacy of the tested compounds was evaluated two weeks after treatment (2 WAT) using the fresh weight reduction method. Data are expressed herein as the per cent of fresh weight reduction with a standard error (SE), which was calculated using the following equation:
1 v/v) at the amount corresponding to the doses of 5440 g and 8160 g of pelargonic acid per 1 ha. These doses accounted for ½ and ¾ of the recommended dose, respectively. The commercial product was a formulation containing 680 g of pelargonic acid in 1 L Beloukha 680 EC (Belchim Crop Protection, Londerzeel, Belgium). It was dissolved in water at doses of 5440 and 8160 g per ha of active substance. The applications were conducted at the 6 leaf growth stage using a moving sprayer (APORO, Poznan, Poland) with a TeeJet® VP 110/02 (TeeJet Technologies, Wheaton, IL, USA) flat-fan nozzle capable of delivering 200 L per ha of spray solution at 0.2 MPa operating pressure. The efficacy of the tested compounds was evaluated two weeks after treatment (2 WAT) using the fresh weight reduction method. Data are expressed herein as the per cent of fresh weight reduction with a standard error (SE), which was calculated using the following equation:
Choice and no-choice tests were carried out in accordance with the methodology developed by Prof. Jan Nawrot.38 Waffle discs (1 cm × 1 mm in diameter) were saturated by immersion in solvent, either methanol (control) or in a 1% solution of the tested compound. After evaporation of the solvent (after 30 min air drying), the discs were given to 3 beetles belonging to the S. granarius species, 20 beetles and 10 larvae belonging to the T. confusum species and 10 larvae belonging to the T. granarium species. Tests were carried out separately for each species. The number of insects used for the test was determined earlier, on the basis of the possibilities and pace of food intake by particular species and their developmental stages. The adult specimens used for the experiments were not divided by gender. Wafer discs were weighed prior to feeding and after a period of 5 days of feeding. Each test was performed in 5 replicates. Three coefficients (relative R, absolute A and total T) were calculated on the basis of the difference in weight of the discs before and after the feeding of the insects, using the formulas:
where C, CC are amount of consumed food from the control discs, and E, EE are amount of consumed food treated with the tested compound.
The measure of the deterrent activity of the tested compounds was the total coefficient of deterrence: T = A + R.
A simplified scheme of testing the deterrent activity of the obtained ILs towards the selected storage pests is shown in Fig. 1.
 | ||
| Fig. 1 Scheme for testing the deterrent activity of the obtained ILs towards the selected storage pests. | ||
The total coefficient T could reach values ranging from −200 to +200, and the following intervals were used to evaluate the biological activity of a given compound:
• Compounds with T values ranging from 151 to 200 were classified as very good deterrents,
• Compounds with T values between 101 and 150 were classified as good deterrents,
• Compounds with T in the range of 51–100 were classified as compounds with medium deterrent properties,
• Compounds with T values below 50 were classified as weak deterrents,
• Negative T values indicated that the compound acts as an attractant.
The biological properties of the studied ILs were compared with the results of the studies of the best known and most practically used compound: azadirachtin.24
The methodology used and described above has been successfully used in previous studies regarding ionic liquids.39–41
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v) was added to the flask. The resulting solution was again gravity filtered and the solvent mixture was evaporated from the filtrate using a rotary vacuum evaporator. The obtained product was dried for 24 h in a vacuum oven at 60 °C.
2 (v/v) was added to the flask. The resulting solution was again gravity filtered and the solvent mixture was evaporated from the filtrate using a rotary vacuum evaporator. The obtained product was dried for 24 h in a vacuum oven at 60 °C.3. Results and discussion
3.1. Synthesis, structure and physicochemical properties of the ILs
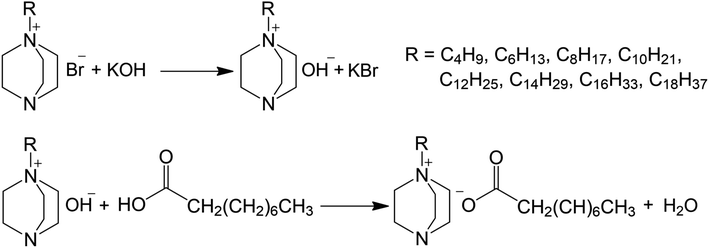
The synthesis of the 1-alkyl-1-azonia-4-azabicyclo[2.2.2]octane pelargonates (1–8) proceeded in three steps, as presented in Scheme 1.The first step was the quaternization of bicyclic tertiary diamine (DABCO), which allowed preparing the 1-alkyl-1-azonia-4-azabicyclo[2.2.2]octane bromides according to the described methodology.42 The synthesized monoalkyl bromides, precursors of the ILs, were characterized in an earlier report, in which the physicochemistry of the compounds and their antimicrobial properties were presented.33 In the second step, the bromide anions were replaced with hydroxide anions in the exchange reaction. The reaction was carried out in anhydrous methanol at room temperature for 60 min, followed by filtration of the precipitated by-product (KBr). In the third step, the obtained solutions of 1-alkyl-1-azonia-4-azabicyclo[2.2.2]octane with hydroxides were neutralized with a stoichiometric amount of a methanolic solution of pelargonic acid. The resulting products were hot dissolved in a mixture of acetone–methanol in ratios from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v), followed by filtration of the inorganic residues. After evaporation of the solvents, the products were dried in a vacuum oven at 60 °C for 24 h. High yields (≥96%) were obtained for 8 new pelargonates, which were liquids with high viscosity at room temperature, and could therefore be classified as ILs. The compounds contained from 4 to 18 carbon atoms in an alkyl substituent in the cation structure. The water content, which was lower than 400 ppm, was determined using the Karl Fischer method. The percentage amounts of precursors and ILs in the products of the individual reaction stages were also determined using the extraction titration method,36 in a two-phase system, commonly used for long-chain compounds. Titration was repeated three times for each compound. Both ILs and the base precursors were characterized by a high purity of ≥97%. The yields of the synthesis and the purity of the ILs are presented in Table 1.
2 (v/v), followed by filtration of the inorganic residues. After evaporation of the solvents, the products were dried in a vacuum oven at 60 °C for 24 h. High yields (≥96%) were obtained for 8 new pelargonates, which were liquids with high viscosity at room temperature, and could therefore be classified as ILs. The compounds contained from 4 to 18 carbon atoms in an alkyl substituent in the cation structure. The water content, which was lower than 400 ppm, was determined using the Karl Fischer method. The percentage amounts of precursors and ILs in the products of the individual reaction stages were also determined using the extraction titration method,36 in a two-phase system, commonly used for long-chain compounds. Titration was repeated three times for each compound. Both ILs and the base precursors were characterized by a high purity of ≥97%. The yields of the synthesis and the purity of the ILs are presented in Table 1.
| IL | R | Short | Yield [%] | Purity [%] |
|---|---|---|---|---|
| 1 | C4H9 | [D4][PEL] | 96 | — |
| 2 | C6H13 | [D6][PEL] | 96 | — |
| 3 | C8H17 | [D8][PEL] | 98 | 97 |
| 4 | C10H21 | [D10][PEL] | 99 | 99 |
| 5 | C12H25 | [D12][PEL] | 96 | 97 |
| 6 | C14H29 | [D14][PEL] | 98 | 97 |
| 7 | C16H33 | [D16][PEL] | 99 | 99 |
| 8 | C18H37 | [D18][PEL] | 99 | 97 |
The structures of the ILs were confirmed by 1H and 13C NMR spectroscopy, which allowed observing signals originating from the cation and the anion. Elemental CHN analysis was also carried out, which confirmed the purity of the ILs. Mass spectroscopy analysis (ESI-Q-TOF) of the obtained ionic liquids was also performed. To record the cations and anions in the obtained ILs, the positive ionization mode and negative ionization mode were applied, respectively. The NMR and ESI-MS spectra of the obtained ILs and a comparison of the IR spectra of the substrates and ionic liquid 6 and a description of the elemental analysis results are included in the ESI (Fig. A.1–A.16 and Table A.1).†
In the proton spectrum, signals originating from the hydrogen atoms in the bicyclic structure could be observed, which could be attributed to the protons in the CH2 groups at the tertiary nitrogen atom occurring at σ = 3.2 ppm (t, 6H) and at the quaternary nitrogen atom at approx. values of σ = 3.4 ppm (t, 6H). Between them, it was possible to observe the signal originating from the protons in the first CH2 group of the alkyl substituent bound to the quaternary nitrogen atom at approx. values of σ = 3.2–3.3 ppm (t, 6H). The carbon atom originating from the carboxylate anion in the ILs structure was visible at a shift value of approx. σ = 181.7–182.5 ppm.
Chemical shifts of the carbon atoms in the bicyclic structure of cation occurred equally at the tertiary and quaternary nitrogen atoms at σ = 46.1–46.3 ppm (3C) and σ = 53.4–53.6 ppm (3C), respectively. The carbon atom of the methylene group attached directly to the quaternary nitrogen atom was present at σ = 65.7–65.9 ppm.
In order to confirm the presence of appropriate functional groups, the transmittance of the infrared spectrum for the pelargonic acid precursor with the tetradecyl substituent in the cation structure and ionic liquid 6 with the same alkyl chain length in the cation were compared. In addition to the standard vibrations originating from the methyl and methylene groups, some differences were noted. The stretching vibrations originating from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the pelargonic acid structure occurred at a value of approx. 1709 cm−1, while the stretching vibrations of the carboxylate anion occurred at lower values of approx. 1566 cm−1. Stretching vibrations in the bicyclic structure of the cation originating from O–N+–R bonds occurred in the spectrum at higher values compared to the vibrations attributed to the bonds of the nitrogen atom with a free electron pair.
O in the pelargonic acid structure occurred at a value of approx. 1709 cm−1, while the stretching vibrations of the carboxylate anion occurred at lower values of approx. 1566 cm−1. Stretching vibrations in the bicyclic structure of the cation originating from O–N+–R bonds occurred in the spectrum at higher values compared to the vibrations attributed to the bonds of the nitrogen atom with a free electron pair.
The solubility of the synthesized ILs was investigated to determine their application potential. All tests were conducted in 10 solvents with a different index of polarity in the Snyder scale. All the tested ILs were completely soluble in the reaction medium, which was methanol. Some of the ILs were soluble in water, while the pelargonic acid was not soluble in this solvent. ILs 1 and 2 with a short alkyl substituent (with 4 and 6 carbons in the alkyl chain) were insoluble in isopropanol, but ILs with longer substituents were partially soluble in this solvent. Almost every IL was completely or partially soluble in acetone, except for 7 and 8. The synthesized ILs were insoluble in DMSO, acetonitrile, ethyl acetate, chloroform, toluene and hexane. The results of the solubility tests are presented in Table 2.
| Compound | Water | Methanol | DMSO | Acetonitrile | Acetone | Chloroform | Isopropanol | Ethyl acetate | Toluene | Hexane |
|---|---|---|---|---|---|---|---|---|---|---|
| a + Complete solubility; ± limited solubility; − insoluble. Bold text – protic solvent. | ||||||||||
| Snyder polarity index43 | 9.0 | 6.6 | 6.5 | 6.2 | 5.4 | 4.4 | 4.3 | 4.3 | 2.3 | 0.0 |
| Pelargonic acid | − | + | + | + | + | + | + | + | + | + |
| 1 | + | + | − | − | ± | − | − | − | − | − |
| 2 | + | + | − | − | + | − | − | − | − | − |
| 3 | + | + | − | − | + | − | ± | − | − | − |
| 4 | ± | + | − | − | + | − | ± | − | − | − |
| 5 | − | + | − | − | ± | − | ± | − | − | − |
| 6 | − | + | − | − | ± | − | ± | − | − | − |
| 7 | − | + | − | − | − | − | ± | − | − | − |
| 8 | − | + | − | − | − | − | ± | − | − | − |
3.2. Surface activity
Surface-tension measurements were carried out to characterize the properties of the interface, to evaluate the adsorption at the boundary surface, and to study the associations in solutions of the surface-active substances. By determining the surface tension as a function of amphiphilic molecule concentration, other parameters, such as the critical micelle concentration (CMC), value of the surface tension at CMC (γCMC), surface excess concentration (Γmax), minimum molecular area (Amin) and adsorption efficiency (pC20), could be calculated. The exact values of the above-mentioned surface-active properties obtained for these series of pelargonates are summarized in Table 3.| IL | Short | CMC (mmol L−1) | γCMC (mN m−1) | pC20 | Γmax × 106 (mol m−2) | Amin × 1019 (m2) | CA [°] |
|---|---|---|---|---|---|---|---|
| 1 | [D4][PEL] | 25.1 | 26.5 | 2.10 | 2.67 | 2.89 | 30.7 |
| 2 | [D6][PEL] | 20.0 | 26.6 | 2.25 | 3.31 | 2.67 | 31.4 |
| 3 | [D8][PEL] | 6.31 | 26.5 | 2.75 | 4.45 | 2.52 | 33.6 |
| 4 | [D10][PEL] | 3.16 | 26.4 | 3.05 | 5.81 | 2.43 | 36.9 |
| 5 | [D12][PEL] | 2.24 | 26.1 | 3.45 | 6.59 | 2.36 | 37.2 |
| 6 | [D14][PEL] | 0.794 | 26.9 | 3.95 | 7.48 | 2.21 | 49.0 |
| 7 | [D16][PEL] | 0.251 | 27.0 | 4.30 | 8.49 | 1.91 | 56.1 |
| 8 | [D18][PEL] | 0.089 | 29.7 | 4.40 | 8.72 | 1.86 | 59.4 |
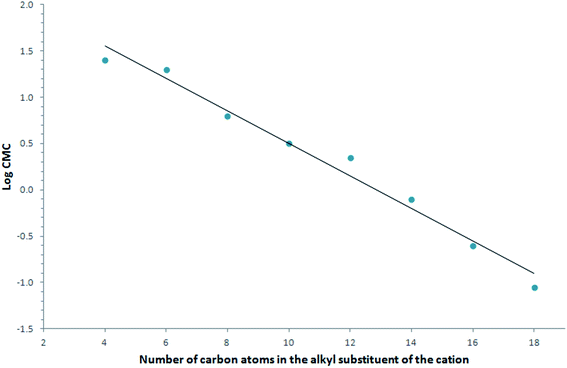
The surface tension of aqueous solutions (γCMC) of the studied ILs decreased from the value for the water–methanol mixture to a minimum located from 26.1 to 29.7 mN m−1. Generally, the γCMC values were very similar, as it was noticed for 8 only that this compound did not lower the surface tension as effectively as the others. At the same time, the CMC of this compound was the lowest. Similar to typical surfactants, the CMC value of the studied ILs was a function of the carbon atom numbers in the alkyl chain (Fig. 2). A significant decrease was observed, from 25.1 (1) to 0.089 mmol L−1 (8). Calculations of the surface excess concentration Γmax and the minimum surface area occupied by a molecule at the interface Amin showed that the values of these parameters differed with the elongation of the alkyl chain. For example, the values of Amin for the ILs decreased with the increasing number of carbon atoms in the alkyl chain of the IL molecule, i.e. from 2.89 × 10−19 to 1.86 × 10−19 m2. In the case of an excess surface concentration, higher values were observed for compounds containing longer alkyl chains, with the lowest values being obtained for 1, while the highest were obtained for 8. The analysis of the results in Table 3 allowed establishing the dependence of both parameters, which is a characteristic of surface-active compounds, whereby with the decrease in the maximum surface excess, the minimum surface per one molecule of surfactant adsorbed on the interface increased.
There was an additional surface activity parameter, the adsorption efficiency, pC20, calculated based on the surface-tension measurements. This parameter is a function of the number of carbon atoms in the alkyl group, and increases as the number of carbon atoms increases. The higher pC20 values showed that the ILs were more efficiently adsorbed at the interface and more effectively reduced the surface tension by 20 mN m−1. The highest value of pC20 was obtained equal to 4.40 (Table 3).
The contact angle values obtained for the studied pelargonates (1–8) ranged from 30.7° to 59.4° (Table 3) and better wetting properties were exhibited by ionic liquids with a shorter alkyl chain. The obtained contact angle values were in the range of 0–90°, which means that the obtained ILs were liquids that could partially wet the paraffin surface.
3.3. Herbicidal activity
Based on our previous studies of HILs, compounds (5–8) containing from 12 to 18 carbon atoms in the alkyl chain of the cation structure were selected to determine the herbicidal activity of new synthetized compounds.33 Another reason for the selection of the compounds was their favourable surface–activity properties. The target weeds were common lambsquarters (Chenopodium album L.) and oilseed rape (Brassica napus L.). The effectiveness of the tested ILs depended on both the plant species and the applied dose of the active substance. The herbicidal activity was also influenced by the length of the alkyl chain in the cation. Common lambsquarters were more susceptible to pelargonic acid than oilseed rape. The reference product applied at the dose of 8160 g of pelargonic acid per 1 ha (¾ of the recommended dose) caused a reduction of the fresh weight by 67% compared to the untreated plants, while it did not cause permanent damage to the oilseed rape plants. Comparing the effectiveness of ILs with a cation that included an alkyl chain ranging from 12 to 16 carbon atoms and the reference product, a significant increase in the activity of the pelargonic acid used in the form of ILs was found. The highest efficacy was observed in the cases of ILs 5 and 6, which respectively contained 12 and 14 carbon atoms in the alkyl chain. IL 5 used at the dose of 5440 g per ha exhibited an effectiveness of 93% for common lambsquarters and 76% for oilseed rape. The efficacy of IL 6 for the same was equal to 87% and 83%, respectively. The reference product used at the same dose of the active substance did not cause a reduction of the fresh mass of the plants compared to the control. Increasing the dose of the above-mentioned compounds to 8160 g per ha resulted in an increase in herbicidal efficacy towards oilseed rape plants by 13–15%, and by 5% in the case of common lambsquarters (6) in comparison to the control. Detailed data on the effectiveness of the tested ILs are presented in Table 4 and Fig. 3.| IL | Short | Fresh weight reduction (![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) ± SE) [%] ± SE) [%] |
|||
|---|---|---|---|---|---|
| Dose = ½ Nb | Dose = ¾ N | ||||
| Common lambsquarters | Oilseed rape | Common lambsquarters | Oilseed rape | ||
a Beloukha 680 EC.b N recommended dose = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 g of pelargonic acid per 1 ha. 880 g of pelargonic acid per 1 ha. |
|||||
| 5 | [D12][PEL] | 93.48 ± 0.92 | 76.30 ± 9.52 | 93.28 ± 1.10 | 91.64 ± 3.20 |
| 6 | [D14][PEL] | 87.26 ± 3.46 | 82.82 ± 3.01 | 92.54 ± 1.57 | 95.93 ± 2.24 |
| 7 | [D16][PEL] | 77.81 ± 4.55 | 66.90 ± 5.50 | 77.95 ± 3.00 | 90.59 ± 3.22 |
| 8 | [D18][PEL] | 47.03 ± 2.93 | 20.70 ± 7.21 | 36.60 ± 3.10 | 44.16 ± 3.25 |
| Referencea | −1.43 ± 4.03 | −10.81 ± 7.13 | 67.40 ± 8.44 | −2.76 ± 5.54 | |
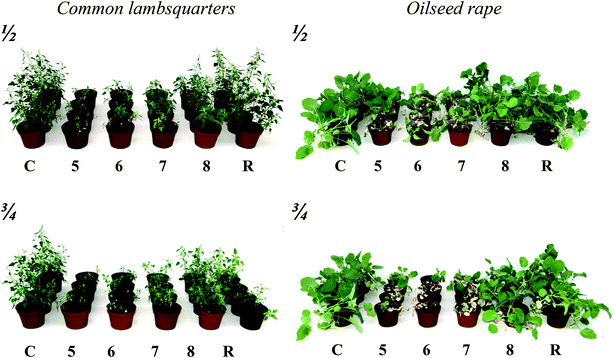 | ||
| Fig. 3 Herbicidal activity of ILs 5–8 tested on common lambsquarters and oilseed rape in ½ and ¾ doses of compounds (C – Control, R – Reference). | ||
3.4. Feeding-deterrent activity
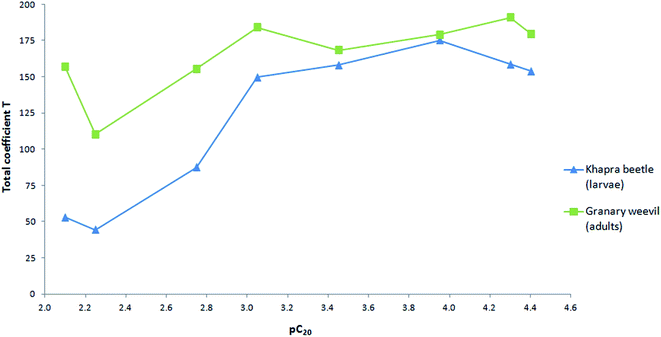
The testing of the deterrent effect of the obtained ILs on the storage pests yielded different results, as presented in Tables 5 and 6, with respect to the criterion presented in Table 7. Individual species of pests were characterized by a different reaction to the presence of the ILs (1–8) in their diet. In the case of the larvae of the granary weevil, ILs 1–3, which contained a lower number of carbon atoms in the alkyl substituent in the cation structure, did not exhibit a higher deterrent activity. Almost all the obtained ILs were characterized by poor activity against the confused flour beetle larvae and beetles. IL 1 acted as an attractant for all individuals of this species, while IL 8 could be described as a good deterrent against the larvae. The ILs with a higher number of carbon atoms in the cation were characterized by a significantly higher activity against all the tested insect species. The grain weevil was the most vulnerable to the presence of ILs in its diet. In the case of this species, 7 out of the 8 tested ILs (1, 3–8) could be classified as very good antifeedants. The relationship between the length of the alkyl chain and the deterrent activity for the larvae of the khapra beetle and the adult specimens of granary weevil are presented in Fig. 4. For these insects, the obtained ILs proved to be the most effective. As many as four of them (4, 6–8) exhibited better activity compared to azadirachtin towards adult specimens of the weevil granary. The appearance of exemplary wafers with the tested insects is presented in Fig. 5.| Compound | Adults granary weevil (Sitophilus granarius) | Adults confused flour beetle (Tribolium confusum) | ||||||
|---|---|---|---|---|---|---|---|---|
| A | R | T | Deterrent activity | A | R | T | Deterrent activity | |
| 1 | 58.5 | 98.7 | 157.2 | Very good | −18.8 | −0.5 | −19.3 | Attracting |
| 2 | 66.8 | 43.4 | 110.2 | Good | −0.7 | 12.5 | 11.8 | Weak |
| 3 | 56.8 | 98.6 | 155.4 | Very good | −18.6 | 39.2 | 20.6 | Weak |
| 4 | 84.0 | 100.0 | 184.0 | Very good | 9.7 | 41.2 | 50.9 | Weak |
| 5 | 71.2 | 97.2 | 168.4 | Very good | 5.1 | 44.1 | 49.2 | Weak |
| 6 | 79.0 | 100.0 | 179.0 | Very good | 29.4 | 32.6 | 62.0 | Medium |
| 7 | 90.9 | 100.0 | 190.9 | Very good | −3.0 | 65.0 | 62.0 | Medium |
| 8 | 79.7 | 100.0 | 179.7 | Very good | 37.4 | 61.0 | 98.4 | Medium |
| Azadirachtin | 74.3 | 100.0 | 174.3 | Very good | 85.0 | 100.0 | 185.0 | Very good |
| Compound | Larvae khapra beetle (Trogoderma granarium) | Larvae confused flour beetle (Tribolium confusum) | ||||||
|---|---|---|---|---|---|---|---|---|
| A | R | T | Deterrent activity | A | R | T | Deterrent activity | |
| 1 | 16.6 | 36.4 | 53.0 | Medium | −33.1 | 22.1 | −11.0 | Attracting |
| 2 | −0.1 | 44.6 | 44.5 | Weak | −27.1 | 48.8 | 21.7 | Weak |
| 3 | 43.5 | 44.0 | 87.5 | Medium | −1.4 | 20.2 | 18.8 | Weak |
| 4 | 68.7 | 80.9 | 149.6 | Good | −25.7 | 68.2 | 42.5 | Weak |
| 5 | 73.2 | 85.0 | 158.2 | Very good | −1.9 | 22.1 | 20.2 | Weak |
| 6 | 80.8 | 94.4 | 175.2 | Very good | −15.5 | 64.0 | 48.5 | Weak |
| 7 | 77.0 | 81.5 | 158.5 | Very good | −16.6 | 90.1 | 73.5 | Medium |
| 8 | 71.8 | 82.1 | 153.9 | Very good | 14.6 | 95.9 | 110.5 | Good |
| Azadirachtin | 94.2 | 100.0 | 194.2 | Very good | 88.4 | 100.0 | 188.4 | Very good |
| Total coefficient T | Deterrent activity |
|---|---|
| 151–200 | Very good |
| 101–150 | Good |
| 51–100 | Medium |
| 50–0 | Weak |
| <0 | Attracting |
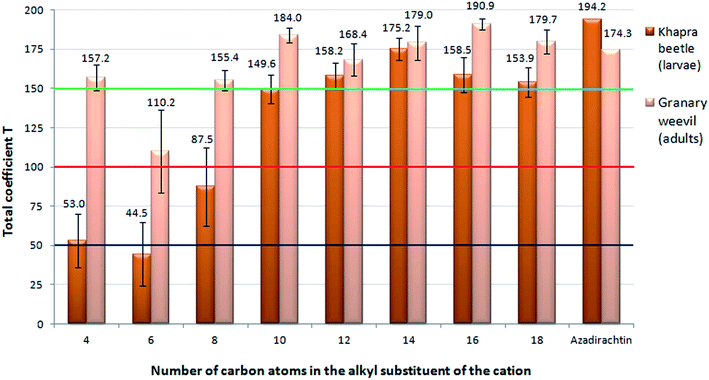 | ||
| Fig. 4 Deterrent activity depending on the number of carbons in the alkyl substituent of the cation in the ILs on the khapra beetle larvae and granary weevil. | ||
Data were analyzed using a one-way ANOVA statistical approach. In cases where the ANOVA test values reached a 5% significance level, the Tukey test was performed and the LSD obtained values are presented in the ESI (Table A.2).†
3.5. Relationship between the surface-tension-reduction efficiency pC20, contact angle and biological activity
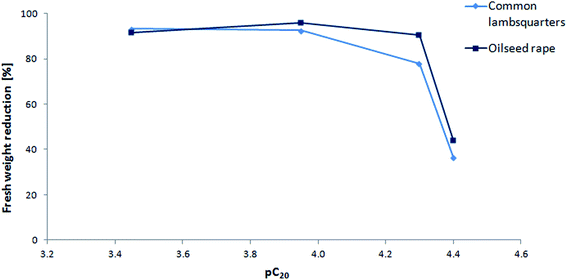
The relationships between both the herbicidal and deterrent activity with the surface tension were determined and are presented in Fig. 6 and 7. The herbicidal activity could be correlated with the surface activity expressed by the pC20 parameter. In general, an inverse relationship was observed, whereby the herbicidal activity decreased with the increasing value of pC20. At the pC20 value of 4.40, the herbicidal activity of the compounds was lost. The relationship between the plant fresh weight reduction and pC20 for ½ N dose of ILs 5–8 is included in the ESI (Fig. A.17)† due to their similarity. It is an interesting observation that in the case of compounds with the number of carbon atoms in the alkyl chain higher than 12, the herbicidal effectiveness decreased, while at the same time their ability to lower the surface tension of the solvent was not significantly impaired. In the case of IL 8, the measured value of γCMC was slightly higher compared to the others (29.7 mN m−1); however, it was not expected that this could be reflected in the loss of herbicidal activity. At the same time, it was noted that compound 8 exhibited the highest value of contact angle (59.4°). The higher the contact angle value, the less the leaf surface is wetted by the ionic liquid solution and the herbicidal efficacy is lower (Fig. 7).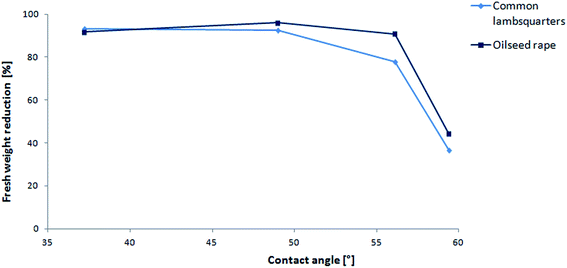 | ||
| Fig. 7 Relationship between the plant's fresh weight reduction and contact angle for ¾ N dose of ILs 5–8. | ||
However, the deterrent activity increased with the higher values of pC20 (Fig. 8). The most effective homologues start with a pC20 value of approx. 3. To the best of our knowledge, this is the first report that presents a dependence between the deterrent activity and surface activity.
4. Conclusion
ILs containing 1-alkyl-1-azonia-4-azabicyclo[2.2.2]octane in the structure as well as an anion derived from the naturally occurring pelargonic acid were designed and synthesized. The three-step synthesis proceeded with high yields, and the structures and purity of the compounds were confirmed using extraction titration, NMR, high-resolution mass spectroscopy, IR spectroscopy and elemental analysis. The compounds contained an even number of carbon atoms from 4 to 18 in the alkyl substituent of the cation.All the ILs were completely soluble in methanol. Some of the ILs were soluble in water (1–4), isopropanol (3–8) and acetone (1–6). The synthesized ILs were insoluble in DMSO, acetonitrile, ethyl acetate, chloroform, toluene and hexane.
The tested ILs were surface-active compounds capable of lowering the surface tension of the solvent at the same level as classical surfactants. The obtained values of contact angles were in the range of 0–90°, which means that they are liquids capable of partially wetting the hydrophobic surface (paraffin). All the ILs were completely soluble in methanol. Some of the ILs were soluble in water (1–4), isopropanol (3–8) and acetone (1–6). The synthesized ILs were insoluble in DMSO, acetonitrile, ethyl acetate, chloroform, toluene and hexane.
The highest herbicidal activity was observed in the cases of ILs 5 and 6, which possessed 12 and 14 carbon atoms in the alkyl chain, respectively, to the following target weed species: common lambsquarters and oilseed rape. Regardless of the dose of the active substance, ILs 5 and 6 were more effective than the commercial formulation used.
Some of the obtained ILs were characterized by very good deterrent properties and effectively limited the feeding of such globally important storage pests as the granary weevil (1, 3–8) and khapra beetle (5–8). The mentioned insects belong to two taxonomically distant super-families: Curculionoidea (weevils) and Bostrichoidea (khapra beetle). The fact that these species are not closely related and react similarly to the synthesized ILs may be the basis for concluding that, in the case of other species, not only storage but also field pests, these substances may find use as an alternative to the currently used, often very toxic and environmentally harmful insecticides.
The obtained correlations allowed observing a reverse dependence, whereby the herbicidal activity decreased with the increasing value of pC20. At the pC20 value of 4.40, the herbicidal activity of the compounds was lost. At the same time, it was noticed that the value of the contact angle increased with the increase in the number of carbon atoms in the alkyl substituent, which resulted in a lower wetting of the leaf surface and, consequently, a lower herbicidal efficacy. During the deterrence tests, the most effective homologues started with a pC20 value of approx. 3.
The obtained results regarding the biological activity allow us to conclude that the synthesized ILs are effective as herbicides and deterrents. Based on this conclusion, these compounds can be referred to as bifunctional ILs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Centre, Poland (Grant 2017/25/B/ST5/01622).References
- L. Rohit Vekariya, J. Mol. Liq., 2017, 227, 44–60 CrossRef.
- A. A. Elgharbawy, F. A. Riyadi, M. Z. Alam and M. Moniruzzaman, J. Mol. Liq., 2018, 251, 150–166 CrossRef CAS.
- W. L. Hough, M. Smiglak, H. Rodriguez, R. P. Swatloski, S. K. Spear, D. T. Daly, J. Pernak, J. E. Grisel, R. D. Carliss, M. D. Soutullo, J. H. Davis Jr and R. D. Rogers, New J. Chem., 2007, 31, 1429–1436 RSC.
- N. Rezki, S. A. Al-Sodies, H. E. A. Ahmed, S. Ihmaid, M. Messali, S. Ahmed and M. R. Aouad, J. Mol. Liq., 2019, 284, 431–444 CrossRef CAS.
- A. Busetti, D. E. Crawford, M. J. Earle, M. A. Gilea, B. F. Gilmore, S. P. Gorman, G. Laverty, A. F. Lowry, M. McLaughlin and K. R. Seddon, Green Chem., 2010, 12, 420–425 RSC.
- R. Jalal, E. K. Goharshadi, S. H. Sajjadi and P. Nancarrow, Phys. Chem. Res., 2014, 2, 260–269 Search PubMed.
- K. Bica, L. R. Cooke, P. Nugent, C. Rijksen and R. D. Rogers, Green Chem., 2011, 13, 2344–2346 RSC.
- J. Pernak, A. Syguda, D. Janiszewska, K. Materna and T. Praczyk, Tetrahedron, 2011, 67, 4838–4844 CrossRef CAS.
- W. Wang, J. Zhu, G. Tang, H. Huo, W. Zhang, Y. Liang, H. Dong, J. Yang and Y. Cao, New J. Chem., 2019, 43, 827–833 RSC.
- J. Pernak, J. Nawrot, M. Kot, B. Markiewicz and M. Niemczak, RSC Adv., 2013, 3, 25019–25029 RSC.
- J. Pernak, B. Łęgosz, F. Walkiewicz, T. Klejdysz, A. Borkowski and Ł. Chrzanowski, RSC Adv., 2015, 5, 65471–65480 RSC.
- M. Niemczak, A. Biedziak, K. Czerniak and K. Marcinkowska, Tetrahedron, 2017, 73, 7315–7325 CrossRef CAS.
- J. Pernak, M. Niemczak, K. Materna, K. Żelechowski, K. Marcinkowska and T. Praczyk, RSC Adv., 2016, 6, 7330–7338 RSC.
- M. Niemczak, R. Giszter, K. Czerniak, K. Marcinkowska and F. Walkiewicz, RSC Adv., 2015, 5, 15487–15493 RSC.
- O. A. Cojocaru, J. L. Shamshina, G. Gurau, A. Syguda, T. Praczyk, J. Pernak and R. D. Rogers, Green Chem., 2013, 15, 2110–2120 RSC.
- G. Ding, Y. Liu, B. Wang, D. Punyapitak, M. Guo, Y. Duan, J. Li and Y. Cao, New J. Chem., 2014, 38, 5590–5596 RSC.
- J. Zhu, G. Ding, Y. Liu, B. Wang, W. Zhang, M. Guo, Q. Geng and Y. Cao, Chem. Eng. J., 2015, 279, 472–477 CrossRef CAS.
- B. Wang, G. Ding, J. Zhu, W. Zhang, M. Guo, Q. Geng, D. Gou and Y. Cao, Tetrahedron, 2015, 71, 7860–7864 CrossRef CAS.
- J. Pernak, M. Niemczak, R. Giszter, J. L. Shamshina, G. Gurau, O. A. Cojocaru, T. Praczyk, K. Marcinkowska and R. D. Rogers, ACS Sustainable Chem. Eng., 2014, 2, 2845–2851 CrossRef CAS.
- H. Choudhary, J. Pernak, J. L. Shamshina, M. Niemczak, R. Giszter, Ł. Chrzanowski, T. Praczyk, K. Marcinkowska, O. A. Cojocaru and R. D. Rogers, ACS Sustainable Chem. Eng., 2017, 5, 6261–6273 CrossRef CAS.
- J. Pernak, M. Niemczak, J. L. Shamshina, G. Gurau, G. Głowacki, T. Praczyk, K. Marcinkowska and R. D. Rogers, J. Agric. Food Chem., 2015, 13, 3357–3366 CrossRef PubMed.
- J. Pernak, K. Czerniak, M. Niemczak, Ł. Ławniczak, D. K. Kaczmarek, A. Borkowski and T. Praczyk, ACS Sustainable Chem. Eng., 2018, 6, 2741–2750 CrossRef CAS.
- G. Tang, B. Wang, G. Ding, W. Zhang, Y. Liang, C. Fan, H. Dong, J. Yang, D. Kong and Y. Cao, Sci. Total Environ., 2018, 616–617, 128–134 CrossRef CAS PubMed.
- J. Pernak, K. Wasiński, T. Praczyk, J. Nawrot, A. Cieniecka-Rosłonkiewicz, F. Walkiewicz and K. Materna, Sci. China: Chem., 2012, 55, 1532–1541 CrossRef CAS.
- D. K. Kaczmarek, K. Czerniak and T. Klejdysz, Chem. Pap., 2018, 72, 2457–2466 CrossRef CAS PubMed.
- B. Łęgosz, A. Biedziak, T. Klejdysz and J. Pernak, Chem.–Eur. J., 2016, 7, 217–224 CrossRef.
- F. Shirini, M. S. N. Langarudi, N. Daneshvar, N. Jamasbi and M. Irankhah-Khanghah, J. Mol. Struct., 2018, 1161, 366–382 CrossRef CAS.
- A. Ziyaei Halimehjani, V. Barati and M. Karimi, Synth. Commun., 2019, 49, 724–734 CrossRef CAS.
- S. Chandrasekhar, C. Narsihmulu, B. Saritha and S. Shameem Sultana, Tetrahedron, 2004, 45, 5865–5867 CrossRef CAS.
- T. Lohar, A. Kumbhar, A. Patil, S. Kamat and R. Salunkhe, Res. Chem. Intermed., 2019, 45, 1639–1651 CrossRef CAS.
- J. Mayr, J. Bachl, J. Schlossmann and D. D. Díaz, Int. J. Mol. Sci., 2017, 18, 303–310 CrossRef PubMed.
- J. Mayr, S. Grijalvo, J. Bachl, R. Pons, R. Eritja and D. D. Díaz, Int. J. Mol. Sci., 2017, 18, 1139–1151 CrossRef PubMed.
- A. Turguła, K. Materna, D. Gwiazdowska, F. Walkiewicz, K. Marcinkowska and J. Pernak, New J. Chem., 2019, 43, 4477–4488 RSC.
- P. E. Månsson, C. Eriksson and K. Sjödin, J. Chem. Ecol., 2005, 31, 989–1001 CrossRef PubMed.
- E. Cohen, V. Stanić and A. Shulov, J. Appl. Entomol., 1974, 76, 303–331 Search PubMed.
- A. Piotrowska, A. Syguda, B. Wyrwas, Ł. Chrzanowski and H. J. Heipieper, Chemosphere, 2017, 167, 114–119 CrossRef CAS PubMed.
- A. I. Vogel, Textbook of Practical Organic Chemistry, ed. B. S. Furniss, A. J. Hannaford, P. W. G. Smith and A. R. Tatchell, Wiley, 1989 Search PubMed.
- J. Nawrot, E. Błoszyk, J. Harmatha, L. Nowotny and B. Drozdz, Acta Entomol. Bohemoslov., 1986, 83, 327–335 CAS.
- M. Niemczak, D. Kaczmarek, T. Klejdysz, D. Gwiazdowska, K. Marchwińska and J. Pernak, ACS Sustainable Chem. Eng., 2018, 7, 1072–1084 CrossRef.
- J. Pernak, B. Łęgosz, T. Klejdysz, K. Marcinkowska, J. Rogowski, D. Kurasiak-Popowska and K. Stuper-Szablewska, RSC Adv., 2018, 8, 28676–28683 RSC.
- T. Klejdysz, B. Łęgosz, D. Czuryszkiewicz, K. Czerniak and J. Pernak, ACS Sustainable Chem. Eng., 2016, 4, 6543–6550 CrossRef CAS.
- R. Engel, J. L. I. Rizzo, C. Rivera, M. Ramirez, M. L. Huang, B. Klaritch-Vrana and J. F. Engel, Chem. Phys. Lipids, 2009, 158, 61–69 CrossRef CAS PubMed.
- L. R. Snyder, J. Chromatogr. A, 1974, 92, 223–230 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Identification of compounds (1H, 13C NMR, ESI-MS and IR spectra, results of elemental analyses), feeding-deterrent activity and the relationship between the surface-tension-reduction efficiency pC20 and biological activity. See DOI: 10.1039/d0ra00766h |
| This journal is © The Royal Society of Chemistry 2020 |