Open Access Article
Open Access ArticlePhotostable near-infrared-absorbing diradical-platinum(II) complex solubilized by albumin toward a cancer photothermal therapy agent†
Ryota Sawamura,
Masataka Sato,
Atsuko Masuya-Suzuki and
Nobuhiko Iki *
*
Graduate School of Environmental Studies, Tohoku University, 6-6-07, Aramaki-Aoba, Aoba-ku, Sendai, 980-8579, Japan. E-mail: iki@tohoku.ac.jp; Fax: +81-22-795-7293
First published on 11th February 2020
Abstract
A hydrophobic diradical-platinum(II) complex was solubilized in aqueous solutions by using bovine serum albumin and exhibited photothermal conversion under near-infrared (NIR) light irradiation. The complex was introduced into cancer cells and induced cell death upon absorption of NIR. These results imply that the complex can function as a photothermal therapeutic agent.
Introduction
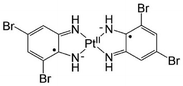
Recently, photothermal therapy (PTT) has attracted attention as a potential new cancer therapy to complement conventional methods such as surgery, chemotherapy, and radiotherapy.1 PTT utilizes near-infrared radiation (NIR, 700–1100 nm) to penetrate biological tissue with minimal damage to normal cells. NIR-absorbing photothermal agents that convert the energy of NIR light into heat can kill cancer cells. If the agents are delivered selectively to cancer cells, heat generation is localized and results in minimal damage to the surrounding tissue. Multiple NIR-absorbing materials have been studied as PTT agents, such as gold nanostructures,2 carbon nanomaterials,3 quantum dots,4 and semiconducting polymers.5 In particular, small-molecule cyanine dyes (indocyanine green (ICG), IR825, etc.) are often used because of their high biocompatibility.6 Cyanine dyes show NIR absorption by the low energy gap between S0–S1 derived from the extended π electron system. However, the photosensitizing property of these dyes towards O2 molecules results in autocleavage and photobleaching.7 Even if stored in the dark, cyanine dyes are easily oxidized and cleaved.8 In addition, the fluorescence of these dyes is disadvantageous because a substantial portion of the energy absorbed as light is emitted as fluorescence and not as heat. Therefore, optical properties of cyanine dyes decrease the efficiency of photothermal conversion.With the aim of developing new photothermal agents, we have studied a series of diradical-platinum(II) complexes consisting of two o-diiminobenzosemiquinonate radical ligands and PtII.9 These complexes absorb NIR light in the region of 700–800 nm with high intensity (ε ≈ 105 M−1 cm−1) by ligand-to-ligand charge transfer.10 Water-soluble diradical complexes have been synthesized as NIR-absorbing bioimaging probes. However, there is a concern that platinum complexes might dissociate through ligand substitution by a bio-thiol, glutathione, which is present in the cytosol at high concentration (0.5–10 mM).11 To avoid dissociation, the complex needs to be introduced into hydrophobic regions of cells such as the cytoplasmic membrane. Past reports indicate that the uncharged complex containing bromo groups (PtL2, Scheme 1) does not show NIR absorption in aqueous solution but does show NIR absorption in hydrophobic environments such as liposome bilayers.12 Moreover, PtL2 does not exhibit fluorescence, suggesting it has a high efficiency of photothermal conversion. Based on these past results, we expected that PtL2 might be localized in cellular membranes in cancer cells and could cause cell death by its photothermal effect.
Here we report that the complex PtL2 can serve as a new small-molecule PTT agent that may replace low photostability organic dyes. The complex solubilized by bovine serum albumin (BSA) was introduced into cancer cells and under NIR laser irradiation, the cells were killed by the photothermal effect of solubilized PtL2.
Results and discussion
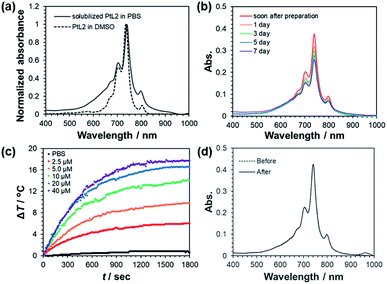
The diradical complex PtL2 was synthesized by two steps (Scheme S1†). The first step was the complexation between PtII and 3,5-dibromo-1,2-diaminobenzene (H2L). Heating the reaction mixture under Ar atmosphere afforded [Pt(H2L)2]2+ without causing deprotonation or oxidation of H2L ligands. Since the obtained complex [Pt(H2L)2]2+ was water-soluble, it was successfully separated from insoluble unreacted starting materials by filtration. In the second step, heating the filtrate in the presence of air promoted the deprotonation and oxidation of the ligands from phenylene H2L to semiquinonate L−. Following this step, blue-violet PtL2 crystals were obtained. The complex showed maximum absorption at 736 nm in dimethyl sulfoxide (DMSO) with the ε value of 1.3 × 105 M−1 cm−1 (Fig. 1a, dashed line).Next, we studied solubilization of the hydrophobic PtL2 by using BSA, a known hydrophilic lipid carrier protein. Absorption spectra of mixtures of PtL2 in DMSO and BSA in phosphate buffer saline (PBS) at different PtL2/BSA molar ratios (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) were measured after heating at 37 °C for 24 h. As the concentration of BSA increased, the absorbance at around 650 nm decreased and that at 740 nm increased (Fig. S1†). When the PtL2/BSA molar ratio was >1, violet precipitates formed in solution. These results suggest that PtL2 was in equilibrium between the insoluble aggregates (absorption band at around 650 nm) and the solubilized form (absorption band at 740 nm). Excess BSA shifted the equilibrium to the solubilized form, suggesting that the hydrophobic cavity of BSA accommodated PtL2 (ref. 9c) thereby increasing solubility. Presence of the equilibrium between aggregated and BSA-bound PtL2 was further confirmed by measuring dynamic light scattering (DLS) of the solution (Fig. S2†). With PtL2/BSA molar ratio > 0.5, particles with 10−6–10−5 m diameters can be seen. But in the presence of 20-fold amount of BSA against PtL2, peaks assignable to particles were not shown. Establishment of the equilibrium took 8 h as measured by the temporal change of the absorption spectra (Fig. S3a†). Following ultrafiltration to remove DMSO, the obtained PBS solution also exhibited NIR absorption (Fig. 1a, solid line). Therefore, PtL2 was successfully solubilized in PBS by using BSA without organic solvents.
5) were measured after heating at 37 °C for 24 h. As the concentration of BSA increased, the absorbance at around 650 nm decreased and that at 740 nm increased (Fig. S1†). When the PtL2/BSA molar ratio was >1, violet precipitates formed in solution. These results suggest that PtL2 was in equilibrium between the insoluble aggregates (absorption band at around 650 nm) and the solubilized form (absorption band at 740 nm). Excess BSA shifted the equilibrium to the solubilized form, suggesting that the hydrophobic cavity of BSA accommodated PtL2 (ref. 9c) thereby increasing solubility. Presence of the equilibrium between aggregated and BSA-bound PtL2 was further confirmed by measuring dynamic light scattering (DLS) of the solution (Fig. S2†). With PtL2/BSA molar ratio > 0.5, particles with 10−6–10−5 m diameters can be seen. But in the presence of 20-fold amount of BSA against PtL2, peaks assignable to particles were not shown. Establishment of the equilibrium took 8 h as measured by the temporal change of the absorption spectra (Fig. S3a†). Following ultrafiltration to remove DMSO, the obtained PBS solution also exhibited NIR absorption (Fig. 1a, solid line). Therefore, PtL2 was successfully solubilized in PBS by using BSA without organic solvents.
Long-term stability of the PBS solution of PtL2 was evaluated from the temporal change of absorption spectra at 4 °C. The absorbance at 740 nm of the solution decreased by ca. 30% over one week (Fig. 1b). Additionally, the proportion of absorption measured at the shorter wavelength increased (Fig. S4†). These results suggest that storage at 4 °C for a week shifted the equilibrium of PtL2 species from the solubilized form to the aggregate form. Aggregates were solubilized by re-heating at 37 °C for 4 h as confirmed by the restoration of the absorption spectrum to the original one (Fig. S5†).
To confirm whether solubilized PtL2 can exhibit photothermal effect and photostability, the temperature of PBS solutions of PtL2 was measured under irradiation by 730 nm laser (2 W cm−2) for 30 min. Temperature change (ΔT, from initial temperature) of the solution increased over time (Fig. 1c) with samples containing higher concentration of the complex showing larger ΔT. For example, at 40 μM PtL2, the solution temperature increased by 17.7 °C. By contrast, in PBS only, the temperature increased by only 0.8 °C. Notably, the absorption spectrum of the PBS solution of the complex was almost unchanged after 30 min irradiation by NIR (Fig. 1d). This implies that the complex remained in the solubilized form without decomposition during irradiation. We compared these results to one using 5.0 μM ICG in PBS (Fig. S6a†). The temperature reached a maximum at ca. 750 s and then decreased gradually. After the irradiation, NIR absorption of the ICG solution greatly diminished (Fig. S6b†). ICG is decomposed under the effects of singlet oxygen generated by the energy transfer from the triplet excited state of ICG to triplet oxygen.13 This suggests that the temperature decrease of the ICG solution after 450 s was caused by a decrease in the number of remaining ICG molecules under NIR irradiation. We further studied the photostability of PtL2 upon laser irradiation by photothermal cycle experiment (Fig. S7a†). As can be seen, PtL2 showed increase and decrease in the solution temperature upon irradiation on and off, respectively. The three cycles seemed to be very similar, suggesting high photostability of PtL2. By contrast, in the same experiment, ICG solution showed that the increase and decrease in the first cycle were not re-produced in the following cycles with diminished temperature changes (Fig. S7b†), suggesting very poor photostability. Therefore, our results suggest that unlike ICG, PtL2 solubilized in PBS can generate heat without decomposition upon NIR laser irradiation.
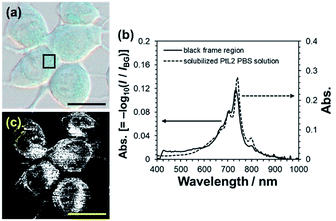
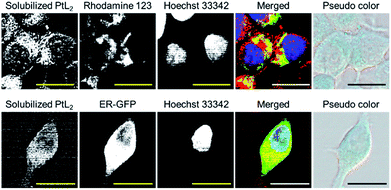
With positive results regarding the photothermal conversion ability and photostability of solubilized PtL2, we introduced the solubilized complex into cancer cells. Human breast cancer cell line MCF-7 was incubated in culture medium containing the solubilized complex (20 μM) for 2 h. The pseudo color image of the cells obtained with a spectral camera showed light blue color (Fig. 2a). The absorption spectrum of the colored area (black frame region) in Fig. 2a was almost the same as that of the complex solubilized in PBS (Fig. 2b). The region showing the NIR absorption characteristic of the complex can be depicted using a spectral angle mapper algorithm (Fig. 2c).14 The solubilized complex showed specific subcellular localization. We then elucidated the subcellular distribution of the solubilized complex by using organelle markers (Fig. 3). The complex was not observed in nuclei but was observed in mitochondria and the endoplasmic reticulum. Therefore, solubilized PtL2 was successfully introduced into MCF-7 cells.
The cytotoxicity of solubilized PtL2 in the dark to MCF-7 cells was analyzed by Calcein AM assay. After incubation for 24 h, more than 50% of the cells containing the solubilized complex at concentration of over 10 μM had died (Fig. S8†). The half maximal inhibitory concentration (IC50) value was estimated to be 8.1 μM (5.9 μg mL−1). Therefore, the solubilized complex has greater cytotoxicity than conventional NIR-absorbing materials but lower cytotoxicity than conventional anti-cancer drugs (Table S1†). For biomedical applications, it would be necessary to improve the biocompatibility of the complex.
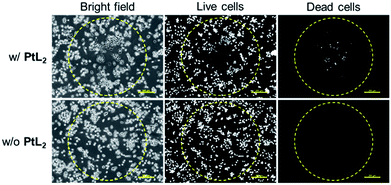
Finally, the cell-killing ability of solubilized PtL2 by NIR irradiation was studied over the course of an incubation time sufficiently short that dark state cytotoxicity did not occur. The MCF-7 cells with or without complex were irradiated by 730 nm laser (0.28 W, spot size: 1 mm) for 15 min. Following irradiation, dead cells were observed at the center of the laser spot (Fig. 4, upper row). In contrast, live cells were observed located in the periphery of the center within the laser spot, suggesting that the irradiation power may not be high enough to kill the cells. In the control, cells without solubilized PtL2 survived both inside and outside the laser spot (Fig. 4, lower row). These results indicate that photothermal conversion of the diradical complex can cause cell death by NIR laser irradiation.
Conclusion
In summary, the hydrophobic complex PtL2 solubilized by BSA functions as a photothermal agent in aqueous environments. During irradiation by NIR laser, the complex solubilized in PBS generated heat without its degradation. In MCF-7 cells, the complex localized to mitochondria and/or endoplasmic reticulum as shown by the NIR absorption of the complex. Cancer cells containing the complex were killed by the photothermal effect of the complex under NIR laser irradiation. This study has demonstrated for the first time that diradical-platinum(II) complex can be applied as a PTT. Further study of tumor-specific delivery of PtL2 by using encapsulation into the amphiphilic copolymer micelles is now underway.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by JSPS KAKENHI grant number 17H03073. One of us (A. M.-S.) was supported by the Naito Foundation Subsidy for Female Researchers after Maternity Leave.Notes and references
- (a) L. Zou, H. Wang, B. He, L. Zeng, T. Tan, H. Cao, X. He, Z. Zhang, S. Guo and Y. Li, Theranostics, 2016, 6, 762 CrossRef CAS PubMed; (b) D. Jaque, L. M. Maestro, B. del Rosal, P. Haro-Gonzalez, A. Benayas, J. L. Plaza, E. M. Rodríguez and J. G. Solé, Nanoscale, 2014, 6, 9494 RSC.
- (a) X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115 CrossRef CAS PubMed; (b) M. R. K. Ali, Y. Wu, Y. Tang, H. Xiao, K. Chen, T. Han, N. Fang, R. Wu and M. A. El-Sayed, Proc. Natl. Acad. Sci., 2017, 114, E5655 CrossRef CAS PubMed; (c) M. Sun, D. Peng, H. Hao, J. Hu, D. Wang, K. Wang, J. Liu, X. Guo, Y. Wei and W. Gao, ACS Appl. Mater. Interfaces, 2017, 9, 10453 CrossRef CAS PubMed.
- (a) K. Yang, S. Zhang, G. Zhang, X. Sun, S. Lee and Z. Liu, Nano Lett., 2010, 10, 3318 CrossRef CAS PubMed; (b) F. Zhou, D. Xing, Z. Ou, B. Wu, D. E. Resasco and W. R. Chen, J. Biomed. Optic., 2009, 14, 021009 CrossRef PubMed; (c) C. Liang, S. Diao, C. Wang, H. Gong, T. Liu, G. Hong, X. Shi, H. Dai and Z. Liu, Adv. Mater., 2014, 26, 5646 CrossRef CAS PubMed.
- (a) Z. Sun, H. Xie, S. Tang, X. Yu, Z. Guo, J. Shao, H. Zhang, H. Huang, H. Wang and P. K. Chu, Angew. Chem., Int. Ed., 2015, 54, 11526 CrossRef CAS PubMed; (b) T. Yang, Y. Tang, L. Liu, X. Lv, Q. Wang, H. Ke, Y. Deng, H. Yang, X. Yang, G. Liu, Y. Zhao and H. Chen, ACS Nano, 2017, 11, 1848 CrossRef CAS PubMed; (c) G. Liang, X. Jin, H. Qin and D. Xing, J. Mater. Chem. B, 2017, 5, 6366 RSC.
- (a) J. Zhou, Z. Liu, X. Zhu, X. Wang, Y. Liao, Z. Ma and F. Li, Biomaterials, 2013, 34, 9584 CrossRef CAS PubMed; (b) K. Yang, H. Xu, L. Cheng, C. Sun, J. Wang and Z. Liu, Adv. Mater., 2012, 24, 5586 CrossRef CAS PubMed; (c) Y. Lyu, C. Xie, S. A. Chechetka, E. Miyako and K. Pu, J. Am. Chem. Soc., 2016, 138, 9049 CrossRef CAS PubMed.
- (a) X. Song, Q. Chen and Z. Liu, Nano Res., 2015, 8, 340 CrossRef CAS; (b) F. Xue, Y. Wen, P. Wei, Y. Gao, Z. Zhou, S. Xiao and T. Yi, Chem. Commun., 2017, 53, 6424 RSC; (c) G. Pan, H. Jia, Y. Zhu, R. Wang, F. Wu and Z. Chen, ACS Biomater. Sci. Eng., 2017, 3, 3596 CrossRef CAS.
- R. R. Nani, J. A. Kelley, J. Ivanic and M. J. Schnermann, Chem. Sci., 2015, 6, 6556 RSC.
- V. Saxena, M. Sadoqi and J. Shao, J. Pharm. Sci., 2003, 92, 2090 CrossRef CAS PubMed.
- (a) A. Masuya, N. Iki, C. Kabuto, Y. Ohba, S. Yamauchi and H. Hoshino, Eur. J. Inorg. Chem., 2010, 22, 3458 CrossRef; (b) K. Tamura, A. Masuya, N. Iki, Y. Ohba, S. Yamauchi and H. Hoshino, Inorg. Chim. Acta, 2011, 378, 81 CrossRef CAS; (c) K. Tamura, A. Masuya, H. Hoshino and N. Iki, Chem. Commun., 2013, 49, 4812 RSC.
- D. Herebian, K. E. Wieghardt and F. Neese, J. Am. Chem. Soc., 2003, 125, 10997 CrossRef CAS PubMed.
- S. Kemp, N. J. Wheate, M. J. Pisani and J. R. Aldrich-Wright, J. Med. Chem., 2008, 51, 2787 CrossRef CAS PubMed.
- Y. Terazono, PhD. thesis, Tohoku University, 1999.
- E. Engel, R. Schraml, T. Maisch, K. Kobuch, B. König, R. Szeimies, J. Hillenkamp, W. Bäumler and R. Vasold, Invest. Ophthalmol. Visual Sci., 2008, 49, 1777 CrossRef.
- X. Liu and C. Yang, in Proceedings of the IEEE International Congress on Image and Signal Processing, CISP 2013, 2013, p. 814 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supporting data on the solubilization of PtL2, stability against NIR laser irradiation of ICG and cytotoxicity of solubilized PtL2. See DOI: 10.1039/d0ra00652a |
| This journal is © The Royal Society of Chemistry 2020 |