Open Access Article
Open Access ArticleMetallo-supramolecular complexes from mPEG/PDPA diblock copolymers and their self-assembled strip nanosheets†
Xiaoyu Zhang,
Yuyang Liu *,
Xin Wang,
Yinxiu Liang and
Lei Yan
*,
Xin Wang,
Yinxiu Liang and
Lei Yan
Key Laboratory of Macromolecular Science and Technology of Shaanxi Province, School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi'an 710072, P. R. China. E-mail: liu_yyang1120@nwpu.edu.cn
First published on 6th March 2020
Abstract
Herein we designed and synthesized mPEG/PDPA copolymers containing two 4-([2,2′:6′,2′′-terpyridin]-4′-yl) phenyl (Tpyp) groups at the junction point of the two blocks (mPEG(-b-Tpyp)2-b-PDPAx, x = 23, 33, and 44). Interestingly, after a hierarchical pattern from the coordination of mPEG(-b-Tpyp)2-b-PDPAx with Ru(II) ions followed by the self-assembly in water, 2D strip nanosheets with a monomolecular layer were obtained. In contrast, mPEG(-b-Tpyp)2-b-PDPAx without coordination self-assembled into spherical micelles in the similar condition. The formation of the rigid and charged ⋯Tpyp-Ru(II)⋯ chain, the brush-shaped polymer architecture and the presence of the hexafluorophosphate (PF6−) counterions should be responsible for the unique self-assembly behavior of the metallo-supramolecular complexes. It is expected that the hierarchical self-assembly pattern can provide a new strategy for preparation of self-assemblies with different morphologies.
1. Introduction
Amphiphilic copolymers can self-assemble into various morphologic nanoaggregates in aqueous solution.1–8 The types of copolymers have therefore potential applications in many fields such as drug delivery9 and microcapsules.10 Therefore, exploration of self-assemblies with novel morphologies and functions attracts many researchers' attentions. Actual morphologies of self-assemblies of amphiphilic copolymers depend on many factors such as compositions and properties of building blocks, macromolecular architecture and self-assembly conditions. As far as architecture of a polymer is concerned, it is usually established by covalent bond linking. However, for some complicated polymer architectures, the synthesis steps are tedious. Therefore, it is interesting to explore novel morphological or functional aggregates from hierarchical supramolecular self-assembly of simple macromolecules.The ligand–metal coordination has currently been used to construct metallo-supramolecular polymers with different molecular architectures such as linear polymers,11–15 block16–22 and star polymers,23–25 dendrimers26 and even hydrogels.25,27 Although the binding strength of ligand–metal interaction in a range of approximately between 25% and 95% of a covalent C–C bond, the coordination bonding usually exhibits directionality and reversibility.12,18,19 Also, the presence of ligand–metal coordination moieties may endow supramolecular polymers with interesting properties or self-assembly behaviors.25,27,28
Macroligand–metal coordination is an important strategy to construct metallo-supramolecular polymers.29–32 For this method, the polymer units containing ligand moieties (macroligands) are first designed and synthesized, and the tailored units are then held together through the coordination with metal ions.12,14–24,30 Among ligand groups, 2,2′:6′,2′′-terpyridine (Tpy) moiety is widely introduced into polymer building blocks for synthesizing macroligands,12,14–23,28–32 because it is able to form stable Tpy-M-Tpy (M, metal ion) complexes with a wide range of metal ions such as Ni(II), Fe(II), Cu(II), Co(II), Zn(II), Cd(II) and Ru(II),29–34 making the formed metallo-supramolecular polymers have a well-defined architecture.15–23,30 Among complex species, Tpy-Ni(II)-Tpy and Tpy-Ru(II)-Tpy types exhibit good stability in water.14 U. S. Schubert et al. have investigated synthesis and coordination self-assembly of Tpy-containing polymers extensively and in-depth.12,14–21,23,29 For example, they constructed amphiphilic copolymers such as PS20-[Ru(II)]-PEO70 (PS, poly(styrene); PEO, poly(ethylene oxide); subscript, degree of polymerization (DP))17 and PS32-b-P2VP13-[Ru(II)]-PEO70 (P2VP, poly(2-vinylpyridine)).18 Liang and Xia et al. also synthesized PPG-[Cu(II)]-mPEG (PPG, poly(propylene glycol); mPEG, methoxypolyethylene glycol) by the similar molecular design.22 The metallo-supramolecular copolymers were able to self-assemble into spherical micelles in water.
Although Tpy-containing macroligands were widely investigated, more attentions were paid on synthesizing different Typ-ended polymers and further constructing copolymers by metal ion coordination linkage. In our opinion, for a A-b-B diblock copolymer, if two Tpy ligand groups are at the junction point of A and B blocks, the Tpy-M-Tpy coordination is able to drive the AB diblock copolymer units to self-assemble to form [-Typ-A(-b-B)-Typ-M-]n metallo-supramolecular architecture. Thus, the AB copolymer units are made directional alignment by metal ion linkage. As a result, ⋯Tpy-M⋯ chains generate, and the A/B blocks are transformed into pendant heterochains of the new supramolecular complexes. When further self-assembly of the complexes in aqueous solution, it is possible to form aggregates with a novel morphology, differing from that of precursor A-b-B copolymer. However, this has not been investigated yet.
Based on the consideration above, we herein designed and synthesized novel diblock copolymers containing two Tpy groups at the junction point of the two blocks, i.e. mPEG(-b-Tpyp)2-b-PDPAx (Mn, mPEG = 4000 g mol−1; PDPA, poly(2-(diisopropylamino)ethyl methacrylate); Tpyp, 4-([2,2′:6′,2′′-terpyridin]-4′-yl) phenyl group). Interestingly, after a hierarchical pattern from the coordination of mPEG(-b-Tpyp)2-b-PDPAx with Ru(II) ions followed by the self-assembly in water, 2D strip nanosheets with a monomolecular layer were obtained and parallel stripes of the nanosheets were observed. This suggests that the self-assembly units adopted directional array. In contrast, mPEG(-b-Tpyp)2-b-PDPAx copolymers without coordination self-assembled into spherical micelles in the similar condition. To our knowledge, so far this type of diblock copolymers, and their metallo-supramolecular complexes and self-assembly of the complexes have not been reported yet.
2. Results and discussion
The synthesis route for mPEG(-b-Tpyp)2-b-PDPAx is shown in Scheme 1 and the experimental methods are shown in ESI.† Herein pentaerythritol was selected as the core, because of its symmetry. It is therefore suitable for constructing AB-type diblock copolymer retaining two hydroxyl groups at the block junction point for linking two ligand groups, to further construct a brush-like metallo-supramolecular architecture with pendant heterochains. Based on this, core @(–OH)3–Cl was firstly synthesized via the reaction of one hydroxyl group of pentaerythritol with 2-chloropropionyl chloride (CPC) (Scheme 1a). Owing to pentaerythritol exhibiting low solubility in DMF at low temperature, the addition of CPC and the followed esterification were done at 50 °C. Compared with other monoesterification method of pentaerythritol,35 the synthesis involved one step reaction. 1H NMR data of @(–OH)3–Cl (Fig. S1, ESI†) confirm that it was synthesized successfully. Then, mPEG-COOH was synthesized via the reaction of mPEG with succinic anhydride in the presence of 4-dimethylaminopyridine (DMAP) (Scheme 1b).36,37 In Fig. S2 (ESI†), it could be observed clearly that the new resonances at chemical shift (δ) = 4.26 and 2.66 ppm are assigned to the protons of –CH2–O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)– and –OC(
O)– and –OC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH2CH2–C(
O)–CH2CH2–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O– groups, respectively, in addition to the characteristic resonances of the mPEG chain at δ = 3.66 and 3.40 ppm. This means that the succinic anhydride esterified the hydroxyl group of mPEG. Thirdly, the esterification of mPEG-COOH with @(–OH)3–Cl in the presence of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI)/DMAP38 afforded mPEG(–OH)2–Cl (Scheme 1c). 1H NMR spectrum of mPEG(–OH)2–Cl (Fig. S3, ESI†) indicates that the resonance intensity ratio of the methoxy group of mPEG to the methyl group of the –CH(CH3)Cl moiety is close to 1
O)O– groups, respectively, in addition to the characteristic resonances of the mPEG chain at δ = 3.66 and 3.40 ppm. This means that the succinic anhydride esterified the hydroxyl group of mPEG. Thirdly, the esterification of mPEG-COOH with @(–OH)3–Cl in the presence of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI)/DMAP38 afforded mPEG(–OH)2–Cl (Scheme 1c). 1H NMR spectrum of mPEG(–OH)2–Cl (Fig. S3, ESI†) indicates that the resonance intensity ratio of the methoxy group of mPEG to the methyl group of the –CH(CH3)Cl moiety is close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, indicating the fact that mPEG(–OH)2–Cl was synthesized successfully. Fourthly, by using mPEG(–OH)2–Cl as the ATRP macroinitiator, three copolymers mPEG(-b-OH)2-b-PDPAx were synthesized in DMF39 (Scheme 1d) through changing mPEG(–OH)2–Cl/DPA (DPA, 2-(diisopropylamino)ethyl methacrylate) feed ratios. The structure information of mPEG(-b-OH)2-b-PDPAx characterized by GPC (Fig. S4, ESI†) and 1H NMR (Fig. S5, ESI†) measurements is shown in Table 1. As seen from Table 1, the molecular weight distributions (Mw/Mn) of the three mPEG(-b-OH)2-b-PDPAx samples are narrow. In Fig. S5 (ESI†), the resonances at δ = 3.85, 3.01 and 2.65 ppm are assigned to the protons of the –C(
1, indicating the fact that mPEG(–OH)2–Cl was synthesized successfully. Fourthly, by using mPEG(–OH)2–Cl as the ATRP macroinitiator, three copolymers mPEG(-b-OH)2-b-PDPAx were synthesized in DMF39 (Scheme 1d) through changing mPEG(–OH)2–Cl/DPA (DPA, 2-(diisopropylamino)ethyl methacrylate) feed ratios. The structure information of mPEG(-b-OH)2-b-PDPAx characterized by GPC (Fig. S4, ESI†) and 1H NMR (Fig. S5, ESI†) measurements is shown in Table 1. As seen from Table 1, the molecular weight distributions (Mw/Mn) of the three mPEG(-b-OH)2-b-PDPAx samples are narrow. In Fig. S5 (ESI†), the resonances at δ = 3.85, 3.01 and 2.65 ppm are assigned to the protons of the –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OCH2–, –CH2N[CH(CH3)]2– and –CH2N[CH(CH3)]2 groups for the DPA units, respectively. The resonances at δ = 0.92 and 1.03 ppm are also assigned to the protons of the methyl groups of the DPA units. According to the resonance strength at δ = 3.40 from the methoxy group of the mPEG block, average x values of the PDPA blocks for the three copolymers were estimated to be 22, 31 and 42, respectively (Table 1). In this paper, the x values of the PDPA blocks were from 1H NMR measurement.
O)OCH2–, –CH2N[CH(CH3)]2– and –CH2N[CH(CH3)]2 groups for the DPA units, respectively. The resonances at δ = 0.92 and 1.03 ppm are also assigned to the protons of the methyl groups of the DPA units. According to the resonance strength at δ = 3.40 from the methoxy group of the mPEG block, average x values of the PDPA blocks for the three copolymers were estimated to be 22, 31 and 42, respectively (Table 1). In this paper, the x values of the PDPA blocks were from 1H NMR measurement.
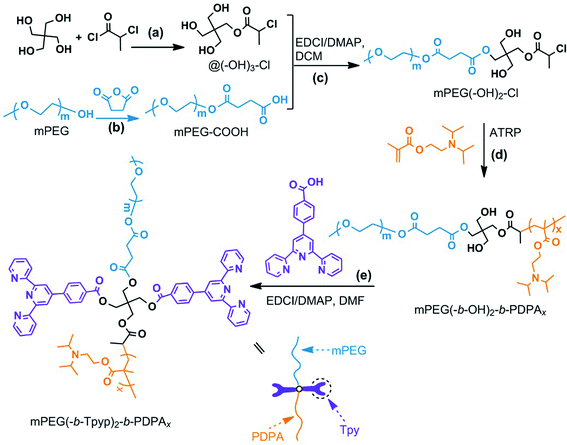 | ||
| Scheme 1 Synthesis route for mPEG(-b-Tpyp)2-b-PDPAx, which included syntheses of (a) @(-OH)3-Cl, (b) mPEG-COOH, (c) mPEG(-OH)2-Cl, (d) mPEG(-b-OH)2-b-PDPAx and (e) mPEG(-b-Tpyp)2-b-PDPAx. | ||
| Synthesis of mPEG(-b-OH)2-b-PDPAx | mPEG(-b-Tpyp)2-b-PDPAx | ||||
|---|---|---|---|---|---|
| x | mPEG(-b-OH)2-Cl/DPA (mol mol−1) | Mn, NMR (g mol−1) | Mn, GPC (g mol−1) | Mw/Mn | x |
| a The subscript x of PDPA refers as DP of the PDPA block, which was determined by 1H NMR spectrum. | |||||
| 22 | 1/15 | 8994 | 7084 | 1.15 | 23 |
| 31 | 1/20 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 911 911 |
7501 | 1.17 | 33 |
| 42 | 1/30 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 254 254 |
8059 | 1.27 | 44 |
mPEG(-b-Tpyp)2-b-PDPAx were synthesized via the esterification of mPEG(-b-OH)2-b-PDPAx with 4-([2,2′:6′,2′′-terpyridin]-4′-yl)benzoic acid in the presence of EDCI/DMAP (Scheme 1e). In 1H NMR spectra of mPEG(-b-Tpyp)2-b-PDPAx by using CDCl3 as the solvent (Fig. S6, ESI†), the resonances at δ = 8.69 (q, u), 8.60 (t), 8.14 (w), 7.94 (s), 7.87 (v) and 7.34 (r) ppm assigned to the protons of the Tpyp groups could be observed clearly in addition to the characteristic those of the mPEG and PDPA blocks. The resonance at δ = 4.58 (peak b′′′) is assigned to the new –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OCH2– groups formed via the esterification. Similarly, according to the resonance strength at δ = 3.40 from the mPEG block, it is confirmed that there are two Tpyp units per mPEG(-b-Tpyp)2-b-PDPAx molecule; and their x values were estimated to be 23, 33 and 44, respectively (Table 1). Compared with those of mPEG(-b-OH)2-b-PDPAx, a slight increase in the x values of mPEG(-b-Tpyp)2-b-PDPAx should be caused by loss of some low molecular weight copolymers via dialysis during the purification.
O)OCH2– groups formed via the esterification. Similarly, according to the resonance strength at δ = 3.40 from the mPEG block, it is confirmed that there are two Tpyp units per mPEG(-b-Tpyp)2-b-PDPAx molecule; and their x values were estimated to be 23, 33 and 44, respectively (Table 1). Compared with those of mPEG(-b-OH)2-b-PDPAx, a slight increase in the x values of mPEG(-b-Tpyp)2-b-PDPAx should be caused by loss of some low molecular weight copolymers via dialysis during the purification.
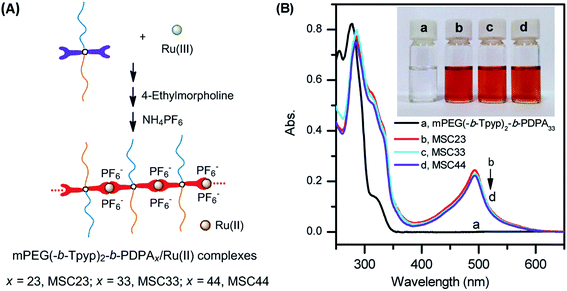
mPEG(-b-Tpyp)2-b-PDPAx/Ru(II) complexes (MSC23, MSC33 and MSC44, Fig. 1A) were prepared in ethanol solution at 75 °C (ESI†).12,16 The solutions of the complexes are red, while mPEG(-b-Tpyp)2-b-PDPAx solution is colorless (Fig. 1B). The formation of Tpy/Ru(II) coordination complexes was investigated by 1H NMR (Fig. S7, ESI†) and UV/vis spectra (Fig. 1B). Fig. S7 (ESI†) shows 1H NMR spectra in aromatic region for the Tpyp groups of sample MSC33 before and after complexing with Ru(II) ions by using CD3CN as the solvent. It is clear that Tpy/Ru(II) coordination led to an evident change in chemical shifts of the Tpyp protons. For example, for protons u, q and t of the Tpy group at δ = 8.54 ppm, after the Tpy group coordinated with Ru(II) ion their chemical shifts shifted to 9.16 (u′), 7.43 (q′) and 8.76 (t′) ppm, respectively. This confirms that mPEG(-b-Tpyp)2-b-PDPA33/Ru(II) complex formed.11,12 In Fig. 1B, the solutions of MSC23, MSC33 and MSC44 all exhibited evident characteristic metal-to-ligand charge-transfer (MLCT) absorption band of bis(terpyridine)-Ru(II) complex at 494 nm.11,16,17 This means that the complexes formed by bis(terpyridine)-Ru(II) coordination. This suggests that multiple mPEG(-b-Tpyp)2-b-PDPAx units were connected through Ru(II) ions. As a result, a rigid ⋯Tpyp-Ru(II)⋯ chain was created and the mPEG/PDPA blocks changed into the pendant heterochains of the new architecture.
Self-assembly of the metallo-supramolecular complexes was carried out through stepwise addition of water into their THF solutions. The THF was afterwards removed by reduced pressure. Interestingly, MSC23 and MSC33 mainly self-assembled into strip nanosheets (Fig. 2a1 and b1), which show several nanometers in width and tens of nanometers in length. But there were evident synechiae among the nanosheets. Under high resolution (e.g. the aggregates in the boxes of Fig. 2a1 and b1), the parallel stripes along the length direction of the nanosheets are observed (Fig. 2a2 and b2). This suggests that the self-assembly of MSC23 and MSC33 adopted highly directional array. The thickness of the nanosheets was determined by AMF images to be ca. 3.5 nm (Fig. 2a3 and b3). Note, the AMF images should also be a result of synechia of multiple strip nanosheets. Although the self-assembly of MSC44 produced spherical micelles (Fig. 2c1) besides some strip nanosheets (Fig. 2c2), the nanosheets still show parallel stripes like those of MSC23 and MSC33 self-assemblies. In Fig. 2c3, images af-3 and af-4 may correspond to sheet and spherical morphologies, respectively. These results imply that x values of the PDPA blocks affected actual self-assembled morphologies of the supramolecular complexes. In contrast, copolymers mPEG(-b-Tpyp)2-b-PDPAx formed spherical micelles (Fig. S8, ESI†). Self-assembly behaviors of analogous copolymers mPEG-b-PDPA (or PEO-b-PDPA) were also investigated by other authors. For example, Yu and Li et al. obtained mPEG114-b-PDPA wormlike micellar structure by a film sonication self-assembly method when mPEG weight fraction of 0.36 ≤ WmPEG ≤ 0.48.40 Wu and Li et al. found that mPEG44-b-PDPA15 could form toruloid aggregates arranged by several single micelles at pH 7.4.41 Xu and Kang et al. prepared mPEG44-b-PDPA80 spherical micelles in aqueous solution.42 Mai et al. prepared PEO44-b-PDPA micelles or polymersomes in water.43 Some metallo-supramolecular block copolymers based on Tpy–metal coordination such as PS20-[Ru(II)]-PEO70 (ref. 17) and PPG-[Cu(II)]-mPEG22 self-assembled into spherical micellar morphology in water. PFS12-[Ru(II)]-PEO70 (PFS, poly-(ferrocenylsilane))20 formed cylindrical micelles in water. Therefore, our metallo-supramolecular complexes should exhibit different self-assembly fashion.
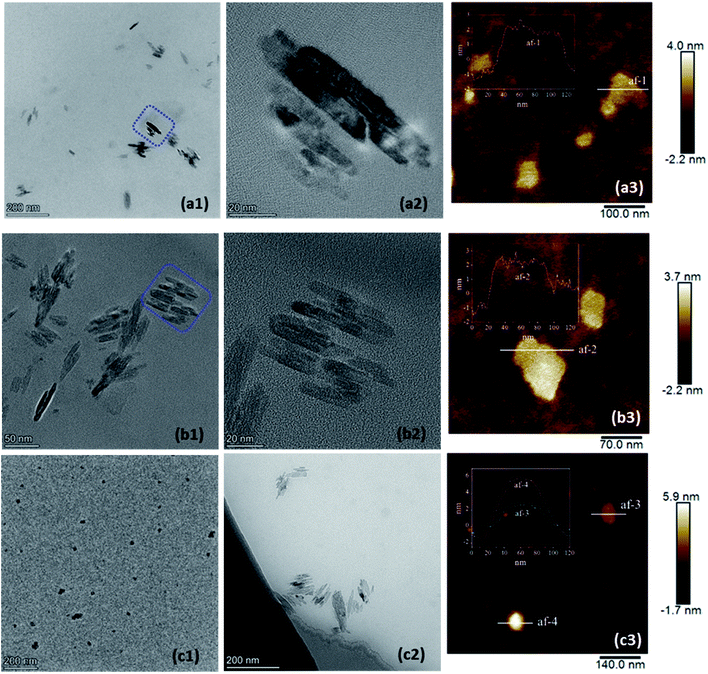 | ||
| Fig. 2 TEM (a1, a2, b1, b2, c1 and c2) and AFM (a3, b3 and c3) images of the self-assembled aggregates from MSC23 (a1–a3), MSC33 (b1–b3) and MSC44 (c1–c3). | ||
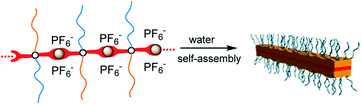
When mPEG(-b-Tpyp)2-b-PDPAx are not coordinated by Ru(II) ions, the large and rigid Tpyp moiety only serves as a hydrophobic pendant group at the block junction point (Scheme 1e). Like some amphiphilic diblock copolymers, mPEG(-b-Tpyp)2-b-PDPAx only self-assembled into spherical micelles in water (Fig. S8, ESI†), a very common and easily prepared morphology. But the formation of bis(terpyridine)-Ru(II) complexes made mPEG(-b-Tpyp)2-b-PDPAx units orientational linkage via Ru(II) ions. Consequently, a positively charged rigid ⋯Tpyp-Ru(II)⋯ chain was created (Fig. 1A), because of rigidity of the Tpyp group itself and orientation of coordination bonding. Furthermore, the charge repulsion of ⋯Tpyp-Ru(II)⋯ chain may also enhance its rigidity. The mPEG/PDPA blocks thus changed into pendant chains of the complexes. Hence, the metallo-supramolecular complexes are polyelectrolytes with positively charged rigid chains along with the hexafluorophosphate (PF6−) counterions11 and with a brush-like polymer architecture. When water induced self-assembly of the complexes, the aggregations of the rigid ⋯Tpyp-Ru(II)⋯ chains carrying the PF6− counterions and the hydrophobic PDPA blocks should be driving forces. Since the mPEG chains show a hydrated state and the PDPA blocks are not rigid in spite of their hydrophobicity, it is highly possible that the regular self-assembly of the metallo-supramolecular complexes was from directional arrangement of the rigid ⋯Tpyp-Ru(II)⋯ chains carrying the hydrophobic PF6− counterions. It was noted that MSC44 formed mixed spherical and sheet morphologies. This may be due to the fact that high DP of the PDPA block lowers the regularity of the supramolecular complex and enhances the intermolecular hydrophobic interaction, perturbing regular array of the rigid ⋯Tpyp-Ru(II)⋯ chains. It was also noted that the nanosheets from MSC33 were more stable than those from MSC23. Therefore, the PDPA block with an appropriate DP may be needed for metallo-supramolecular complex to self-assemble into a nanosheet morphology. According to the thickness of ca. 3.5 nm, it is speculated that the nanosheets should be a 2D monomolecular layer structure.8 The self-assembly mechanism may be that the intermolecular aggregation of the rigid ⋯Tpyp-Ru(II)⋯ chains first formed a sheet, whose driving forces should be the hydrophobic and π–π interactions of the rigid chains. And the mPEG/PDPA pendant chains accordingly emanated from the two sides of the sheet. The mPEG chains showed an extended state, guaranteeing water-solubility of the self-assemblies, while the PDPA blocks gradually formed inward collapsed hydrophobic domains and stabilized the nanosheets. A proposed self-assembly pattern for the nanosheets is shown in Scheme 2.
Owing to pH-sensitivity of the PDPA block (pKa of protonated DPA homopolymer, ∼6),44 The influence of change in environmental pH on the morphologies of the self-assemblies above was also investigated by TEM observation. When the pH adjusted to 8.5, the morphologies of the self-assemblies didn't change evidently (Fig. S9a, S10a and S11a, ESI†) compared with those of their parent samples. Interestingly, when the pH of the solutions adjusted to 3.2, most of the strip nanosheet morphologies were destroyed due to the PDPA blocks changing into hydrophilicity, but nanosized aggregates were still observed from the three samples (Fig. S9b, S10b and S11b, ESI†). Wu and Li et al. reported that mPEG44-b-PDPA15 could not form apparent nanostructure at pH 5.5.41 The observable aggregates of our samples should be attributed to the hydrophobic aggregation of the ⋯Tpyp-Ru(II)⋯ chains. Therefore, the hydrophobic interactions for the self-assembly of the complexes were from both the rigid ⋯Tpyp-Ru(II)⋯ chains and the PDPA blocks. And the formation and stability of the strip nanosheets were closely related to the hydrophobic interaction of the PDPA blocks.
The hydrophobic domains of the self-assemblies of the metallo-supramolecular complexes were further detected by fluorescence probe Nile Red (NR) (ESI†), because NR is fluorescent in hydrophobic environment but weak one in polar condition.38,45 Herein the NR dosage used should not lead to visible precipitate when the THF was removed from the systems for self-assembly. The NR-loaded samples were diluted with water to 10 times for fluorescent spectrum measurement (Fig. S12, ESI†). The results indicate that samples NR/MSC44 (1.75/100, weight ratio), NR/MSC33 (0.75/100) and NR/MSC23 (1/100) showed fluorescent emission peaks at λem, max = 571–574 nm (Fig. S12, ESI†).38 Judged from the fluorescence intensity, a higher PDPA content formed a stronger hydrophobic domain in the aggregates (Fig. S12, ESI†). The TEM observations show that NR/MSC23 (1/100) self-assembled into mixed spherical micellar and nanosheet morphologies (Fig. S13a, ESI†) and NR/MSC44 (1.75/100) produced spherical micelles containing a small number of strip nanosheets (Fig. S13c, ESI†); while NR/MSC33 (0.75/100) mainly formed strip nanosheets (Fig. S13b, ESI†). It was found that the fluorescent intensities of the NR-loaded samples at λem, max remained 75–80% after the incubation at 37 °C for 19.5 h. Then, the pH of the solutions was adjusted to 3.2, their fluorescent intensity lowered to 4–7% of initial values when measured after 30 min. This indicates that the NR molecules were initially mainly encapsulated in the hydrophobic PDPA domains and the NR in the domains was therefore quickly released into polar water medium when the PDPA blocks changing into hydrophilicity at pH 3.2.
3. Conclusion
In summary, mPEG(-b-Tpyp)2-b-PDPAx (x = 23, 33 and 44) block copolymer units were synthesized. After a hierarchical pattern of the coordination of the copolymers with Ru(II) ions followed by the self-assembly in water, strip nanosheets were obtained. And the parallel stripes along the length direction of the nanosheets were observed. Estimated from the thickness of ca. 3.5 nm for the nanosheets, they should be a 2D monomolecular layer structure. In contrast, mPEG(-b-Tpyp)2-b-PDPAx without coordination formed spherical micelles. The formation of the strip nanosheets should be closely related to the architecture of the metallo-supramolecular complexes, i.e. the formation of the rigid ⋯Tpyp-Ru(II)⋯ chains, the PDPA block with an appropriate DP and its hydrophobic interaction, even the presence of the PF6− counterions. It is expected that the hierarchical self-assembly pattern can provide a new strategy of preparation of self-assemblies with different morphologies.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21871222).Notes and references
- H. Qiu, Z. M. Hudson, M. A. Winnik and I. Manners, Science, 2015, 347, 1329–1332 CrossRef CAS PubMed.
- G. Rizis, T. G. M. van de Ven and A. Eisenberg, Angew. Chem., Int. Ed., 2014, 53, 9000–9003 CrossRef CAS PubMed.
- L. Cheng, G. Zhang, L. Zhu, D. Chen and M. Jiang, Angew. Chem., Int. Ed., 2008, 47, 10171–10174 CrossRef CAS PubMed.
- F. Xu, D. Wu, Y. Huang, H. Wei, Y. Gao, X. Feng, D. Yan and Y. Mai, ACS Macro Lett., 2017, 6, 426–430 CrossRef CAS.
- P. Zhou, Y.-Y. Liu, L.-Y. Niu and J. Zhu, Polym. Chem., 2015, 6, 2934–2944 RSC.
- L. Niu, Y. Liu, Y. Hou, W. Song and Y. Wang, Polym. Chem., 2016, 7, 3406–3415 RSC.
- Y. Hirai, T. Terashima, M. Takenaka and M. Sawamoto, Macromolecules, 2016, 49, 5084–5091 CrossRef CAS.
- Y. Wei, J. Tian, Z. Zhang, C. Zhu, J. Sun and Z. Li, Macromolecules, 2019, 52, 1546–1556 CrossRef CAS.
- J. Zhang, J. Liu, Y. Zhu, Z. Xu, J. Xu, T. Wang, H. Yu and W. Zhang, Chem. Commun., 2016, 52, 12044–12047 RSC.
- Z. Wang, J. Gao, V. Ustach, C. Li, S. Sun, S. Hu and R. Faller, Langmuir, 2017, 33, 7288–7297 CrossRef CAS PubMed.
- S. Kelch and M. Rehahn, Macromolecules, 1999, 32, 5818–5828 CrossRef CAS.
- H. Hofmeier, S. Schmatloch, D. Wouters and U. S. Schubert, Macromol. Chem. Phys., 2003, 204, 2197–2203 CrossRef CAS.
- L. He, J. Liang, Y. Cong, X. Chen and W. Bu, Chem. Commun., 2014, 50, 10841–10844 RSC.
- B. G. G. Lohmeijer and U. S. Schubert, Macromol. Chem. Phys., 2003, 204, 1072–1078 CrossRef CAS.
- H. Hofmeier, R. Hoogenboom, M. E. L. Wouters and U. S. Schubert, J. Am. Chem. Soc., 2005, 127, 2913–2921 CrossRef CAS PubMed.
- B. G. G. Lohmeijer and U. S. Schubert, Angew. Chem., Int. Ed., 2002, 41, 3825–3829 CrossRef CAS PubMed.
- J.-F. Gohy, B. G. G. Lohmeijer and U. S. Schubert, Macromolecules, 2002, 35, 4560–4563 CrossRef CAS.
- J.-F. Gohy, B. G. G. Lohmeijer, S. K. Varshney, B. Décamps, E. Leroy, S. Boileau and U. S. Schubert, Macromolecules, 2002, 35, 9748–9755 CrossRef CAS.
- M. Chiper, M. A. R. Meier, D. Wouters, S. Hoeppener, C.-A. Fustin, J.-F. Gohy and U. S. Schubert, Macromolecules, 2008, 41, 2771–2777 CrossRef CAS.
- J.-F. Gohy, B. G. G. Lohmeijer, A. Alexeev, X.-S. Wang, I. Manners, M. A. Winnik and U. S. Schubert, Chem.–Eur. J., 2004, 10, 4315–4323 CrossRef CAS PubMed.
- C. Ott, R. Hoogenboom, S. Hoeppener, D. Wouters, J.-F. Gohy and U. S. Schubert, Soft Matter, 2009, 5, 84–91 RSC.
- B. Liang, R. Tong, Z. Wang, S. Guo and H. Xia, Langmuir, 2014, 30, 9524–9532 CrossRef CAS PubMed.
- J.-F. Gohy, M. Chiper, P. Guillet, C.-A. Fustin, S. Hoeppener, A. Winter, R. Hoogenboomb and U. S. Schubert, Soft Matter, 2009, 5, 2954–2961 RSC.
- N. Hosono, M. Gochomori, R. Matsuda, H. Sato and S. Kitagawa, J. Am. Chem. Soc., 2016, 138, 6525–6531 CrossRef CAS PubMed.
- W. Zheng, L.-J. Chen, G. Yang, B. Sun, X. Wang, B. Jiang, G.-Q. Yin, L. Zhang, X. Li, M. Liu, G. Chen and H.-B. Yang, J. Am. Chem. Soc., 2016, 138, 4927–4937 CrossRef CAS PubMed.
- C. Kim and H. Kim, J. Organomet. Chem., 2003, 673, 77–83 CrossRef CAS.
- X. Yan, S. Li, T. R. Cook, X. Ji, Y. Yao, J. B. Pollock, Y. Shi, G. Yu, J. Li, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2013, 135, 14036–14039 CrossRef CAS PubMed.
- Q. Zheng, Z. Ma and S. Gong, J. Mater. Chem. A, 2016, 4, 3324–3334 RSC.
- A. Winter and U. S. Schubert, Chem. Soc. Rev., 2016, 45, 5311–5357 RSC.
- Y.-J. He, T.-H. Tu, M.-K. Su, C.-W. Yang, K. V. Kong and Y.-T. Chan, J. Am. Chem. Soc., 2017, 139, 4218–4224 CrossRef CAS PubMed.
- Y. Yan and J. Huang, Coord. Chem. Rev., 2010, 254, 1072–1080 CrossRef CAS.
- R. Shunmugam, G. J. Gabriel, K. A. Aamer and G. N. Tew, Macromol. Rapid Commun., 2010, 31, 784–793 CrossRef CAS.
- G. Gröger, W. Meyer-Zaika, C. Böttcher, F. Gröhn, C. Ruthard and C. Schmuck, J. Am. Chem. Soc., 2011, 133, 8961–8971 CrossRef PubMed.
- X. Lu, X. Li, K. Guo, T.-Z. Xie, C. N. Moorefield, C. Wesdemiotis and G. R. Newkome, J. Am. Chem. Soc., 2014, 136, 18149–18155 CrossRef CAS PubMed.
- A. B. Padias and H. K. Hall, Jr, Macromolecules, 1982, 15, 217–223 CrossRef CAS.
- B. Treetharnmathurot, C. Ovartlarnporn, J. Wungsintaweekul, R. Duncan and R. Wiwattanapatapee, Int. J. Pharm., 2008, 357, 252–259 CrossRef CAS PubMed.
- B. Parrish and T. Emrick, Macromolecules, 2004, 37, 5863–5865 CrossRef CAS.
- Y. Wang, Y. Liu, J. Liang and M. Zou, RSC Adv., 2017, 7, 11691–11700 RSC.
- K. Dayananda, M. S. Kim, B. S. Kim and D. S. Lee, Macromol. Res., 2007, 15, 385–391 CrossRef CAS.
- H. Yu, Z. Xu, D. Wang, X. Chen, Z. Zhang, Q. Yin and Y. Li, Polym. Chem., 2013, 4, 5052–5055 RSC.
- W. Wu, W. Wang, S. Li, J. Wang, Q. Zhang, X. Li, X. Luo and J. Li, J. Polym. Res., 2014, 21, 494 CrossRef.
- Z. Xu, P. Xue, Y.-E. Gao, S. Liu, X. Shi, M. Hou and Y. Kang, J. Colloid Interface Sci., 2017, 490, 511–519 CrossRef CAS PubMed.
- B. T. Mai, M. Barthel, R. Marotta and T. Pellegrino, Polymer, 2019, 165, 19–27 CrossRef CAS.
- V. Bütün, S. P. Armes and N. C. Billingham, Polymer, 2001, 42, 5993–6008 CrossRef.
- M. K. Gupta, J. R. Martin, T. A. Werfel, T. Shen, J. M. Page and C. L. Duvall, J. Am. Chem. Soc., 2014, 136, 14896–14902 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods, 1H NMR and fluorescence spectra, GPC traces, TEM images and DLS measurements. See DOI: 10.1039/d0ra00431f |
| This journal is © The Royal Society of Chemistry 2020 |